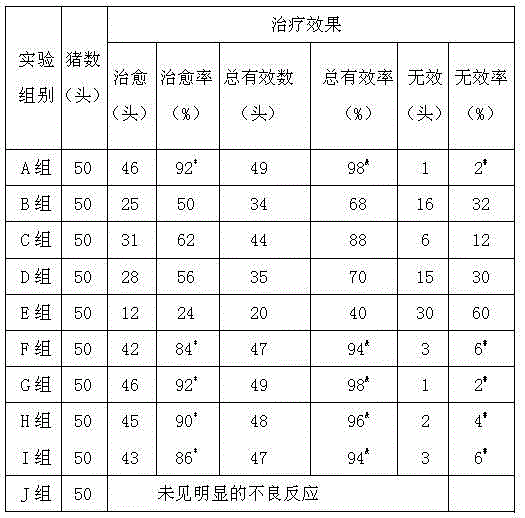
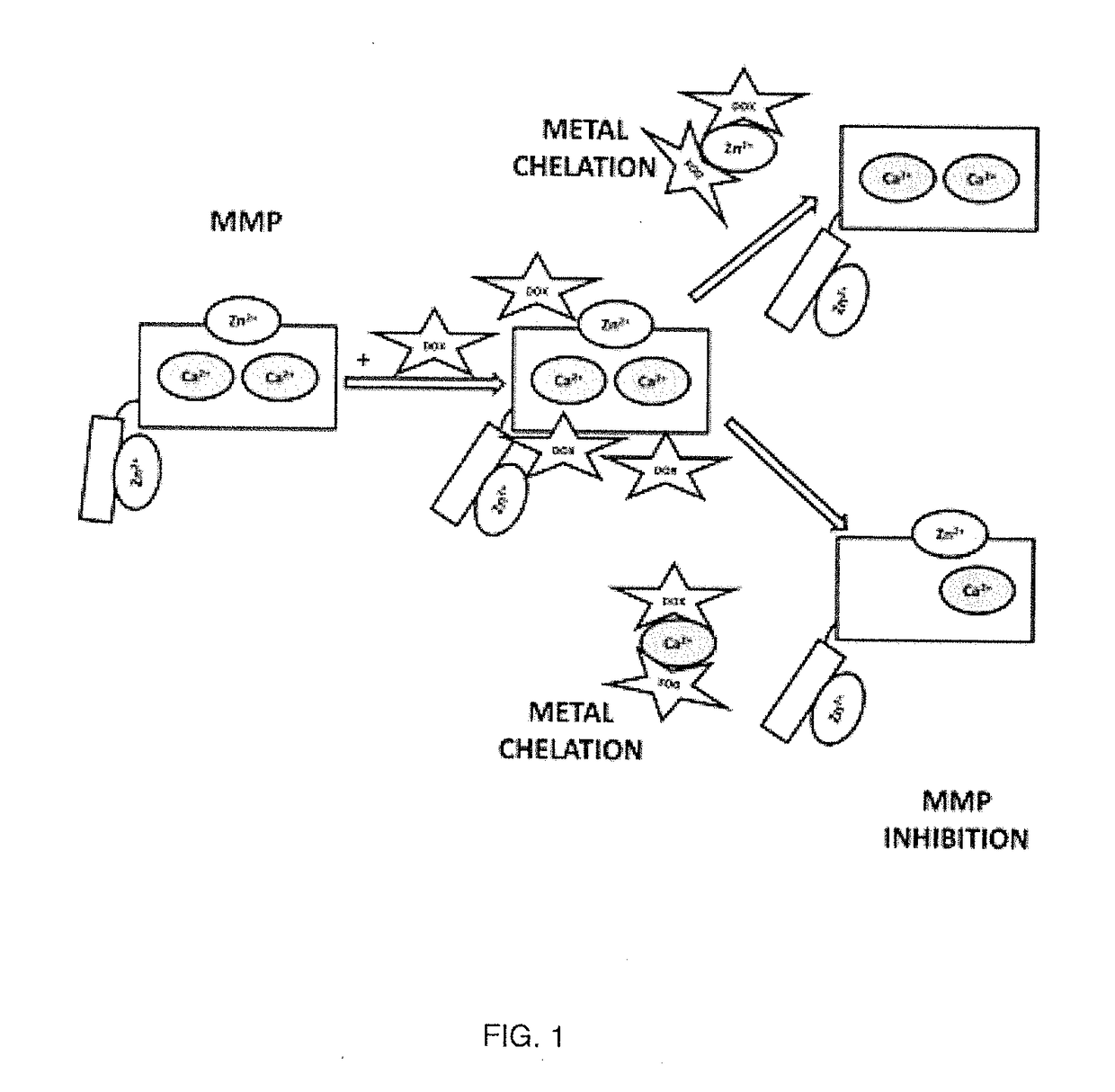
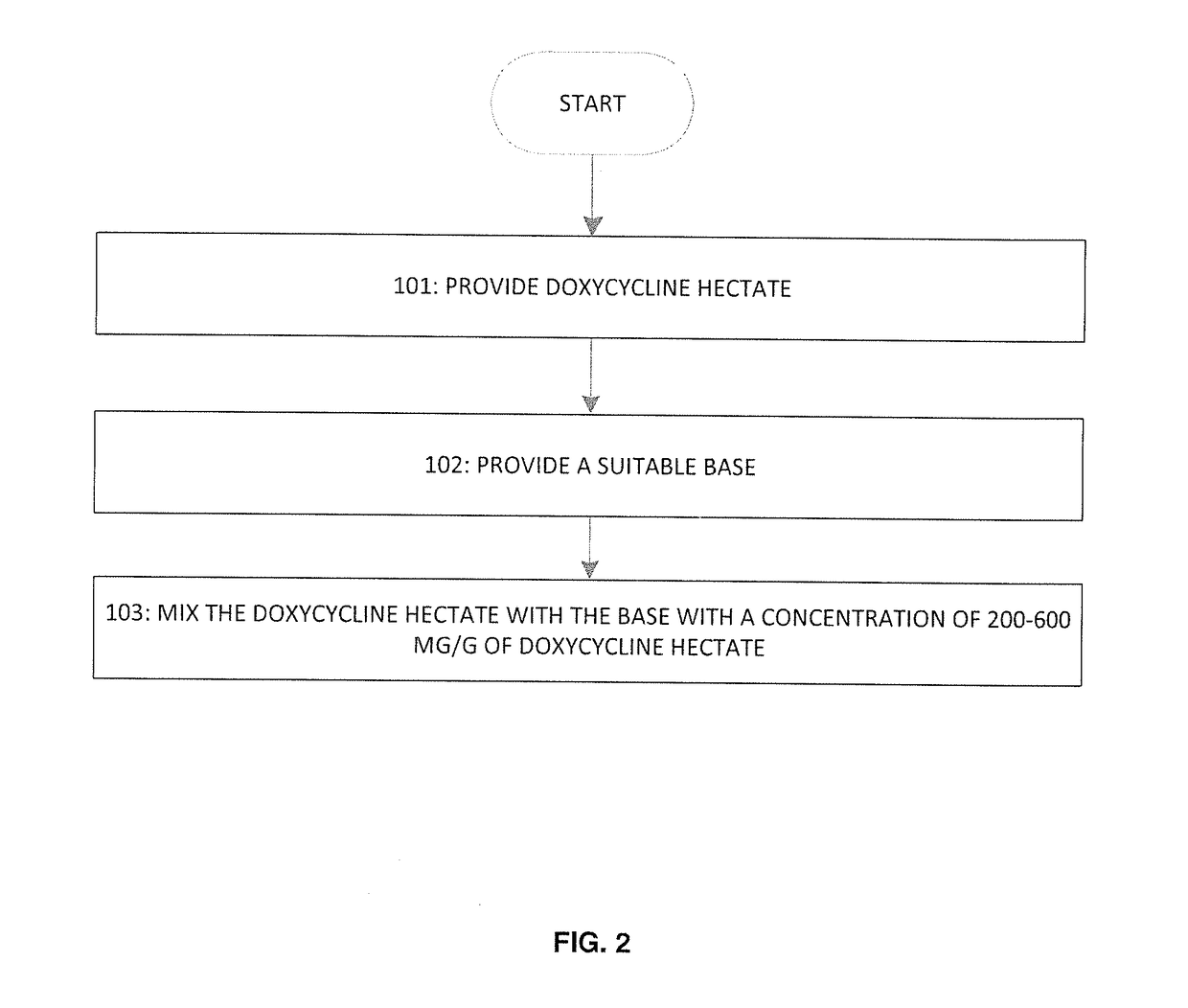
Patents
Literature
31 results about "Doxycycline Hyclate" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
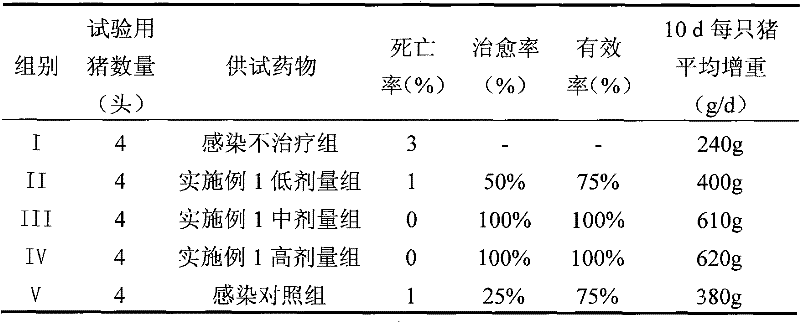
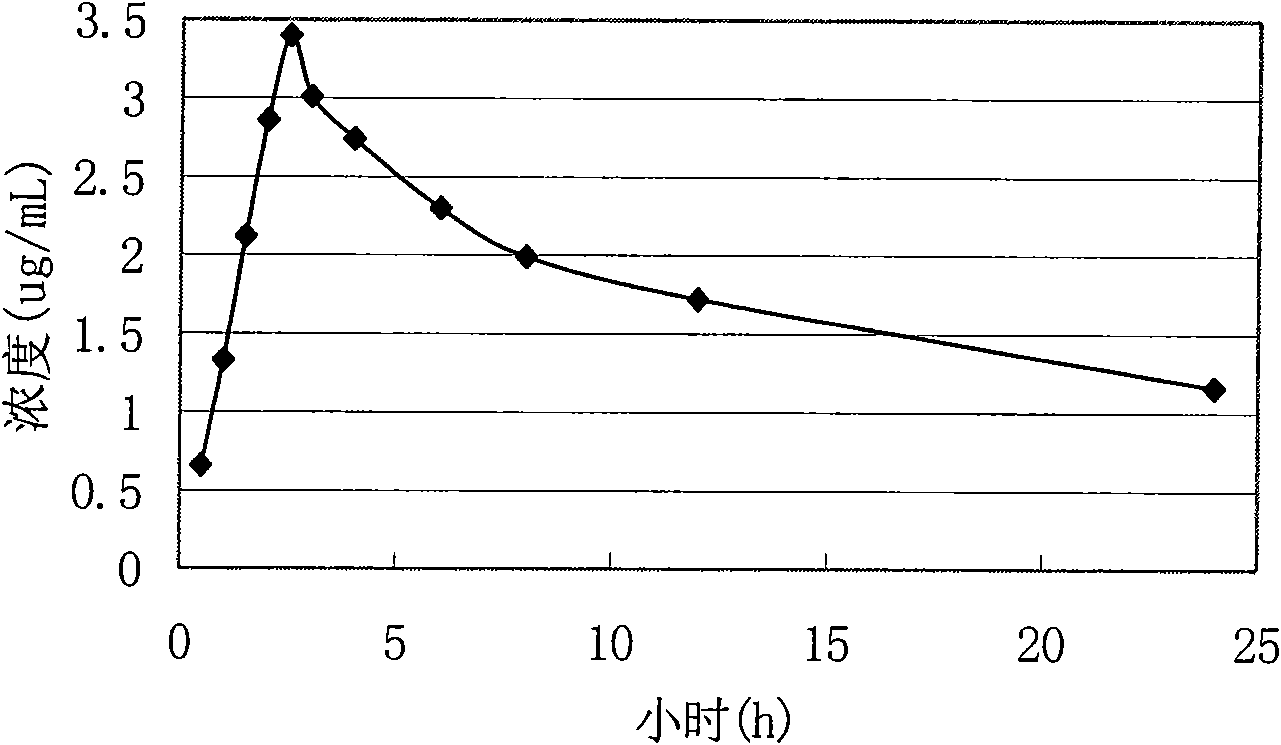
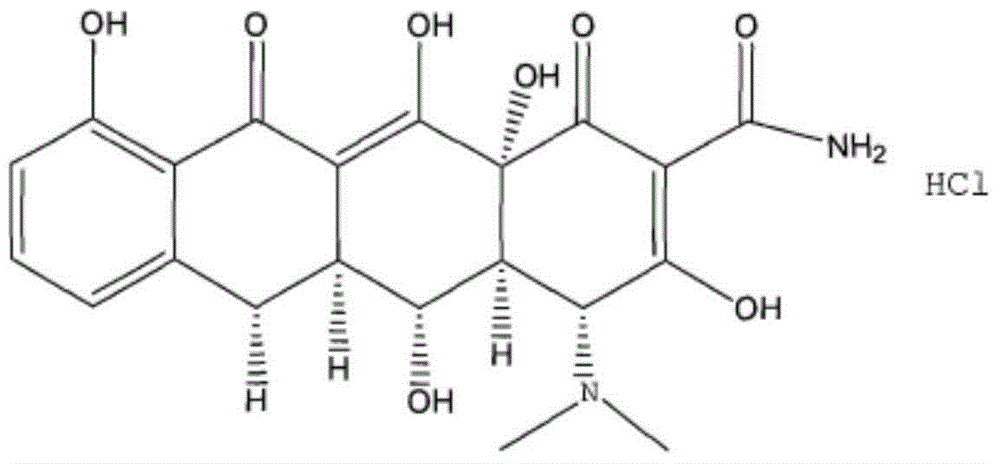
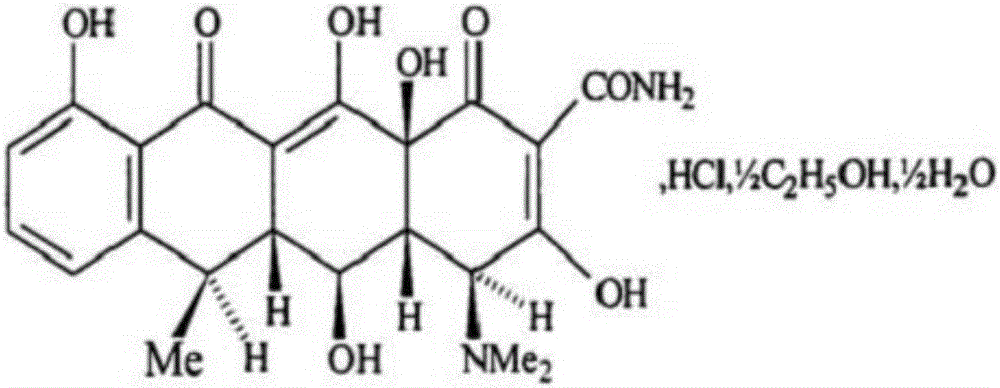
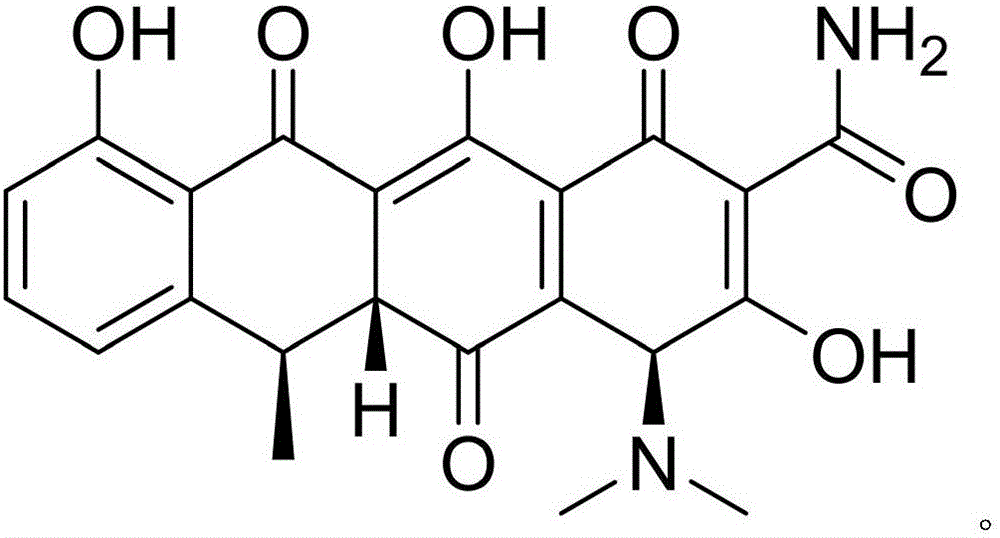
The hyclate salt form of doxycycline, a synthetic, broad-spectrum tetracycline antibiotic exhibiting antimicrobial activity. Doxycycline hyclate binds reversibly to the 30S ribosomal subunit, possibly to the 50S ribosomal subunit as well, thereby blocking the binding of aminoacyl-tRNA to the mRNA-ribosome complex. This leads to an inhibition of protein synthesis. In addition, this agent has exhibited inhibition of collagenase activity.
