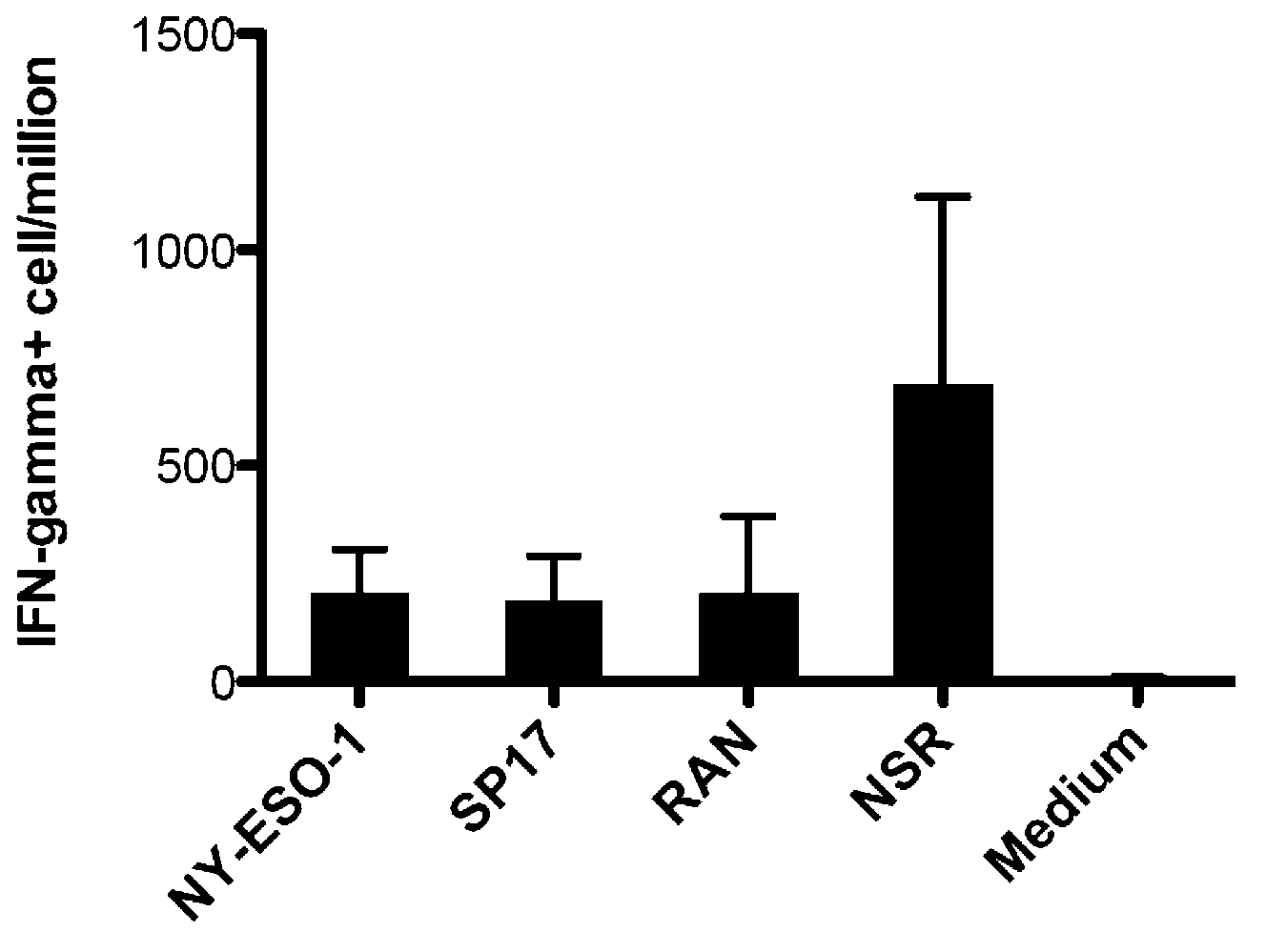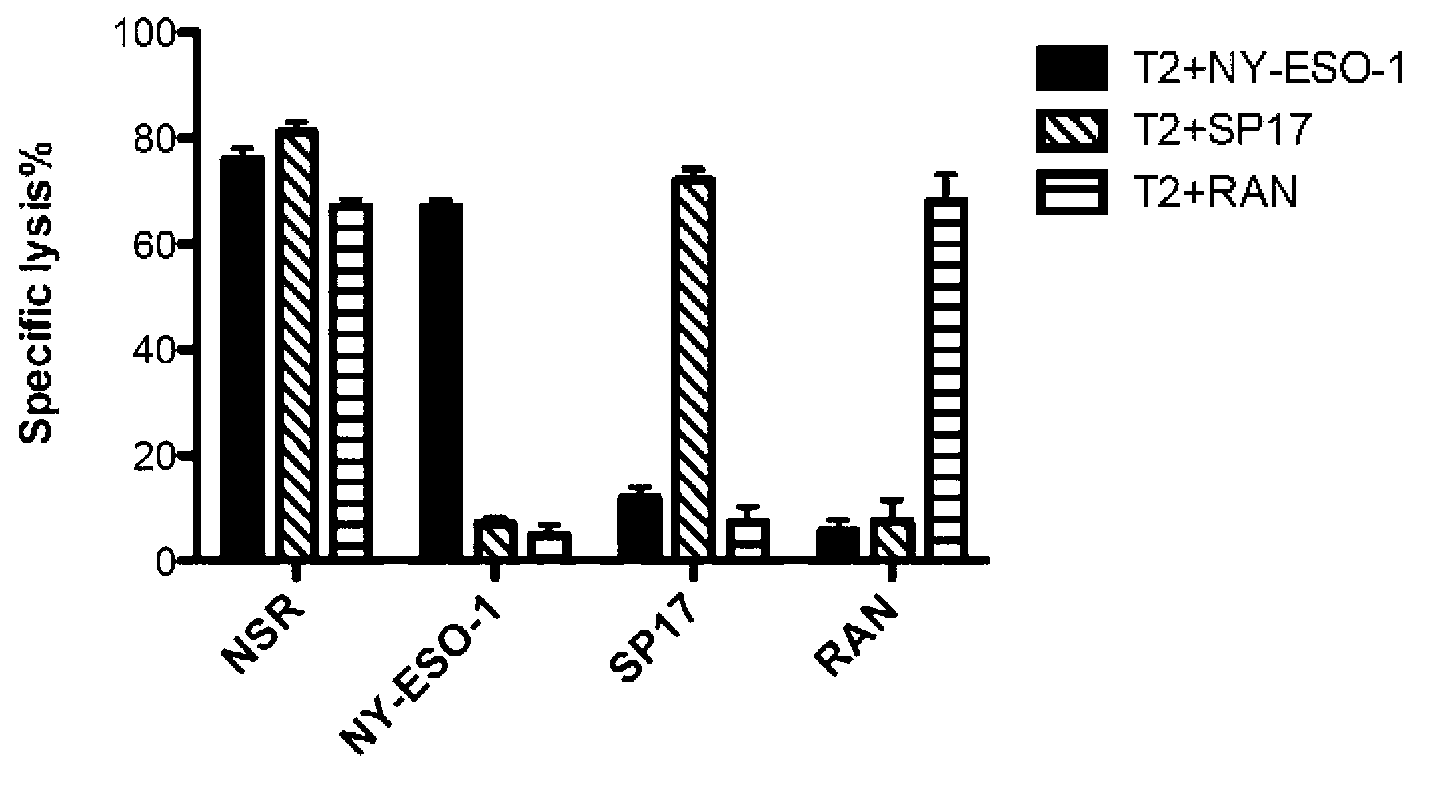Combined CTL antigenic epitope and its application
An antigenic epitope and immunogen technology, applied in the field of immunology, can solve the problems such as difficult breakthrough of CTL, achieve low maximum blood drug concentration, improve compliance, and improve the quality of life
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Embodiment 1 synthetic peptide
[0028] The peptide shown as SEQ ID NO: 4 was entrusted to Hangzhou Zhongpei Biochemical Co., Ltd. to synthesize it using a standard Fmoc protocol, and to use directional high-performance liquid chromatography for purification and purity analysis, and mass spectrometry for identification and molecular weight determination. The result shows that the purity of the polypeptide is higher than 95%, and the molecular weight is consistent with the theoretical value.
[0029] Specific synthesis steps: 1. Deprotection: The Fmoc-protected column and monomer must use a basic solvent, such as piperidine, to remove the amino protection group. 2. Activation and cross-linking: The carboxyl group of the next amino acid is activated and dissolved by the activator, and the activated monomer and the free amino group are cross-linked under the action of the cross-linking agent to form a peptide bond. 3. Cycle: The first two steps are repeated until the enti...
Embodiment 2
[0030] Example 2 Derivatives of Modified Polypeptides
[0031]Polyethylene glycol (PEG) is polymerized from ethylene oxide and consists of repeating oxyethylene groups. PEG not only has good water solubility, but also can be dissolved in organic solvents such as dichloromethane, N-N-dimethylacylphenol, benzene, acetonitrile and ethanol.
