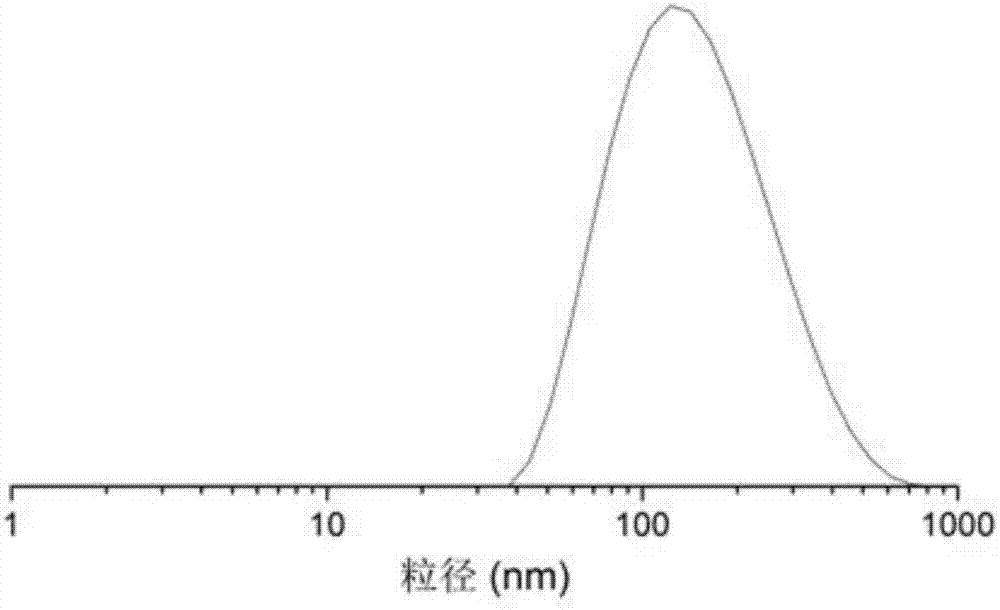
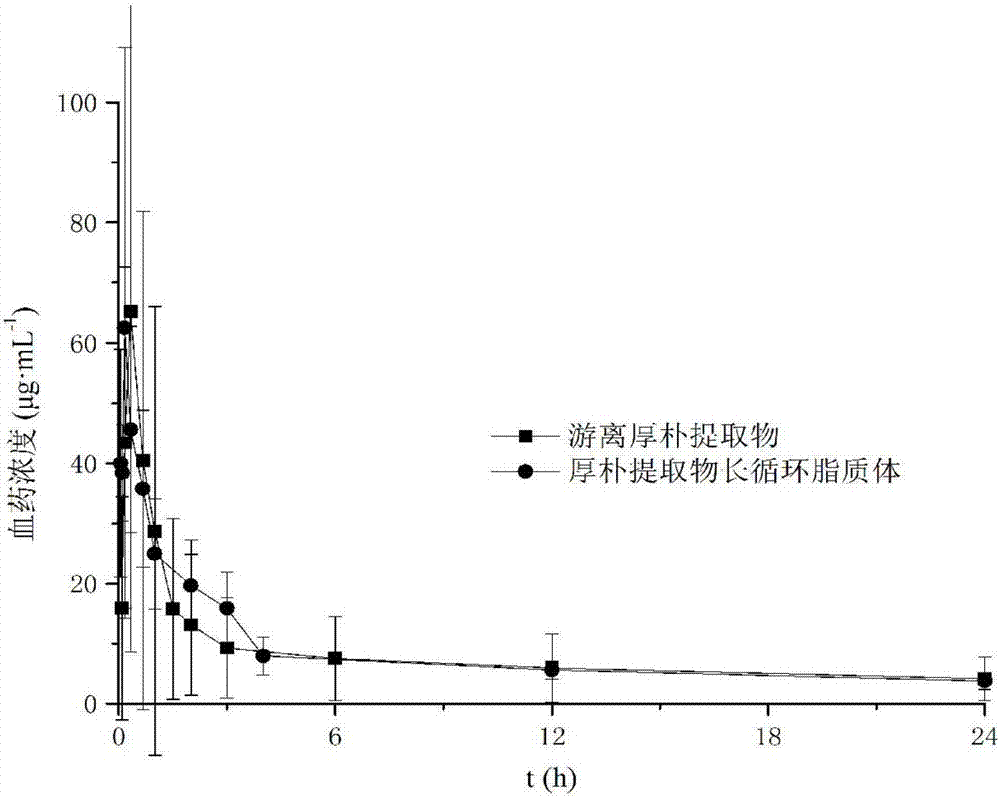
Long-circulating liposome freeze-drying oral preparation of magnolia cortex total phenol and preparation method of freeze-drying oral preparation
A long-circulation liposome and oral preparation technology, which is applied in liposome delivery, freeze-drying delivery, anti-inflammatory agents, etc., can solve the problems of no new dosage form of magnolol total phenols and limit the clinical application of magnolia , to achieve the effects of prolonging the residence time, reducing the number of medications and high blood drug concentration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
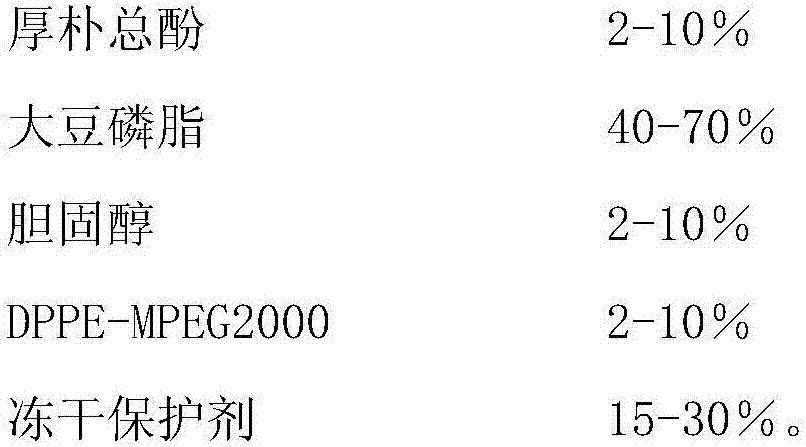
[0032] Magnolol long-circulating liposome lyophilized oral preparation, the composition of which is as follows:
[0033]
[0034] Preparation method: Weigh 6 mg of magnolol total phenols, 48 mg of soybean lecithin, 6 mg of cholesterol, and 6 mg of DPPE-MPEG2000, add them to 220 ml of methanol-chloroform mixed organic solvent (4:1, v / v), place in an eggplant-shaped bottle, Stir at room temperature until completely dissolved to obtain a mixed solution with a total concentration of 0.3 mg / ml. The mixed solution was rotary evaporated under reduced pressure at 40° C. to remove the organic solvent until a thin film was formed on the inner wall of the bottle, and the vacuum was continued for 1 h to remove the residual organic solvent. Add 20ml of ultrapure water to the dried eggplant-shaped bottle to fully submerge the film on the wall of the bottle, stir and hydrate at 60°C for 1 hour, and then ultrasonicate at intervals in an ice bath (power 80W, ultrasonic at intervals of 4 s...
Embodiment 2