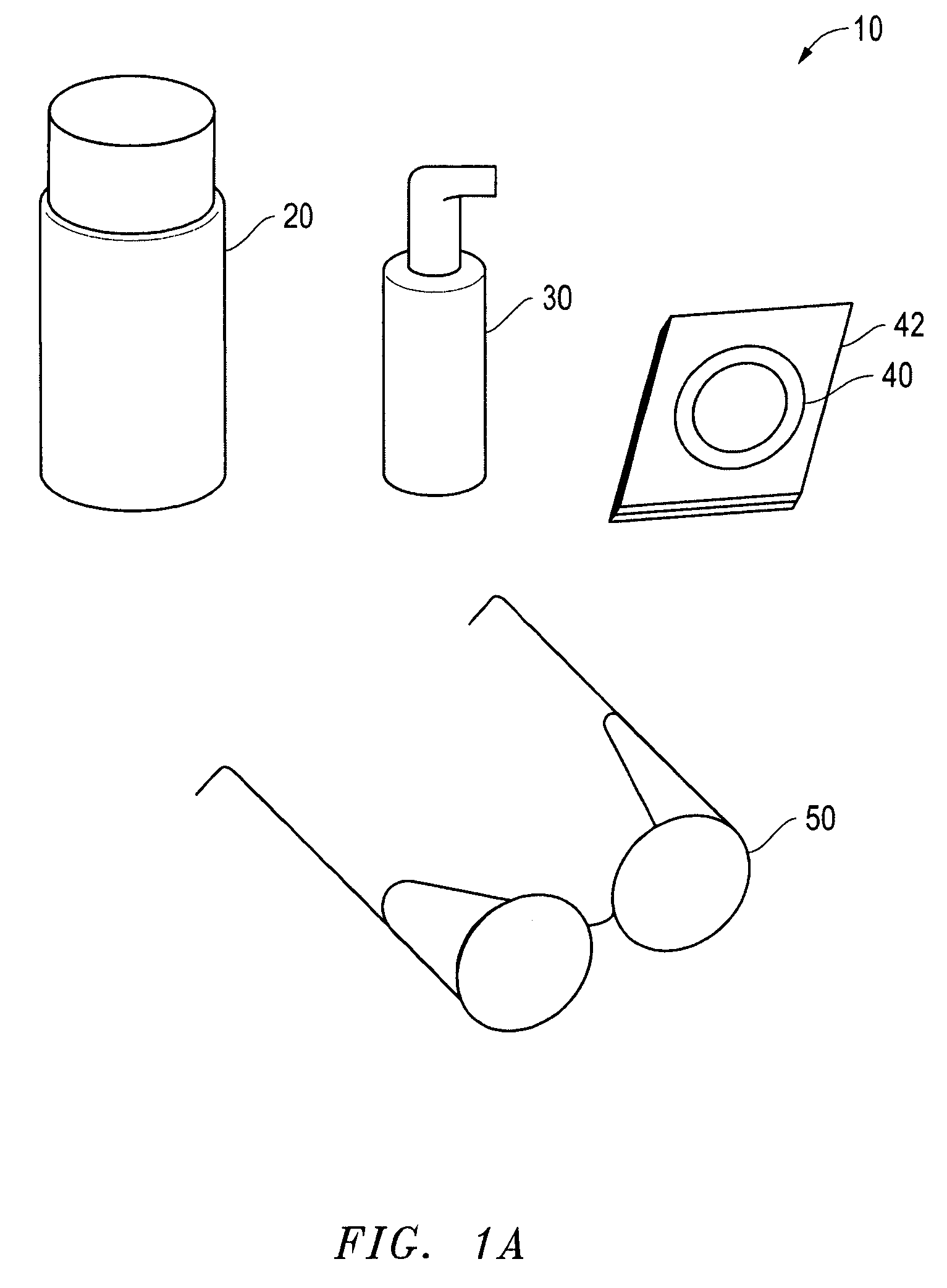
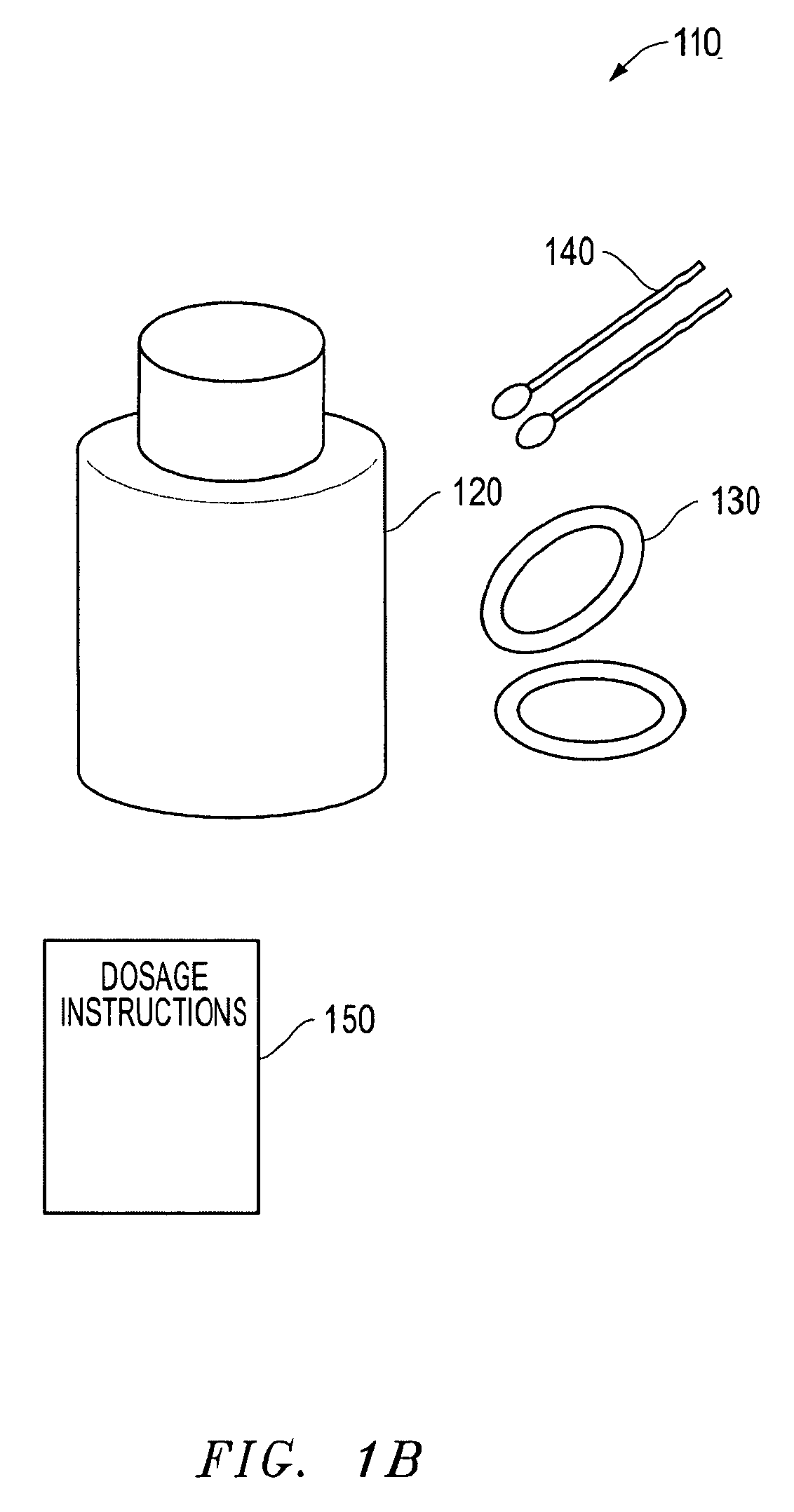
Convenience kit for eyelid treatment
ActiveUS8202853B2Improving overall eyelid hygieneConvenient treatmentAntibacterial agentsCosmetic preparationsEyelidMoisture chamber goggles
The present invention offers an eyelid treatment kit used for convenient combination therapy for improving overall eyelid hygiene while also providing for adjunctive eyelid therapy. The eyelid treatment kit comprises low dose doxycycline hyclate tablets, a non-irritating eyelid cleansing composition, an anti-bacterial eyelid preparation and at least one pair of moist heat goggles and / or one pair of moisture chamber goggles. The eyelid treatment kit further comprises instruction sheets containing dosage and administration information on the doxycycline hyclate coupled with information on improving eyelid hygiene. The various embodiments of the eyelid treatment kit of the invention facilitate treatment of dry eyes due to infected eyelids, and proper cleansing of the eyelids to prevent recurring infections.
