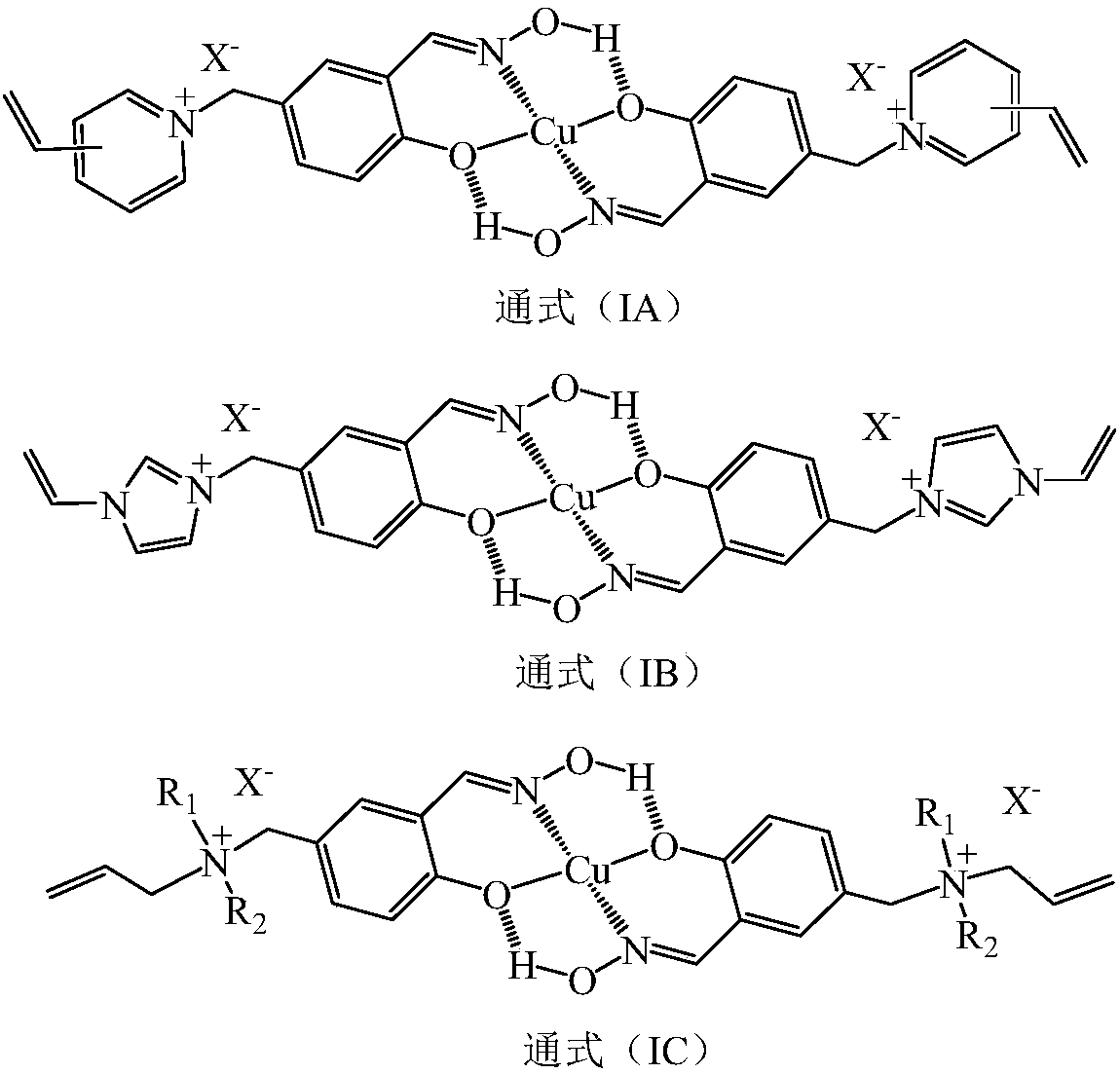
Anion-cation double exchange type copper (II) ion surface imprinted polymer and preparation method thereof
An anion and cation, surface imprinting technology, applied in ion exchange, zwitterion exchange, ion exchange water/sewage treatment, etc., can solve the problems of waste pollution, prone to hydrolysis reaction, deep pores of ion imprinted particles, etc., to achieve stability high effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Preparation of the copper (II) ion surface imprinted polymer (IA-1) of the anion-cation double exchange of embodiment 1 hollow sphere
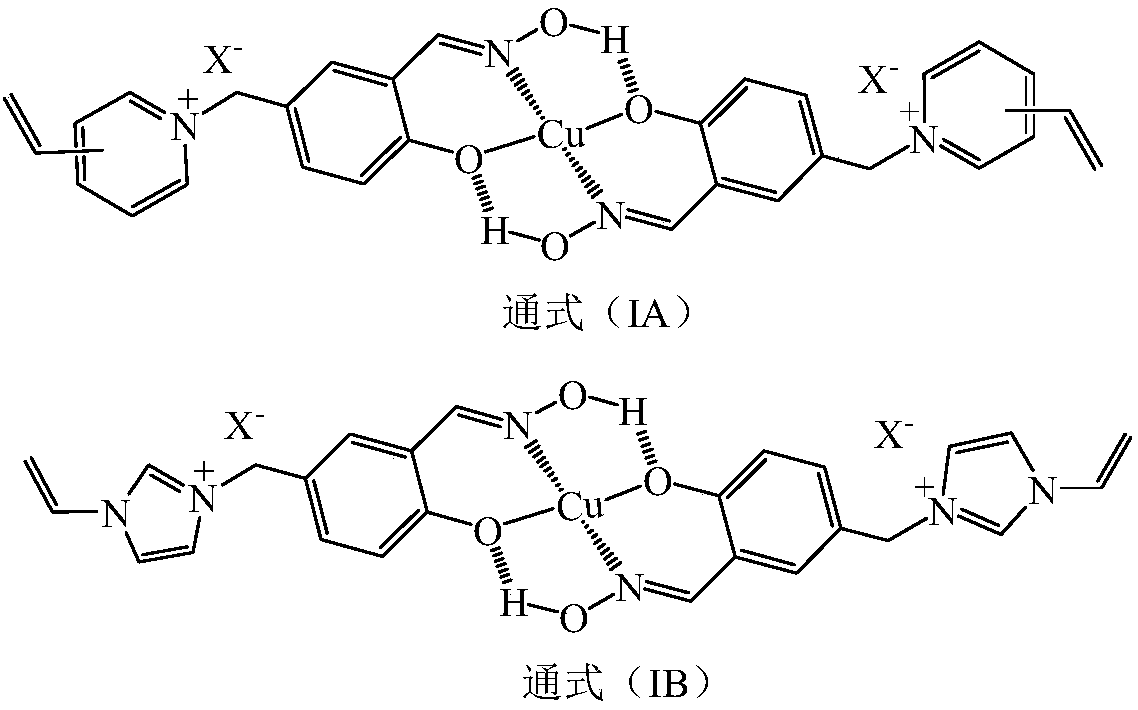
[0036] In the dry reaction bottle, drop into chloromethyl polystyrene macroporous cross-linked resin ball 50 grams (chlorine content 13.4%) and 80 grams of chlorobenzene that average particle diameter is 1.8 millimeters, after room temperature swelling 2 hours, temperature control 30 ~60°C, add 20 grams of triallylamine, react for 4 hours, cool down, filter out polystyrene resin balls, wash with water until neutral, and dry in vacuum to obtain triallyl ammonium salified cross-linked polystyrene hollow resin balls 66.2 grams. In another reaction bottle, put 100 grams of N,N-dimethylacetamide and 3.8 grams of azobisisobutyronitrile, start stirring, and add 50 grams of functional monomer (Ia-1) and triallylamine hydrochloride under stirring. 20 grams of salt, 10 grams of N,N-dimethylacrylamide and 50 grams of triallyl ammonium salted cros...
Embodiment 2