[0036] Magnolol long-circulating liposome lyophilized oral preparation, the composition of which is as follows:
[0037]
[0038] Preparation:
[0039] (1) Total magnolol, soybean lecithin, cholesterol, DPPE-MPEG2000 are added in methanol-chloroform mixed organic solvent (6:1, v / v), make total magnolol, soybean lecithin, cholesterol, DPPE-MPEG2000 The total concentration is 0.5mg / ml, stirred at room temperature until completely dissolved;
[0040] (2) Rotate the mixed solution under reduced pressure at 45°C to remove the organic solvent and form a thin film;
[0041] (3) Add water to the film, stir at 50°C, then sonicate in an ice bath, and finally pass through a microporous membrane (0.22 μm) to obtain a liposome suspension;
[0042] (4) Adding a lyoprotectant (trehalose: mannitol = 5:1) to the liposome suspension, freeze-dried to obtain.
Embodiment 3
[0044] Magnolol long-circulating liposome lyophilized oral preparation, the composition of which is as follows:
[0045]
[0046] Preparation:
[0047] (1) Total magnolol, soybean lecithin, cholesterol, DPPE-MPEG2000 are added in methanol-chloroform mixed organic solvent (10:1, v / v), make total magnolol, soybean lecithin, cholesterol, DPPE-MPEG2000 The total concentration is 0.7mg / ml, stirred at room temperature until completely dissolved;
[0048] (2) Rotate the mixed solution under reduced pressure at 50°C to remove the organic solvent and form a thin film;
[0049] (3) Add water to the film, stir at 70°C, then sonicate in an ice bath, and finally pass through a microporous membrane (0.22 μm) to obtain a liposome suspension;
[0050] (4) Adding a lyoprotectant (trehalose: mannitol = 9:1) to the liposome suspension and freeze-drying.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Aperture | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com