Owner:OCUSOFT
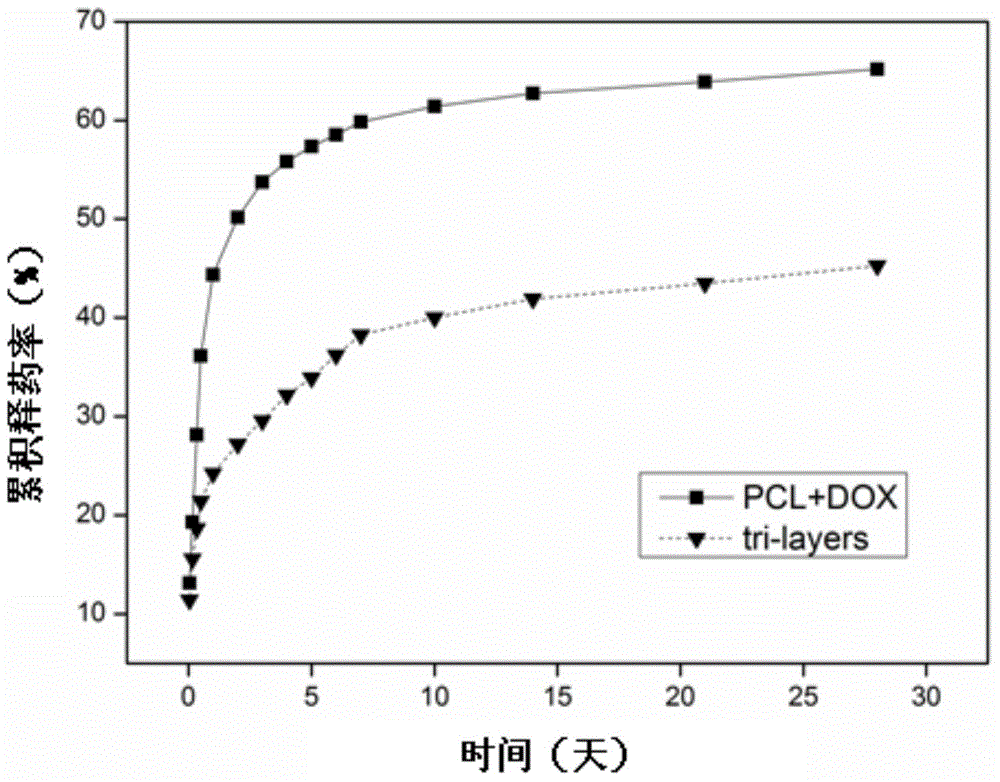
Doxycycline-hyclate-carried GTR/GBR composite membrane and preparation method thereof
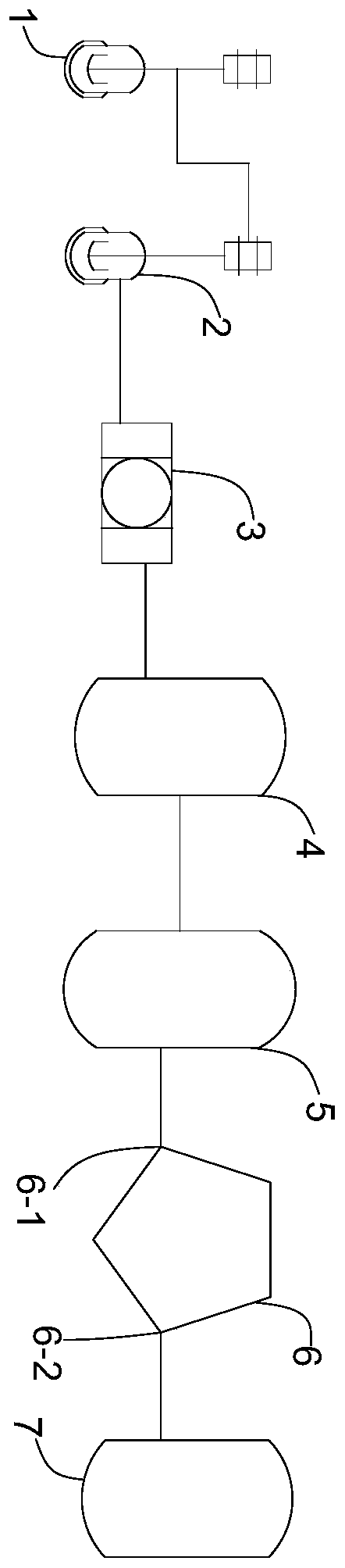
The invention discloses a doxycycline-hyclate-carried GTR (Guide Tissue Regeneration) / GBR (Guide Bone Regeneration) composite membrane and a preparation method thereof and belongs to the technical field of medicines. The GTR / GBR composite membrane adopts a three-layer structure, wherein the upper surface layer, the lower surface layer and the sandwich layer are all manufactured through sequence electrospinning; the upper surface layer and the lower surface layer are prepared from a natural polymer material and a mixed solution through which the polymer material is synthesized; the sandwich layer is a membrane layer prepared from a doxycycline-hyclate-carried solution through which the polymer material is synthesized. The uniaxial sequence electrospinning method is adopted to prepare the composite membrane adopting the three-layer structure, so that the medicine entrapment efficiency of fibers is improved, the preliminary burst effect of a medicine is reduced / eliminated, and continuously slow release of the medicine is realized. The two surface layers of the three-layer medicine-carried composite membrane can not only serve as porous barriers to control the medicine release rate so as to realize continuously slow release of the medicine but also prevent direct contact between the medicine and tissues to improve the compatibility between the tissues and the membrane so as to be more conducive to tissue repair and regeneration.
Owner:FOURTH MILITARY MEDICAL UNIVERSITY
Compound doxycycline hyclate suspension injection and preparation method thereof
InactiveCN102440998AFacilitated releaseHigh speedAntibacterial agentsTetracycline active ingredientsDoxycycline HyclateAntioxidant
The invention discloses a compound doxycycline hyclate suspension injection and a preparation process thereof. The suspension injection is prepared by taking doxycycline hyclate and tilmicosin phosphate as main medicines and adding appropriate medical accessories. The compound doxycycline hyclate suspension injection comprises the following components: 5-20 percent (W / V) of doxycycline hyclate, 5-20 percent (W / V) of tilmicosin phosphate, 0.5-2 percent (W / V) of a suspending aid, 0.01-0.2 percent (W / V) of an antioxidant, 0.1-2 percent (V / V) percent of a thickening agent and injection oil which supplements the balance to reach 100 percent. According to the invention, the doxycycline hyclate and the tilmicosin phosphate are evenly suspended into a solvent by a special process, and therefore the compound doxycycline hyclate suspension injection has good dispersibility and high bioavailability. Meanwhile, a feasible approach is provided for broadened production, and the advantages of increasing medical efficacy, reducing medication frequency and saving manpower and material resources are achieved.
Owner:TIANJIN RINGPU BIO TECH
Doxycycline hyclate enteric-coated pellet
ActiveCN101596162AAvoid local irritationAvoid adverse reactionsPowder deliveryTetracycline active ingredientsPeristalsisMedicine
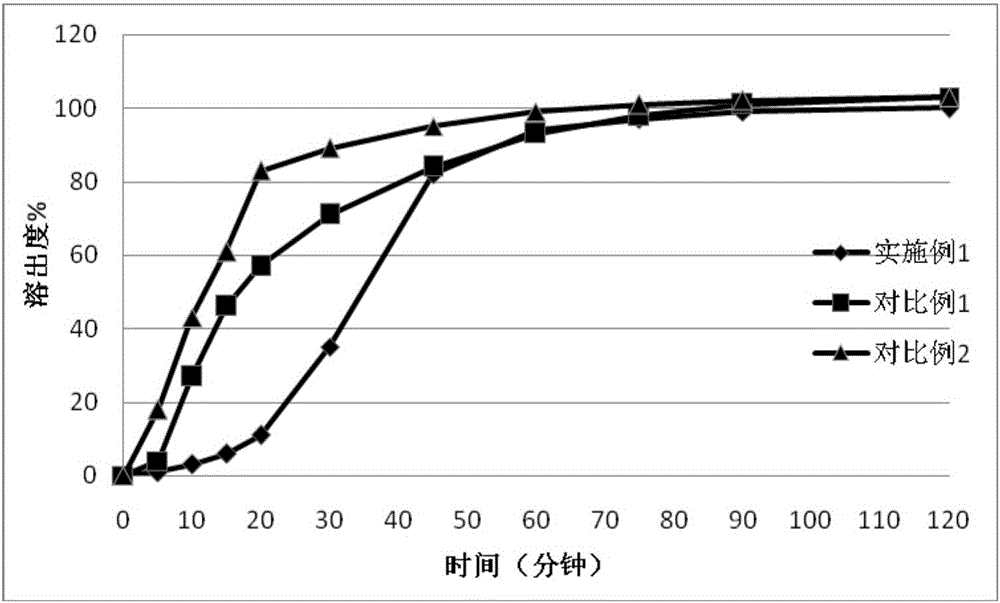
The invention discloses a doxycycline hyclate enteric-coated pellet which comprises the following components in percentage by weight from inside to outside: 48-58 blank core pellet, 35-40 medicinal layer and 4-13 enteric-coated layer. The invention also discloses a method for preparing the doxycycline hyclate enteric-coated pellet. The doxycycline hyclate enteric-coated pellets prepared by the method are filled into a capsule, and an obtained enteric-coated preparation has the advantages of increased contact area with the intestinal tract, high bioavailability, good stability, steady release in vitro and vivo, no influence by gastrointestinal peristalsis and food intake, uniform absorption, smaller difference of bioavailability among individuals, and the like.
Owner:德全药品(江苏)股份有限公司
Preparation method of doxycycline hyclate freeze-dried powder injection
ActiveCN105078905ASolve technical problems that are difficult to freeze-dryImprove freeze-drying efficiencyAntibacterial agentsPowder deliveryAtrophyFreeze-drying
The invention discloses a preparation method of doxycycline hyclate freeze-dried powder injection. The method includes steps of material preparing, freeze drying and the like, wherein pre-freezing is performed in three steps, the temperature of materials is fast decreased to about minus 20 DEG C, then the temperature of the materials is decreased to about minus 30 DEG C at a constant speed, and the temperature of the materials is finally fast decreased to about minus 40 DEG C and the temperature is kept; sublimation is performed in two steps, the temperature of the materials is increased to about minus 20 DEG C and the temperature is kept for 5-10 hours, and then the temperature of the materials is increased to about minus 5 DEG C and the temperature is kept for 1-3 hours. The method has the advantages that the special freeze drying process is used to solve the technical problem that the doxycycline hyclate liquid is difficult in freeze drying, doxycycline hyclate liquid solid content can reach above 50%, freeze drying efficiency is increased, freeze drying cycle is shortened, and products are full in appearance, free of atrophy, collapse, fracture and the like, and good in re-dissolving performance and mass stability.
