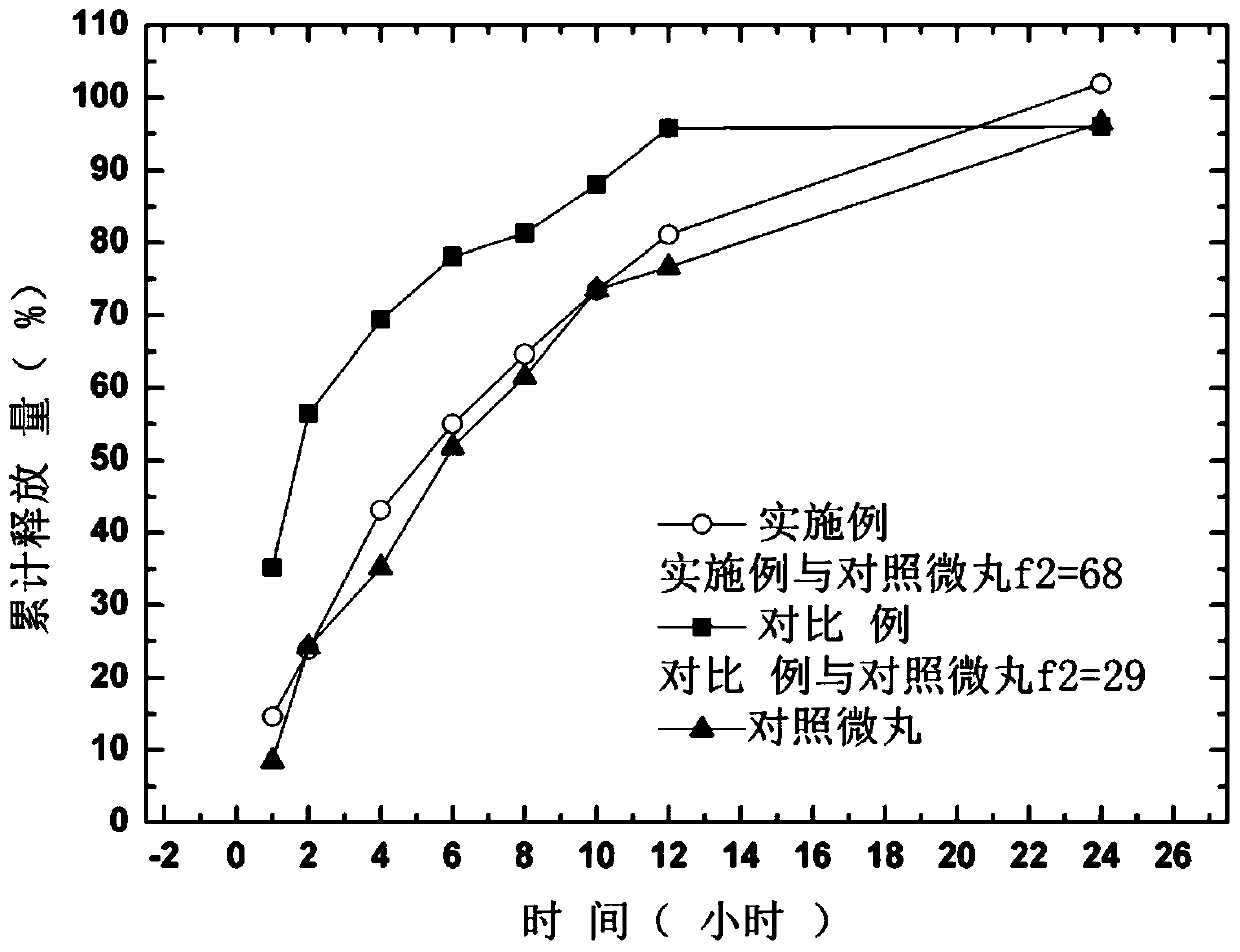
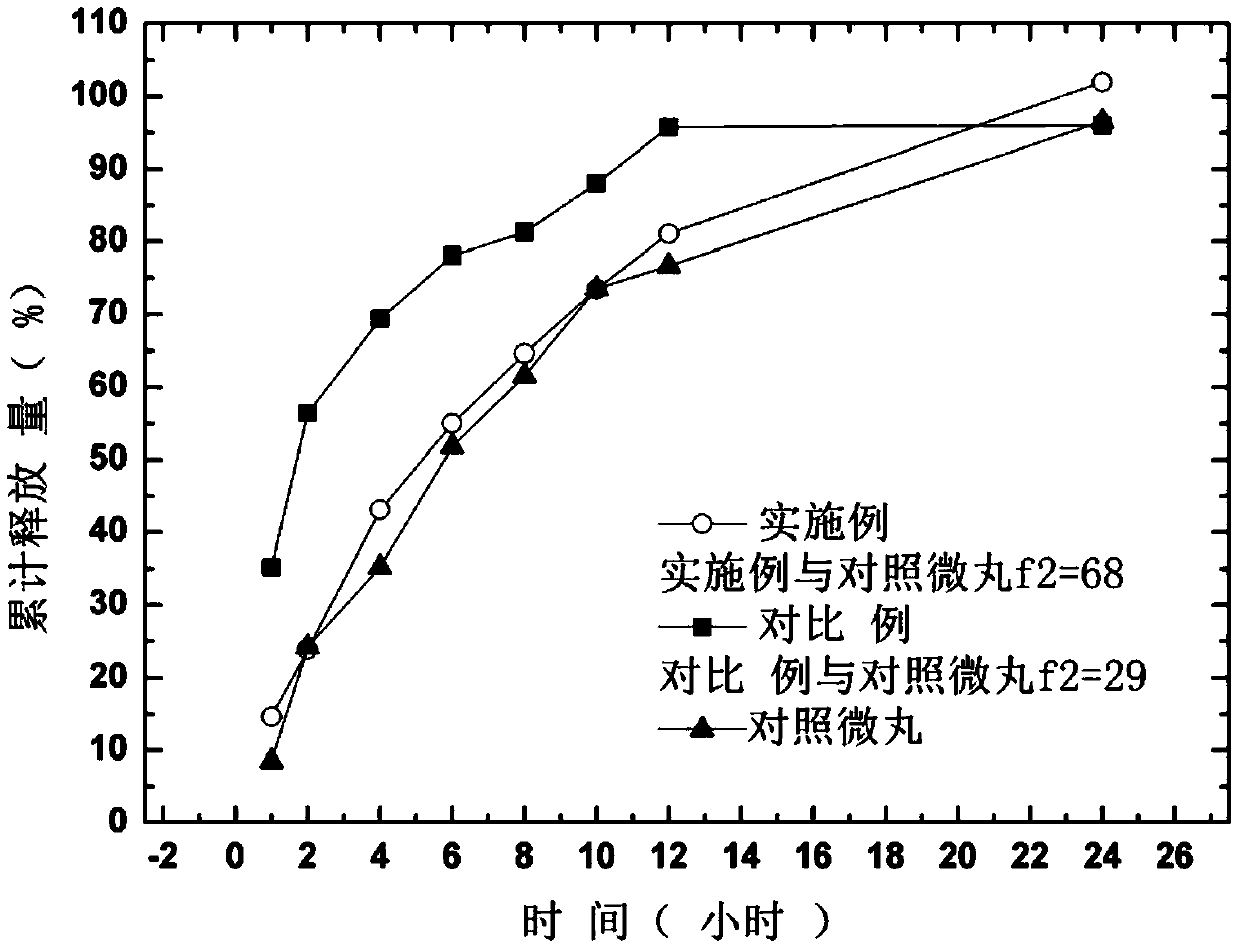
A kind of memantine hydrochloride sustained-release pellet tablet and preparation method thereof
A technology of memantine hydrochloride and sustained-release micro-pill tablets, which is applied in the directions of pill delivery, microcapsules, nervous system diseases, etc., can solve the problems of uncontrollable release, difficult to mix uniformly, unqualified drug content uniformity in tablets, etc. The effect of good compressibility, good drug content uniformity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
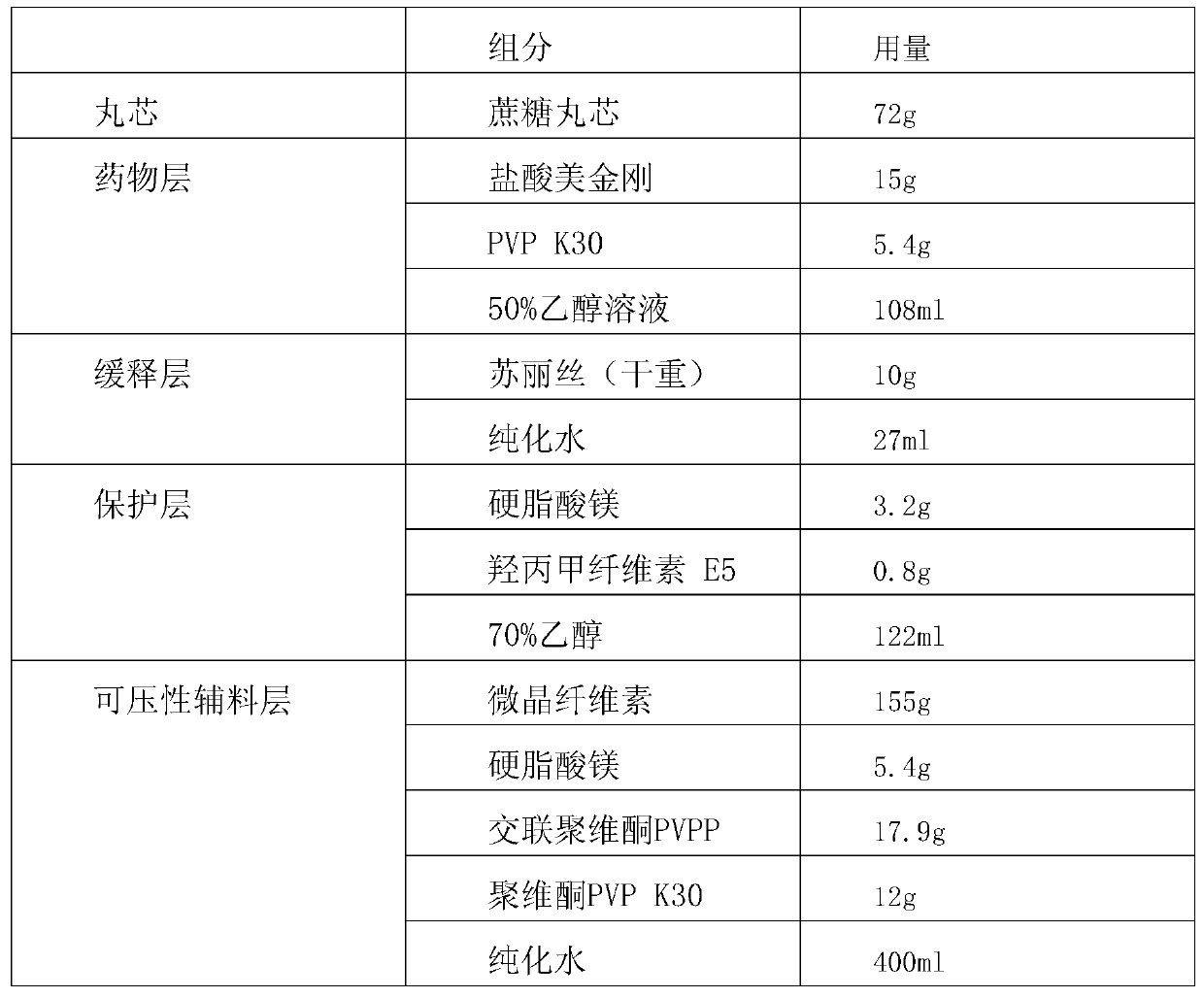
[0032] The composition of self-protected multi-layer pellets is shown in the following table:
[0033]
[0034] Preparation:
[0035] Drug coating layer: dissolve memantine hydrochloride and PVP K30 in 50% ethanol solution to prepare drug solution containing adhesive. Put the sucrose ball core into the fluidized bed coating machine, use the bottom spray method to wrap the above drug solution on the surface of the ball core, the atomization pressure is controlled at 0.1MPa, and the ventilation volume is 50m 3 / h, material temperature 32-38 ℃. After the drug application is completed, the atomization pressure is turned off and the micropills are kept in a fluidized state for 15 minutes to prepare the micropills containing the drug layer for subsequent use.
