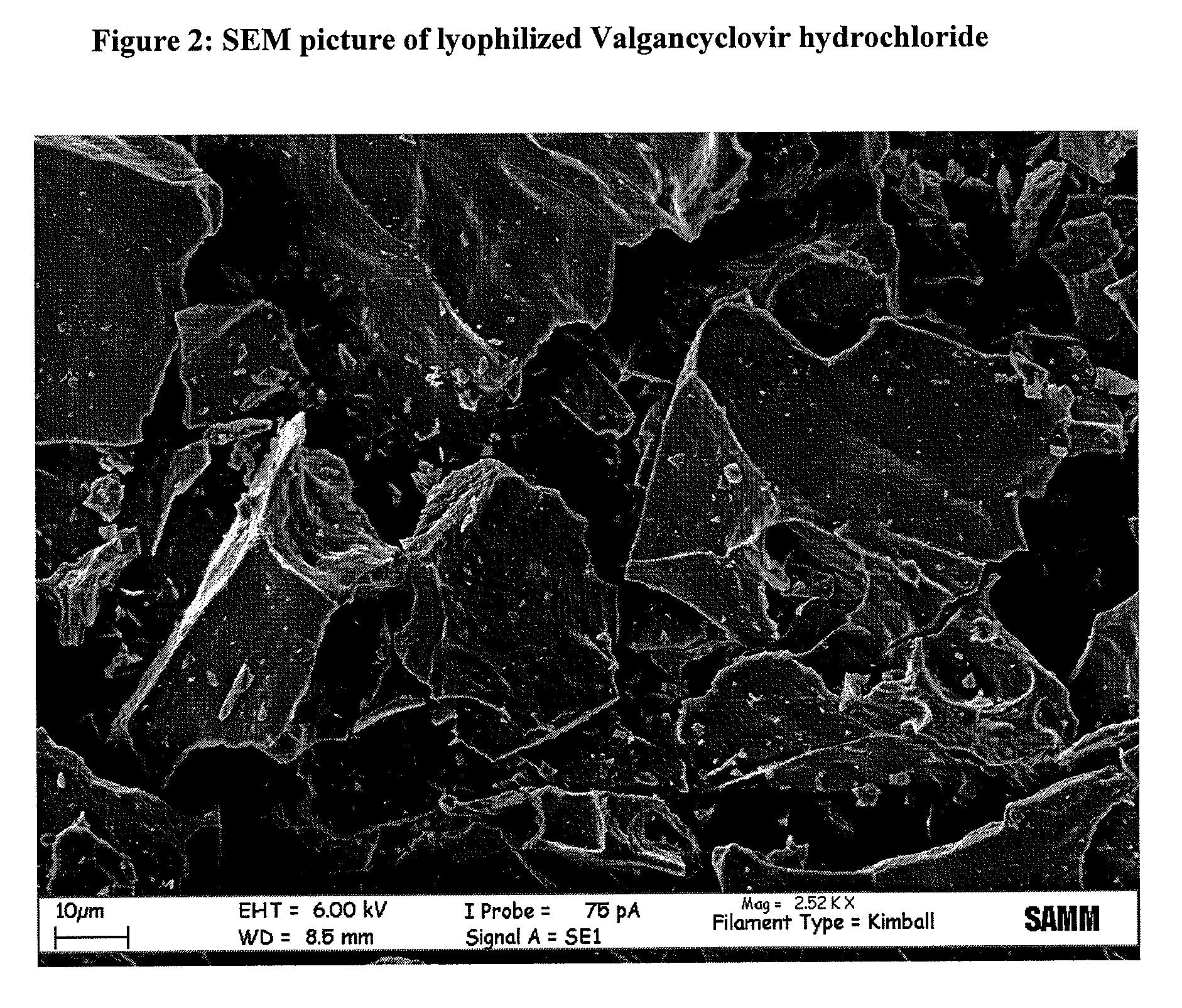
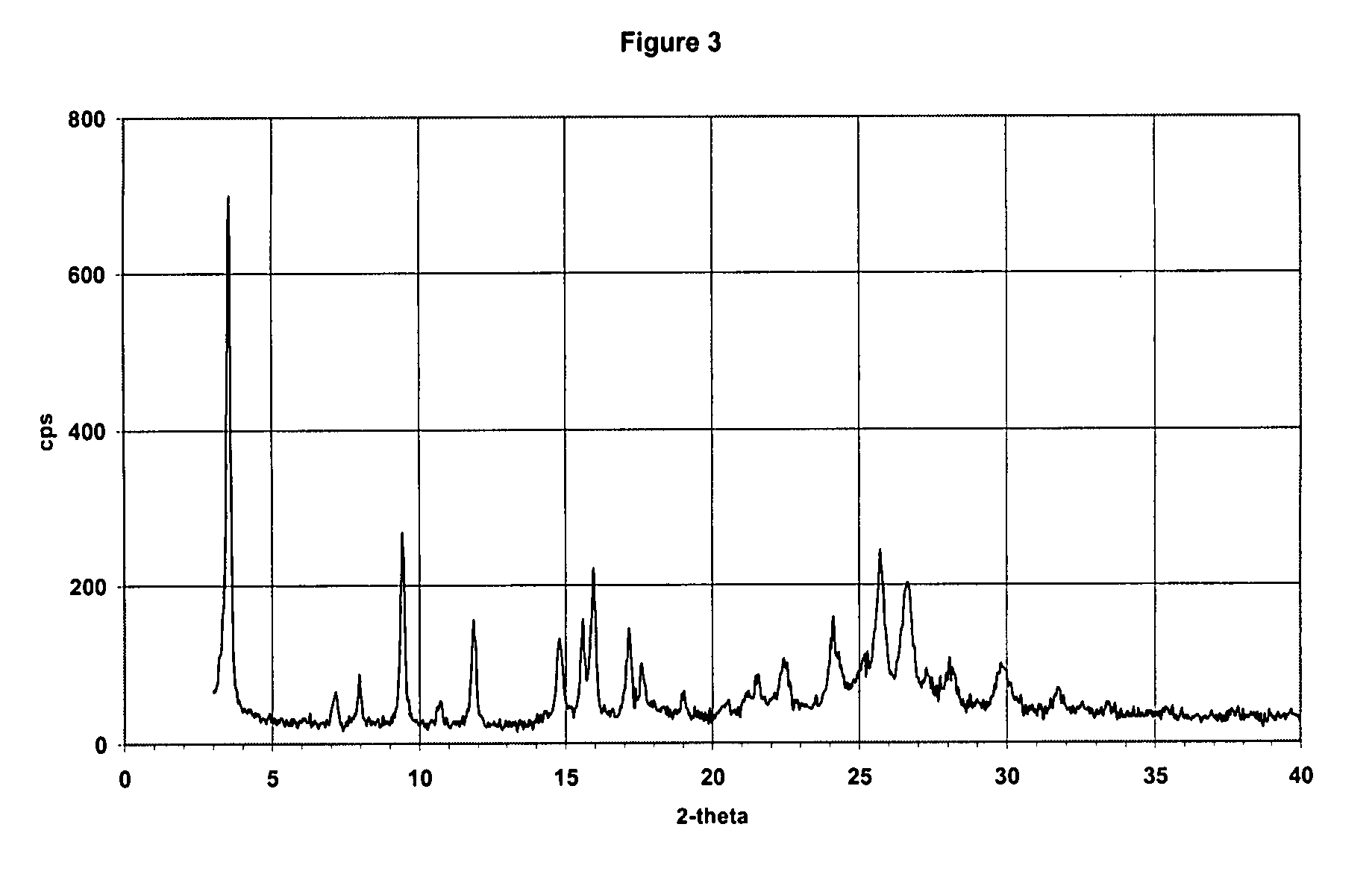