Owner:HAINAN GENERAL & KANGLI PHARMA
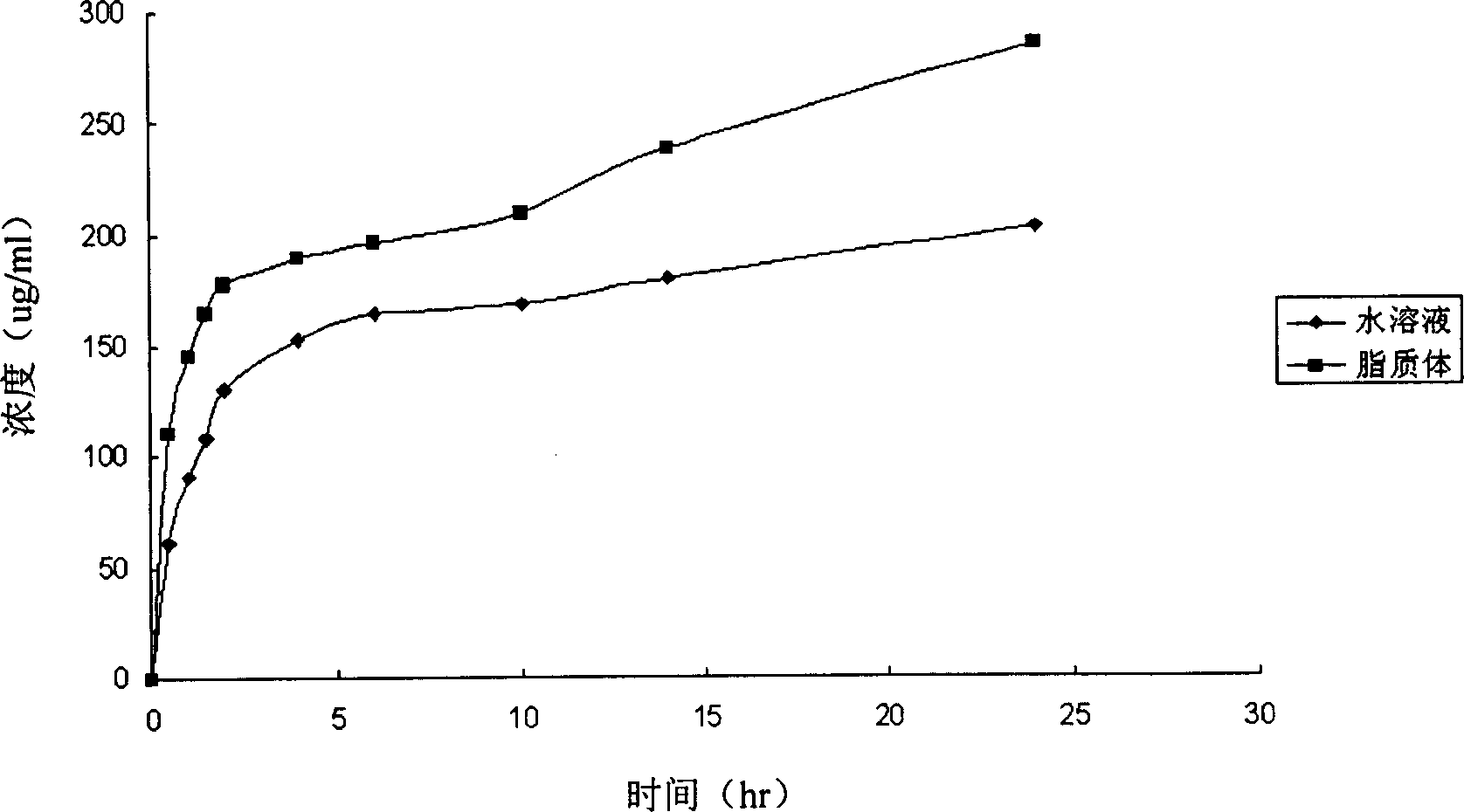
Doxycycline hyclate liposome and production thereof
InactiveCN1853640AIncrease concentrationImprove adhesionAntibacterial agentsTetracycline active ingredientsCholesterolDoxycycline Hyclate
A doxycycline hydrochloride liposome with high optical and thermal stability, high power to pass through skin and mucosa, and high antibacterial effect is prepared from phosphatide, cholesterol, doxycycline hydrochloride and emulsifier by film dispersing method, injection method, or reverse evaporation method.
Owner:SHANGHAI INST OF PHARMA IND CO LTD +1
Soluble compound doxycycline hyclate powder for animals and preparation method thereof
InactiveCN101590019AAvoid damageReduce stress responsePowder deliveryTetracycline active ingredientsDiseaseDoxycycline Hyclate
The invention discloses soluble compound doxycycline hyclate powder for animals and a preparation method thereof, which aim to provide compound doxycycline hyclate powder which is used for treating the mixed infection of swine plague mycoplasma pneumonia, has quick effect and can treat both symptoms and root causes, lighten stress reactions when pigs fall ill and improve the weight growth ratio, and a preparation method which has simple process and is easy to realize. The soluble powder comprises the following components by the weight percent: 1-20 of doxycycline hyclate, 0.5-5 of ofloxacin, 5 of aminophylline and the balance of anhydrous dextrose. The soluble compound doxycycline hyclate powder adopts antibiotics, antibacterial drugs and antiasthmatic drugs in a reasonable proportion so as to have multiple actions, can quickly ease clinic symptoms caused by the swine plague and the mycoplasma pneumonia, reduce damages caused by the two diseases to organisms, quicken the recovery, kill pathogens fundamentally, reduce the probability of the drug resistance of pathogenic microorganisms and treat both the symptoms and the root causes, and has quick effect.
Owner:TIANJIN SHENGJI GRP CO LTD
Preparation method of doxycycline hyclate and doxycycline hyclate prepared through method
ActiveCN109134291AHigh yieldHigh purityOrganic compound preparationSulfonic acids salts preparationAlcoholDoxycycline Hyclate
The invention provides a preparation method of doxycycline hyclate and doxycycline hyclate prepared through the method. Through a reaction between chlorinated acetanilide and oxytetracycline, raw materials are provided. The yield of doxycycline hyclate is increased, production of by-products is reduced, and meanwhile, acetanilide, ethyl alcohol, methyl alcohol and the like generated in a preparation process can be recycled. Therefore, environmental pollution and resource waste are reduced, the production cost is decreased, and the yield of products in the preparation process is stable. Additionally, doxycycline hyclate prepared through the preparation method is high in purity and great in luster.
Owner:河南后羿制药有限公司
Compound florfenicol injection and preparation method thereof
ActiveCN102755337AToxicShould not be used in combinationAntibacterial agentsTetracycline active ingredientsDoxycycline HyclateOfloxacinum
The invention discloses compound florfenicol injection and relates to the field of veterinary medicine preparation. The compound florfenicol injection is prepared from florfenicol, doxycycline hyclate, ofloxacin, dimethylacetamide (DMAC), propylene glycol and absolute ethyl alcohol. The invention also provides a preparation method of the compound florfenicol injection. As a novel veterinary compound preparation, the compound florfenicol injection has the characteristics of strong synergistic effect and low dosage, can effectively treat porcine infectious pleuropneumonia and has the important significance for sustainable development of the animal husbandry.
Owner:山东中牧兽药有限公司
Medicine for treating chickens and pigs infected by mixed mycoplasma and other bacteria
InactiveCN1915229AMixed infection works wellAntibacterial agentsTetracycline active ingredientsBacilliDoxycycline Hyclate
A veterinary medicine for treating the infection of the mixture of mould body and other bacteria to chicken and pig contains florfenicol, fumarate trevisin, doxycycline hyclate and lactate TMP.
Owner:杨建彬
Doxycycline hyclate enteric-coated pellet
ActiveCN101596162BAvoid local irritationAvoid adverse reactionsPowder deliveryTetracycline active ingredientsDoxycycline HyclatePeristalsis
The invention discloses a doxycycline hyclate enteric-coated pellet which comprises the following components in percentage by weight from inside to outside: 48-58 blank core pellet, 35-40 medicinal layer and 4-13 enteric-coated layer. The invention also discloses a method for preparing the doxycycline hyclate enteric-coated pellet. The doxycycline hyclate enteric-coated pellets prepared by the method are filled into a capsule, and an obtained enteric-coated preparation has the advantages of increased contact area with the intestinal tract, high bioavailability, good stability, steady release in vitro and vivo, no influence by gastrointestinal peristalsis and food intake, uniform absorption, smaller difference of bioavailability among individuals, and the like.
Owner:Yung Shin Pharm Ind (Kunshan) Co Ltd
Veterinary compound florfenicol injection and preparation method thereof
ActiveCN102133213ADoes not affect weight gainQuick resultsAntibacterial agentsInorganic phosphorous active ingredientsDoxycycline HyclatePhosphoric acid
The invention discloses compound florfenicol injection and a preparation method thereof. The compound florfenicol injection comprises the following components: 500g of florfenicol, 500g of doxycycline hyclate, 500g of tilmicosin phosphate, 3,000g of polyvinylpyrrolidone, 4,000g of N, N-dimethylacetylamide, 200g of magnesium chloride, 20g of sodium formaldehyde sulphoxylate and 10,000 milliliters of water for injection. The preparation method comprises the following steps of: (1) preparing; (2) adding doxycycline into the polyvinylpyrrolidone, heating to 80 DEG C, stirring until a mixture is clarified, adding the tilmicosin phosphate, and stirring until a mixture is clarified; (3) heating dimethylacetylamide to 50 DEG C, adding the florfenicol, and stirring for dissolving; (4) adding 1,000 milliliters of water for injection, adding the magnesium chloride and the sodium formaldehyde sulphoxylate in turn, and stirring for dissolving; and (5) mixing solution prepared in the step (2) and the step (3), gradually adding solution prepared in the step (4) under stirring, replenishing the water for injection until the total volume of the water for injection is 10,000 milliliters, uniformly stirring, filtering, filling nitrogen, encapsulating, and sterilizing at the temperature of between 110 and 115 DEG C for 30 minutes to prepare the compound florfenicol injection.