[0039] Example 2 Preparation of Hollow Resin Spherical Anion-Cation Double Exchanged Copper(II) Solid Phase Imprinting Dual-position Extractant (IB-1)
[0040] According to the method and operating steps of Example 1, the functional monomer (Ia-1) of Example 1 was replaced with a functional monomer (Ib-1), and an anion-cation double-exchanged copper (II) surface imprinted polymer ( IB-1) Hollow resin ball. According to the experiment, at 25°C, the time for the imprinted polymer (IB-1) on the surface of the anion-cation double-exchange copper (II) imprinted polymer (IB-1) to saturate absorb copper (II) ions is 1.7 minutes, and the saturated adsorption capacity of copper (II) ions is 38.8mg / g.
[0041]
Embodiment 3
[0042] Example 3 Preparation of Hollow Resin Spherical Anion-Cation Double Exchanged Copper (II) Surface Imprinted Polymer (IC-1)
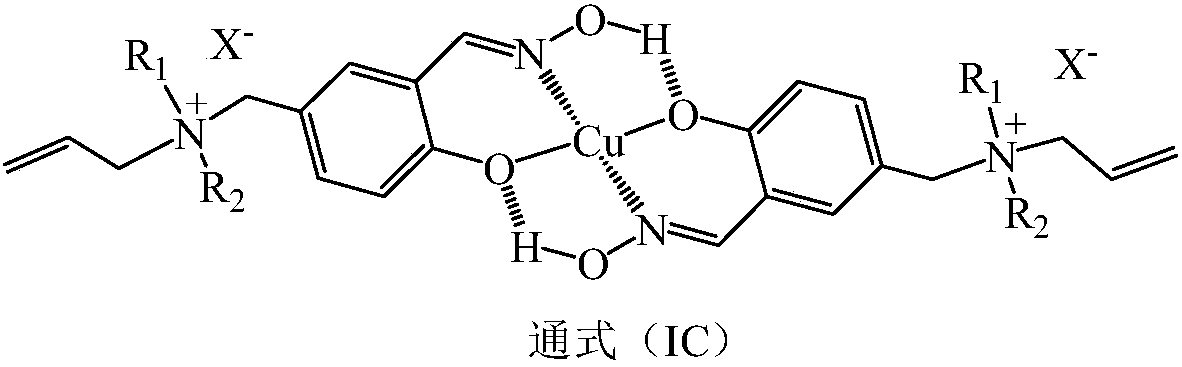
[0043] According to the method and operating steps of Example 1, the functional monomer (Ia-1) of Example 1 was replaced with a functional monomer (Ic-1), and an anion-cation double-exchanged copper (II) surface imprinted polymer ( IC-1) Hollow resin ball. According to experiments, at 25°C, the saturated adsorption time of copper (II) ions on the copper (II) surface imprinted polymer (IC-1) with anion and cation double exchange is 1.1 minutes, and the saturated adsorption capacity of copper (II) ions is 40.1mg / g.
[0044]
PUM
| Property | Measurement | Unit |
|---|---|---|
| adsorption capacity | aaaaa | aaaaa |
| adsorption capacity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com