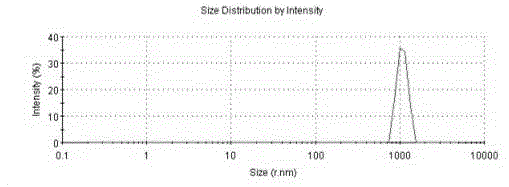
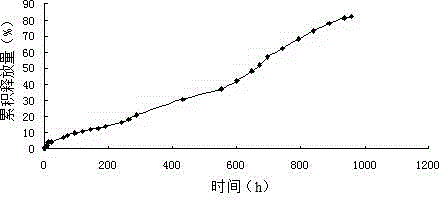
Burst release-free irinotecan microsphere and preparation method thereof
A technology of irinotecan and effect, which is applied in the direction of medical preparations with non-active ingredients, medical preparations containing active ingredients, and pharmaceutical formulas. It can solve the problems of short drug effect time, short half-life, and poor water solubility, and achieve the appearance Uniformity, extended dosing interval, pain-reducing effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0016] 200mg irinotecan and 5.0g PLGA were co-dissolved in 25mL dichloromethane as the oil phase; 2.5g PVA was dissolved in 250mL distilled water as the water phase; the oil phase was slowly added to the water phase under the treatment of a high-shear dispersing emulsifier to form Milky dispersion system, the ratio of oil phase to water phase is 1:10, the speed of high shear dispersing emulsifier is 8000rpm, and the stirring time is 30s. The organic solvent was evaporated by rotary steaming at 30°C for 50 minutes, and the irinotecan microspheres were collected after washing with low-temperature high-speed centrifugation.
Embodiment 2
[0018] 200mg of irinotecan and 6.0g of PLGA were co-dissolved in 30mL of dichloromethane as the oil phase; 3.0g of PVA was dissolved in 300mL of distilled water as the water phase; the oil phase was slowly added to the water phase under the treatment of a high-shear dispersing emulsifier to form an emulsion Cloudy dispersion system, the ratio of oil phase to water phase is 1:10, the speed of high shear dispersing emulsifier is 8000rpm, and the stirring time is 30s. The organic solvent was evaporated by rotary steaming at 30°C for 50 minutes, and the irinotecan microspheres were collected after washing with low-temperature high-speed centrifugation.
Embodiment 3
[0020] 200mg irinotecan and 7.0g PLGA were co-dissolved in 35mL dichloromethane as the oil phase; 3.5gPVA was dissolved in 350mL distilled water as the water phase; the oil phase was slowly added to the water phase under the treatment of a high-shear dispersing emulsifier to form an emulsion Cloudy dispersion system, the ratio of oil phase to water phase is 1:10, the speed of high shear dispersing emulsifier is 8000rpm, and the stirring time is 30s. The organic solvent was evaporated by rotary steaming at 30°C for 50 minutes, and the irinotecan microspheres were collected after washing with low-temperature high-speed centrifugation.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com