Substituted di(amino mercapto) benzene hydrochloride and process for preparing same
A technology of dimethylcyclohexane dihydrochloride and cyclohexane dihydrochloride, which is applied in mercaptan preparation, organic chemistry, etc., can solve the problem of reducing intermolecular attraction and chain rigidity, difficulty in film formation, and polymerization Problems such as easy fiber formation, to achieve the effect of improving solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
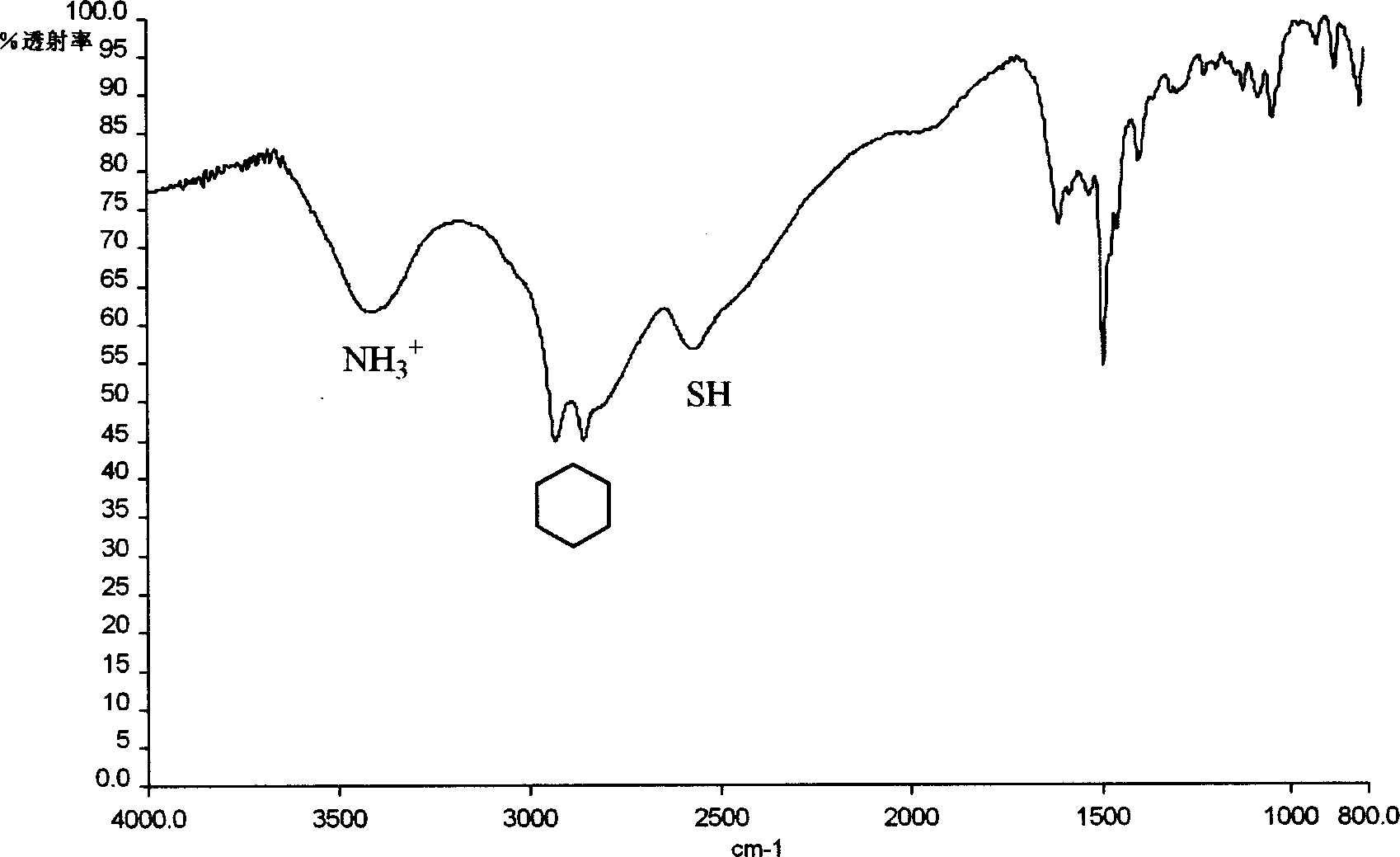
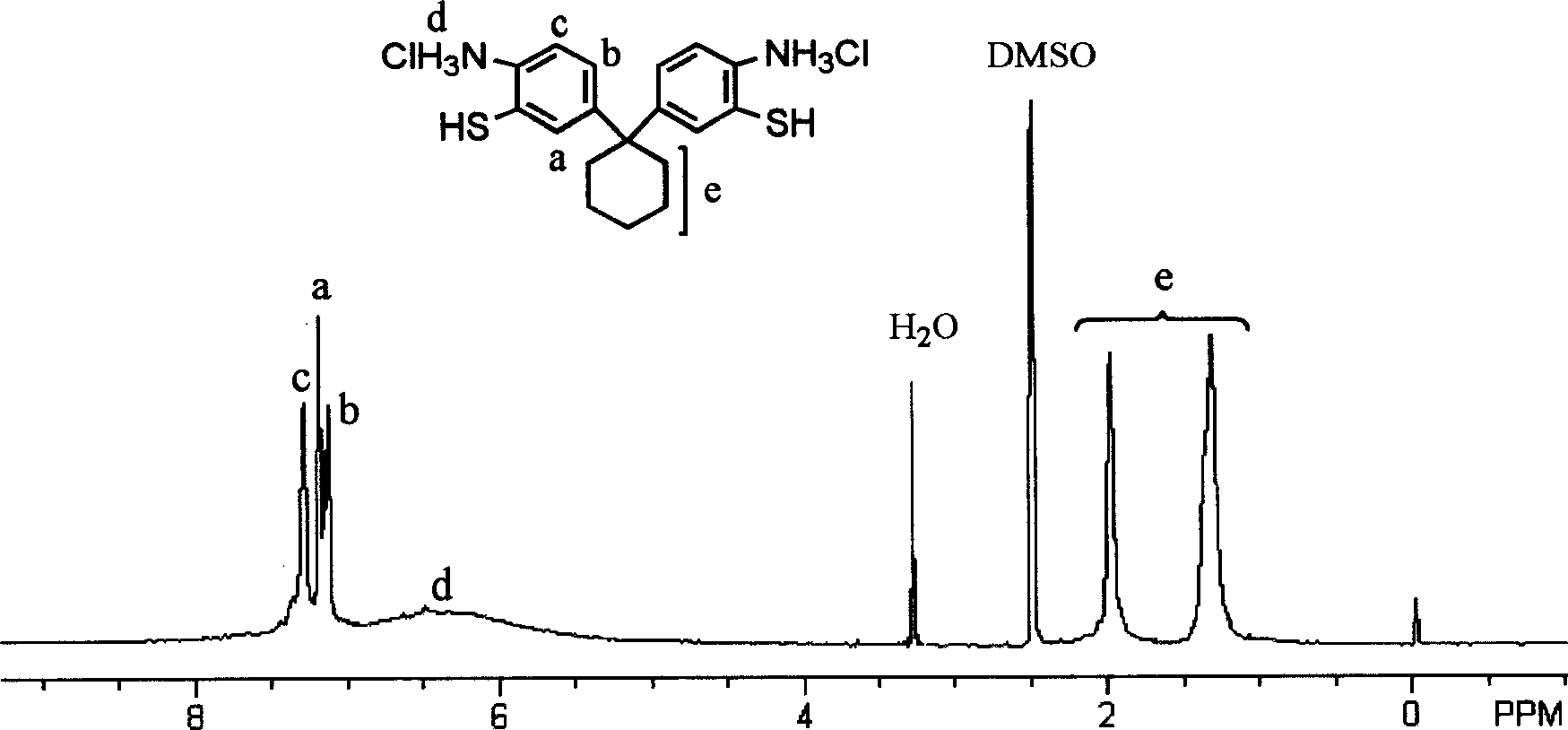
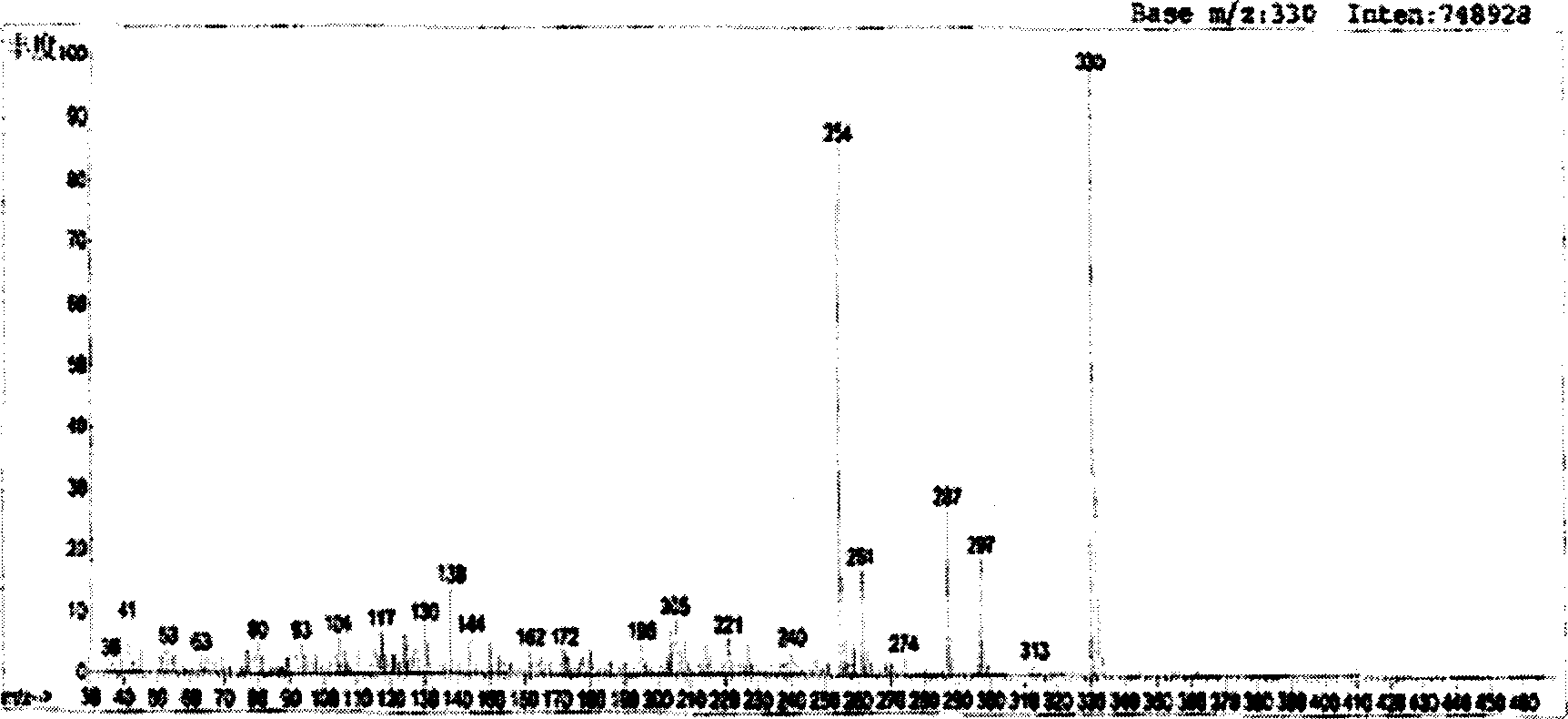
Image
Examples
Embodiment 1
[0031] (a) Add 37.3g (0.4mol) of aniline and 37mL of 35wt% HCl solution into a three-necked flask equipped with a magnetic stirrer, a nitrogen inlet and outlet, and a reflux condenser, and stir to dissolve at 60°C. After the solution was clarified, 9.8 