Owner:HUBEI WUDANG ANIMAL PHARMA
Ointment for treating acne and preparation method and application thereof
InactiveCN106539817ALess reactiveLess likely to cause side effectsAntibacterial agentsAntipyreticClinical efficacyVitamin C
The invention relates to an ointment, in particular to an ointment for treating acne and a preparation method and application thereof. The ointment for treating acne comprises a base material and active pharmaceutical ingredients, wherein the active pharmaceutical ingredients comprise 500-2000 parts of doxycycline hyclate, 800-2500 parts of chloramphenicol, 500-2000 parts of triamcinolone acetonide acetate, 500-2000 parts of vitamin C, 10-100 parts of glucocorticoid, 10-100 parts of vitamin B2 and 5-100 parts of cyproheptadine hydrochloride. The ointment for treating acne is unique in composition and significant in therapeutic effect; through the unique compatibility of the composition, various medicines in the active pharmaceutical ingredients not only can achieve effects of the original medicine but also can be further improved in clinical effect after being combined; sebum secretion is effectively regulated, toxins and hazardous substances in hair follicles are discharged, and symptoms such as acne and comedo can be eliminated fundamentally; and meanwhile the ointment has significant therapeutic effects on acne mark fading and acne pit smoothing.
Owner:杜光彦
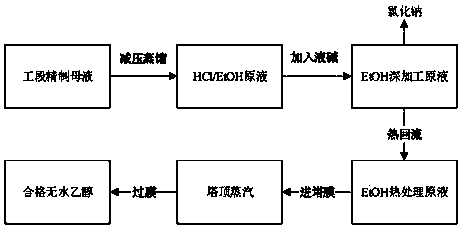
Device and method for recycling ethyl alcohol in refined mother solution of doxycycline hyclate
PendingCN110294665AEmission reductionReduce pollutionChemical industryHydroxy compound separation/purificationRecovery methodAlcohol
The invention discloses a device and method for recycling ethyl alcohol in a refined mother solution of doxycycline hyclate. The specific device for recycling ethyl alcohol comprises a pressure reduction distillation kettle, a neutralization kettle, a press filter, a to-be-recycled ethyl alcohol intermediate tank, a distillation tower, a vaporization membrane separation device and a finished product tank which are connected in sequence; the distillation tower is connected with a side material solution inlet of the vaporization membrane separation device, and a side material solution outlet ofthe vaporization membrane separation device is connected with the finished product tank. According to the device and method for recycling the ethyl alcohol in the refined mother solution of the doxycycline hyclate, the rate of recycling absolute ethyl alcohol reaches 85% or above, the content of the recycled ethyl alcohol reaches 99.5% or above, the moisture content is smaller than 0.5%, the content of chloroethane is lower than 200 ppm, and the recycle device and method accord with the standard of recycle application. The technology is stable, the recycle rate is high, the production cost isgreatly lowered, low-temperature pressure reduction distillation is adopted, the energy is saved, the emission amount of the ethyl alcohol is lowered, and the environmental pollution is reduced.
Owner:CHANGZHOU PHARMA FACTORY
Ureaplasma urealyticum/mycoplasma hominis combined rapid culture and drug sensitivity detection kit
InactiveCN104988206AResolve detectionResolve accuracyMicrobiological testing/measurementMicroorganism based processesPenicillinArginine
Owner:姜洪波 +1
Dental compositions
ActiveUS20150328236A1Enhanced antibacterial substantivitySevere stainingAntibacterial agentsBiocideDoxycycline HyclateDentistry
A dental composition comprising a therapeutically effective amount of doxycycline hyclate and a therapeutically effective amount of triamcinolone acetonide and use in dental treatment.
Owner:OZDENT
Doxycycline hyclate injection and preparation process thereof
InactiveCN105748404AImprove solubilityImprove stabilityAntibacterial agentsTetracycline active ingredientsAntioxidantDoxycycline Hyclate
The invention belongs to the technical field of veterinary medicine and discloses doxycycline hyclate injection and a preparation process thereof.Every 100ml of the doxycycline hyclate injection comprises 4.2-12.4g of magnesium chloride complexing agent, 5-15g of doxycycline hyclate, 20-30ml of alpha-pyrrolidone, 10-20ml of dimethyl formamide, 10-20ml of glycerin methylal, 0.1-0.2g of antioxidant, 5-10g of cosolvent and the balance of injection water.The prepared doxycycline hyclate injection has the advantages that the doxycycline hyclate injection is light in color, good in stability, quick in action, definite in curative effect, convenient in veterinary clinical application and easy in dose control, the doxycycline hyclate injection has a certain long-acting effect and cannot be easily affected by other medicine, and the treatment effect and efficiency are increased.
Owner:安徽省兽药研发中心
Doxycycline hyclate enteric-coated tablet and preparation method thereof
ActiveCN105796517AGuaranteed content uniformityWon't splinterAntibacterial agentsTetracycline active ingredientsCross-linkTreatment effect
The invention belongs to the technical field of medicine and particularly relates to a doxycycline hyclate enteric-coated tablet and a preparation method thereof. The enteric-coated tablet is prepared from, by weight, 28-32% of doxycycline hyclate enteric-coated pellets, 55-65% of lactose anhydrous, 4-8% of corn starch, 0.1-5% of cross-linking povidone and 0.1-1% of magnesium stearate. The diameter of the doxycycline hyclate enteric-coated pellets ranges from 0.35 mm to 1.00 mm, lactose anhydrous includes lactose anhydrous bodies with two different particle sizes, the particle size of the small-particle-size lactose anhydrous ranges from 45 microns to 150 microns, and the particle size of the large-particle-size lactose anhydrous ranges from 250 microns to 400 microns. The doxycycline hyclate enteric-coated tablet has the advantages that the preparation technology is simple, hardness is moderate, disintegration is quick, content uniformity is good and the release degree accords to quality standards, the treatment effect is good, and adverse effects are small.
Owner:华益泰康药业股份有限公司
A kind of doxycycline hydrochloride enteric-coated tablet and preparation method thereof
ActiveCN105796517BSimple preparation processModerate hardnessAntibacterial agentsTetracycline active ingredientsCoated tabletsDoxycycline Hyclate
The invention belongs to the technical field of medicine and particularly relates to a doxycycline hyclate enteric-coated tablet and a preparation method thereof. The enteric-coated tablet is prepared from, by weight, 28-32% of doxycycline hyclate enteric-coated pellets, 55-65% of lactose anhydrous, 4-8% of corn starch, 0.1-5% of cross-linking povidone and 0.1-1% of magnesium stearate. The diameter of the doxycycline hyclate enteric-coated pellets ranges from 0.35 mm to 1.00 mm, lactose anhydrous includes lactose anhydrous bodies with two different particle sizes, the particle size of the small-particle-size lactose anhydrous ranges from 45 microns to 150 microns, and the particle size of the large-particle-size lactose anhydrous ranges from 250 microns to 400 microns. The doxycycline hyclate enteric-coated tablet has the advantages that the preparation technology is simple, hardness is moderate, disintegration is quick, content uniformity is good and the release degree accords to quality standards, the treatment effect is good, and adverse effects are small.
Owner:华益泰康药业股份有限公司
Compound Chinese medicine injection for treating porcine respiratory disease
InactiveCN102441014AImprove pharmacological activityNo gainEther/acetal active ingredientsRespiratory disorderDiseaseDoxycycline Hyclate
The invention relates to a compound Chinese medicine injection for treating a porcine respiratory disease. The compound Chinese medicine injection mainly comprises common andrographis herb and Alpha-asarin. The compound doxycycline hyclate suspension injection has the functions of clearing away fever and detoxicating, relieving cough and asthma, calming and relieving spasm and the like, has the advantages of simple and convenience preparation process and stable performance and is suitable for industrial production.
Owner:TIANJIN RINGPU BIO TECH
Compound florfenicol injection and preparation method thereof
ActiveCN102755337BToxicShould not be used in combinationAntibacterial agentsTetracycline active ingredientsDoxycycline HyclateOfloxacinum
The invention discloses compound florfenicol injection and relates to the field of veterinary medicine preparation. The compound florfenicol injection is prepared from florfenicol, doxycycline hyclate, ofloxacin, dimethylacetamide (DMAC), propylene glycol and absolute ethyl alcohol. The invention also provides a preparation method of the compound florfenicol injection. As a novel veterinary compound preparation, the compound florfenicol injection has the characteristics of strong synergistic effect and low dosage, can effectively treat porcine infectious pleuropneumonia and has the important significance for sustainable development of the animal husbandry.
Owner:山东中牧兽药有限公司
Formulation including doxycycline hyclate and method for administering the same
ActiveUS20190008877A1Nervous disorderTetracycline active ingredientsDoxycycline HyclateAntibiotic effect
A topical formulation for transdermal delivery of sub-acute does of doxycycline hyclate to treat pain and inflammation may include: a base suspension; and doxycycline hyclate. The doxycycline hyclate is mixed with the base suspension in a proportion such that the formulation lacks any antibiotic effect and the concentration of doxycycline hyclate is between 200 and 600 mg / g inclusive. A method of applying the formulation is also disclosed.
Owner:ROSEMAN BRUCE
Dental compositions
ActiveUS9861646B2Enhanced antibacterial substantivitySevere stainingAntibacterial agentsImpression capsDoxycycline HyclateDentistry
A dental composition comprising a therapeutically effective amount of doxycycline hyclate and a therapeutically effective amount of triamcinolone acetonide and use in dental treatment.