[0036] Including slow-release layer: disperse Surelease in an appropriate amount of water to make an aqueous dispersion with a solid content of 15%, and stir for more than 15 minutes. Put the drug-containing pellets int...
Embodiment 2
[0042] The composition of self-protected multi-layer pellets is shown in the following table:
[0043]
[0044]
[0045] Preparation:
[0046] Drug coating layer: dissolve memantine hydrochloride and PVP K30 in 50% ethanol solution to prepare drug solution containing adhesive. Put the sucrose ball core into the fluidized bed coating machine, use the bottom spray method to wrap the above drug solution on the surface of the ball core, the atomization pressure is controlled at 0.15MPa, and the ventilation volume is 60m 3 / h, material temperature 32-37°C. After the drug application is completed, the atomization pressure is turned off to allow the micropills to continue to maintain the fluidized state for 10 minutes, and the micropills containing the drug layer are prepared for subsequent use.
[0047] Including slow-release layer: disperse Surelease in an appropriate amount of water to make an aqueous dispersion with a solid content of 15%, and stir for more than 15 minute...
Embodiment 3
[0053] The lubricant in the protective layer of the self-protecting multi-layer pellets is selected from sodium stearate fumarate, and the composition of the pellets is shown in the table below.
[0054]
[0055] Preparation:
[0056] Drug coating layer: dissolve memantine hydrochloride and PVP K30 in 50% ethanol solution to prepare drug solution containing adhesive. Put the sucrose ball core into the fluidized bed coating machine, use the bottom spray method to wrap the above drug solution on the surface of the ball core, the atomization pressure is controlled at 0.1MPa, and the ventilation volume is 70m 3 / h, material temperature 34-39 ℃. After the drug application is completed, the atomization pressure is turned off to allow the micropills to continue to maintain the fluidized state for 30 minutes, and the micropills containing the drug layer are prepared for subsequent use.
[0057] Including slow-release layer: disperse Surelease in appropriate amount of water to mak...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| hardness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com