[0032] One end is blocked with a methyl group as methoxypolyethylene glycol (mPEG), and the molecular formula of linear mPEG is CH3-(O-CH 2 -CH 2 )n-OH, the derivatives of mPEG are most widely used in the study of PEGylation modification of polypeptides. Polyethylene glycol is a neutral, non-toxic high molecular polymer with unique physical and chemical properties and good biocompatibility. It is also one of the very few synthetic polymers approved by the FDA that can be used as a drug for intracorporeal injection. Polyethylene glycol is highly hydrophilic. When coupled to drug molecules, it can impart its excellent properties to the modif...
Embodiment 3
[0037] Example 3 Cytotoxic activity and the ability of vaccines to induce CTL-mediated cellular immunity in vitro
[0038] Effector cells: HLA-A * Anticoagulated fresh whole blood of 0201 positive healthy people was separated by conventional polysucrose-diatrizoate layered solution gradient centrifugation to obtain peripheral blood mononuclear cells, which were cultured with RPMI1640 containing 10% fetal bovine serum by volume solution to adjust the cell concentration to 1.0 x 10 6 / mL, inoculated into 24-well culture plate, 1 mL per well, each group stimulated PBMC 3 times continuously with epitopes with a concentration of 10 μg / mL and recombinant interleukin-2 (rhIL-2) with a concentration of 50 U / mL, weekly Once, the cells were collected on the 3rd day after the last stimulation to obtain epitope-induced specific CTLs, and the cell concentration was adjusted with RPMI1640 culture medium containing 10% fetal bovine serum by volume as effector cells.
[0039] 1 Multi-epitop...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com