Owner:OZDENT
Medicinal composition for treating avian mixed infection and method for preparing the same
InactiveCN101112377AGrowth inhibitionStrong dosingTetracycline active ingredientsAntiinfectivesCoccidiosisProtozoa
The present invention relates to a drug compound to treat the poultry mixed infection and the preparation method thereof, and the components and the mix ratios by weight of the drug component are 0.5 to 1.0 percent doxycycline hyclate, 10 to 20 percent metronidazole, 0.5 to 1.0 percent mequindox and 0.3 to 0.8 percent halofuginone respectively, while the rest is the excipients; the preparation method thereof is to smash and screen the raw materials of the drug, then the raw materials are mixed in line with the mix ratios and the excipients are added to 100 percent. The drug compound of the invention can be used for material mixing or drinking, which has easy usage, strong effects of anti-bacterial, anti-coccidiosis and anti-protozoa, etc., and good effects of prevention and treatment of a variety of mixed infection, small dosage, safety and low toxicity.
Owner:张兴龙
Medicine for treating chickens and pigs infected by mixed mycoplasma and other bacteria
InactiveCN100434067CMixed infection works wellAntibacterial agentsTetracycline active ingredientsBacteroidesDoxycycline Hyclate
A veterinary medicine for treating the infection of the mixture of mould body and other bacteria to chicken and pig contains florfenicol, fumarate trevisin, doxycycline hyclate and lactate TMP.
Owner:杨建彬
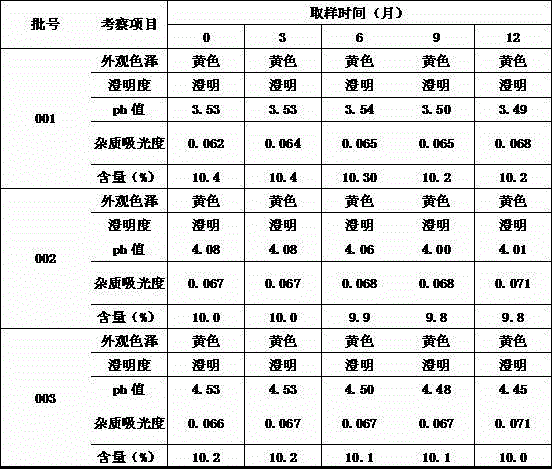
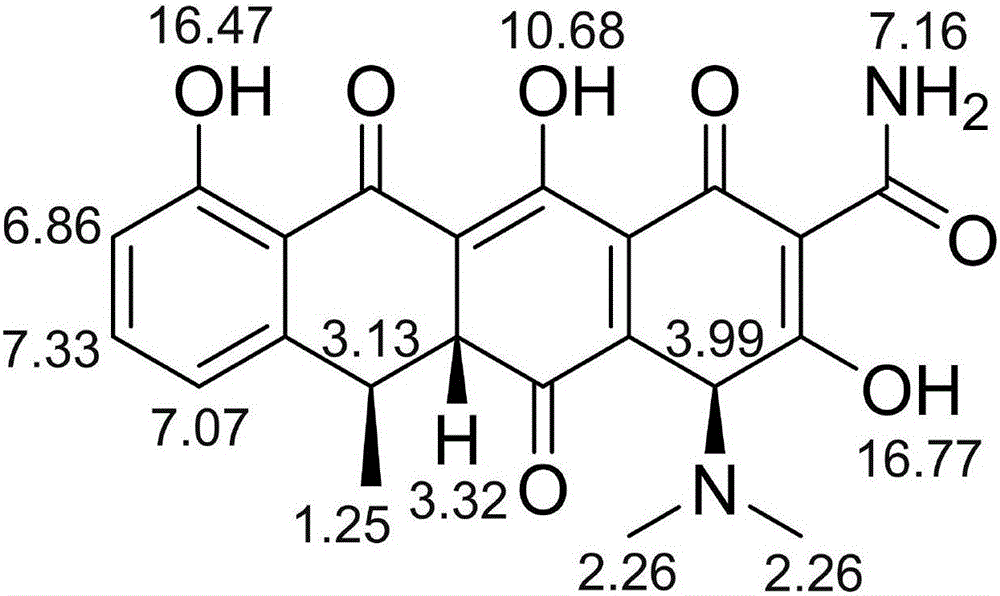
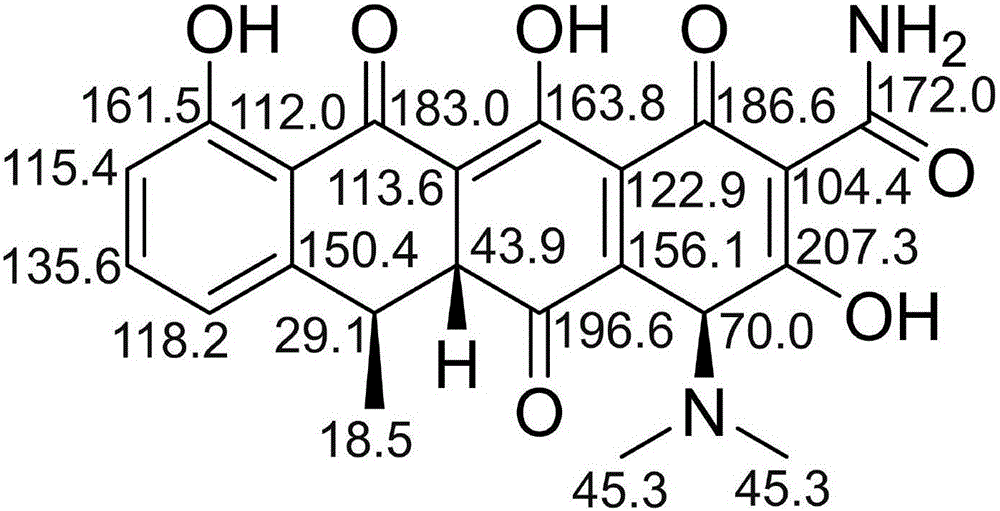
Substance related to doxycycline hyclate as well as analysis and detection method thereof
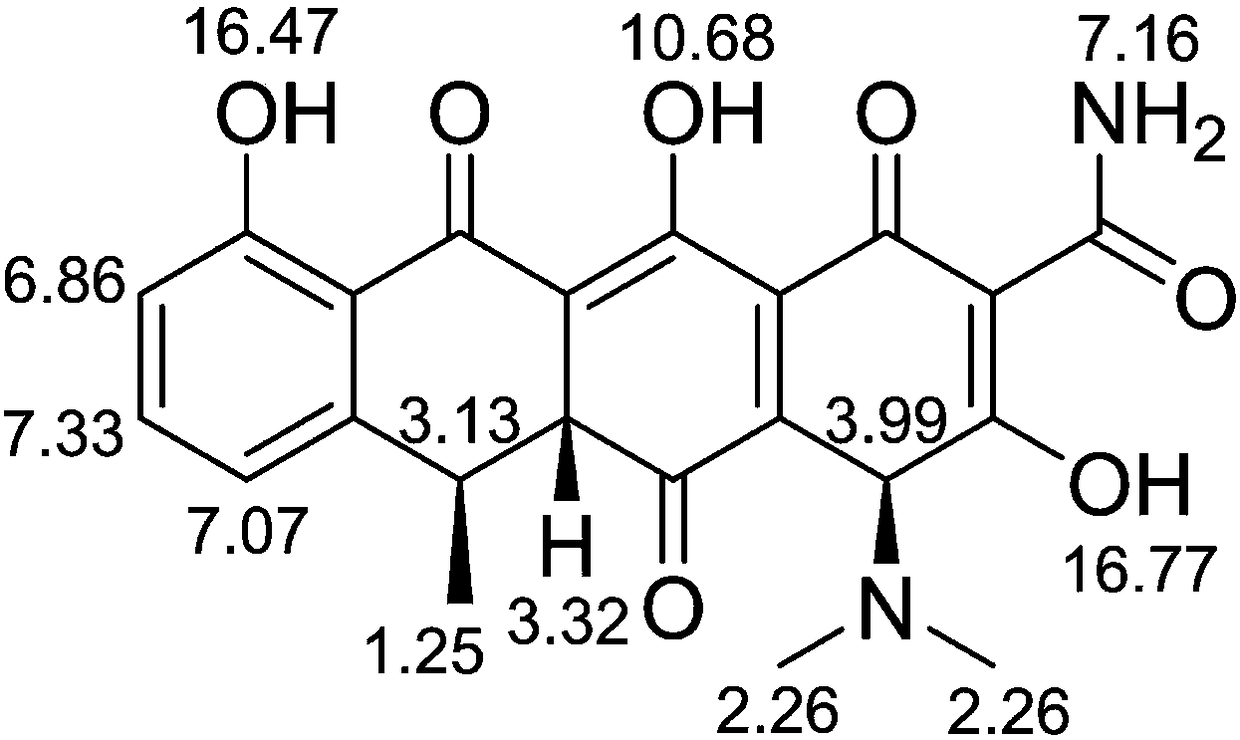
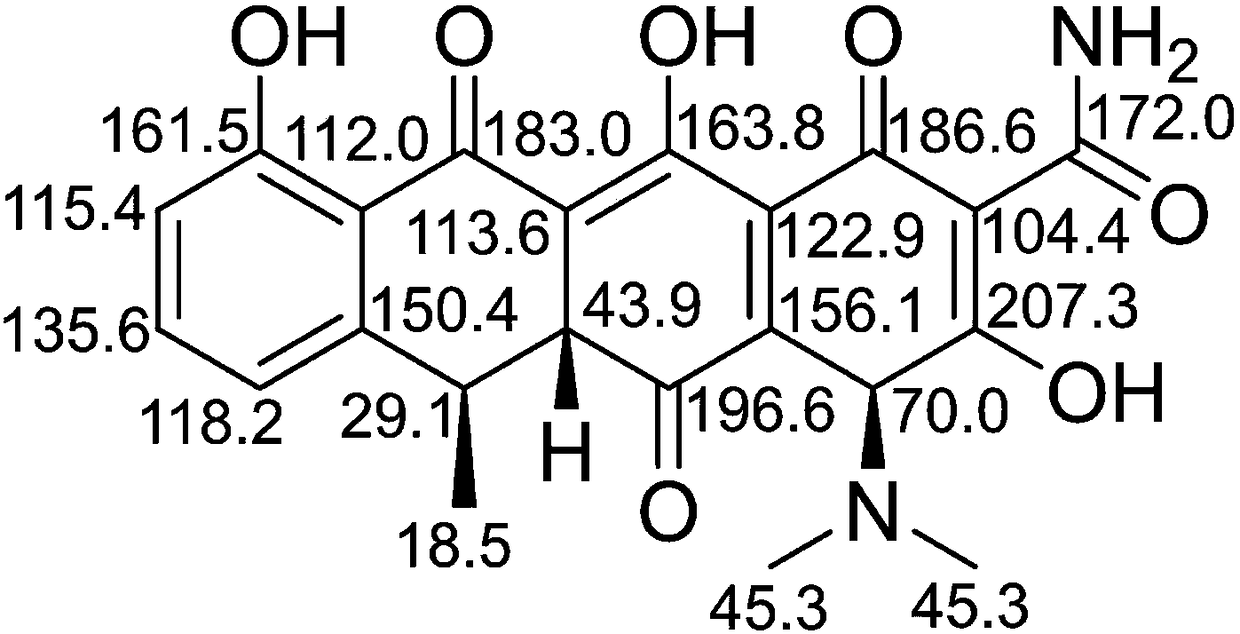
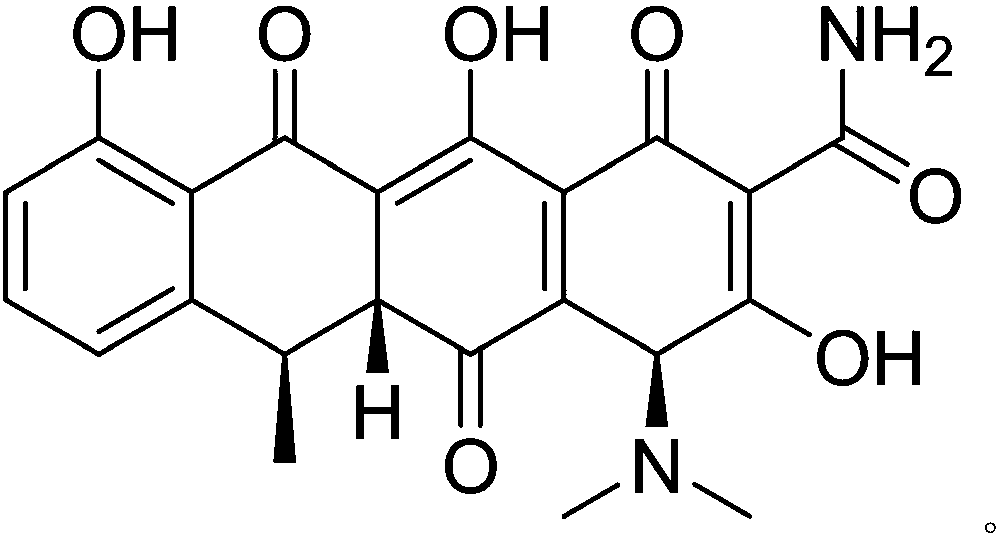
ActiveCN105929046ASimple manufacturing methodImprove the detection rateComponent separationDoxycycline HyclateLength wave
The invention discloses a substance related to doxycycline hyclate as well as an analysis and detection method thereof. The invention discovers a novel photodegradation product of doxycycline hyclate, and the structure of the photodegradation product is reported for the first time. The invention provides a preparation method of the photodegradation product, the method is simple and easy to operate, and a large number of the photodegradation products can be prepared. A liquid phase analysis method is provided for rapidly detecting the photodegradation products, and a maximum absorption wavelength of the photodegradation products is used as a detection wavelength, so that the detection rate of the photodegradation products is improved, and the result is accurate and reliable; the photodegradation products can be used for detecting substances related to doxycycline hyclate and preparations thereof, further improving quality standards of doxycycline hyclate and preparations thereof, and improving safety and controllability thereof.
Owner:ZHENJIANG GAOHAI BIOLOGICAL PHARMA CO LTD
Veterinary compound florfenicol injection and preparation method thereof
ActiveCN102133213BDoes not affect weight gainQuick resultsAntibacterial agentsInorganic phosphorous active ingredientsDoxycycline HyclatePhosphoric acid
The invention discloses compound florfenicol injection and a preparation method thereof. The compound florfenicol injection comprises the following components: 500g of florfenicol, 500g of doxycycline hyclate, 500g of tilmicosin phosphate, 3,000g of polyvinylpyrrolidone, 4,000g of N, N-dimethylacetylamide, 200g of magnesium chloride, 20g of sodium formaldehyde sulphoxylate and 10,000 milliliters of water for injection. The preparation method comprises the following steps of: (1) preparing; (2) adding doxycycline into the polyvinylpyrrolidone, heating to 80 DEG C, stirring until a mixture is clarified, adding the tilmicosin phosphate, and stirring until a mixture is clarified; (3) heating dimethylacetylamide to 50 DEG C, adding the florfenicol, and stirring for dissolving; (4) adding 1,000 milliliters of water for injection, adding the magnesium chloride and the sodium formaldehyde sulphoxylate in turn, and stirring for dissolving; and (5) mixing solution prepared in the step (2) and the step (3), gradually adding solution prepared in the step (4) under stirring, replenishing the water for injection until the total volume of the water for injection is 10,000 milliliters, uniformly stirring, filtering, filling nitrogen, encapsulating, and sterilizing at the temperature of between 110 and 115 DEG C for 30 minutes to prepare the compound florfenicol injection.