g (0.1 mol) of cyclohexanone was added dropwise. The mixture was reacted at 120°C for 24 hours. The solution was cooled to room temperature, 10% sodium hydroxide solution was added to pH = 10, the organic layer was separated and washed with water until neutral. Dry over anhydrous magnesium sulfate, distill off excess aniline under reduced pressure, and recrystallize from toluene to obtain 22.1 g of 1,1-bis(4-aminophenyl)cyclohexane (83.1% yield).
[0032] (b) 26.6g (0.1mol) 1,1-bis((4-amino)phenyl)cyclohexane, 398mL glacial acetic acid were added to a three-necked flask equipped with mechanical stirring, nitrogen inlet and dropping funnel, After stirring to dissolve, add 48.5g (0.51mol) potassium thiocyanate. Under the condit...
Embodiment 2
[0035] (a) 15.4g (0.1mol) 4-tert-butylcyclohexanone, 51.8g (0.4mol) aniline hydrochloride and 102.3g (1.1mol) aniline were added to a three-necked flask with magnetic stirring and nitrogen inlet, and the mixture was Stir and reflux at 140° C. for 36 hours, cool the solution to room temperature, add 10 wt % potassium hydroxide solution to pH = 10, and reflux the mixture for 10 minutes. The organic layer is separated and washed with water until neutral. Dry over anhydrous magnesium sulfate, distill off excess aniline under reduced pressure, and recrystallize from benzene to obtain 20.6 g of 1,1-bis(4-aminophenyl-4-tert-butylcyclohexane (63.9% yield).
[0036] (b) Add 16.1g (0.05mol) 1,1-bis(4-aminophenyl)-4-tert-butylcyclohexane and 240mL glacial acetic acid into a three-necked flask equipped with mechanical stirring, nitrogen inlet and dropping funnel In, stirring and dissolving, add 29.1g (0.3mol) potassium thiocyanate. Under the conditions of cooling in an ice bath and mecha...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com