Owner:HUBEI WUDANG ANIMAL PHARMA
A kind of related substance of doxycycline hydrochloride and its analysis and detection method
ActiveCN105929046BSimple manufacturing methodImprove the detection rateOrganic chemistryComponent separationDoxycycline HyclateDoxycycline hydrochloride
The invention discloses a substance related to doxycycline hyclate as well as an analysis and detection method thereof. The invention discovers a novel photodegradation product of doxycycline hyclate, and the structure of the photodegradation product is reported for the first time. The invention provides a preparation method of the photodegradation product, the method is simple and easy to operate, and a large number of the photodegradation products can be prepared. A liquid phase analysis method is provided for rapidly detecting the photodegradation products, and a maximum absorption wavelength of the photodegradation products is used as a detection wavelength, so that the detection rate of the photodegradation products is improved, and the result is accurate and reliable; the photodegradation products can be used for detecting substances related to doxycycline hyclate and preparations thereof, further improving quality standards of doxycycline hyclate and preparations thereof, and improving safety and controllability thereof.
Owner:ZHENJIANG GAOHAI BIOLOGICAL PHARMA CO LTD
Preparation technology for improving purity of doxycycline hyclate injection
InactiveCN105287218AImprove solubilityImprove cleanlinessPharmaceutical product form changeWater bathsOrganic solvent
The invention relates to a preparation technology for improving the purity of a doxycycline hyclate injection. The preparation technology comprises following steps: grinding doxycycline hyclate into powder by the use of a ball mill, and dissolving the powder in water so as to form a solution A; dissolving ethyl cellulose in an organic solvent so as to form a solution B; pouring the solution A into the solution B under a stirring condition, wherein an emulsion is formed after the solution A disperses in the solution B; dissolving gelatin in distilled water under a water-bath condition at 60-80 DEG C so as to prepare a protection solution D which is standby at the constant temperature of 30 DEG C; drooping the emulsion C into the protection solution D under a stirring condition so as to form a solution E, and stirring the solution under a condition that the temperature of the water-bath is raised to 50 DEG C from 30 DEG C so that the organic solvent volatilizes; and then performing suction filtration on the solution E, washing the solution E, and drying the solution E so as to obtain the white powder doxycycline hyclate. According to the invention, problems that the dissolving speed is relatively slow in a conventional preparation process of doxycycline hyclate preparations and the doxycycline hyclate for injection contains impurities in the conventional preparation process are solved.
Owner:CHINA CHENGDU ANIMAL HUSBANDRY IND BIOPHARM
Doxycycline hyclate liposome and production thereof
InactiveCN1853640BImprove stabilityImprove adhesionAntibacterial agentsTetracycline active ingredientsCholesterolDoxycycline Hyclate
A doxycycline hydrochloride liposome with high optical and thermal stability, high power to pass through skin and mucosa, and high antibacterial effect is prepared from phosphatide, cholesterol, doxycycline hydrochloride and emulsifier by film dispersing method, injection method, or reverse evaporation method.
Owner:SHANGHAI INST OF PHARMA IND CO LTD +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com