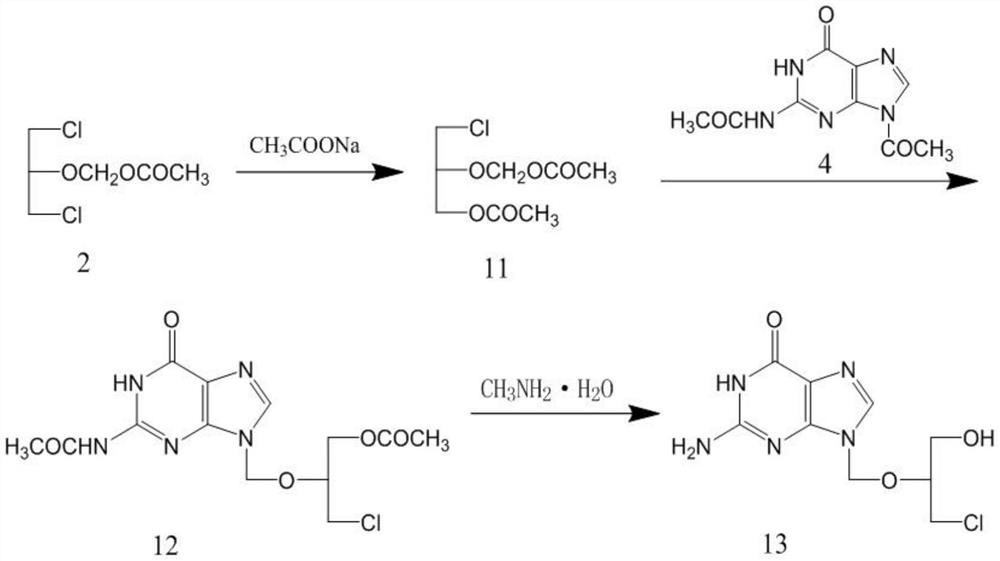
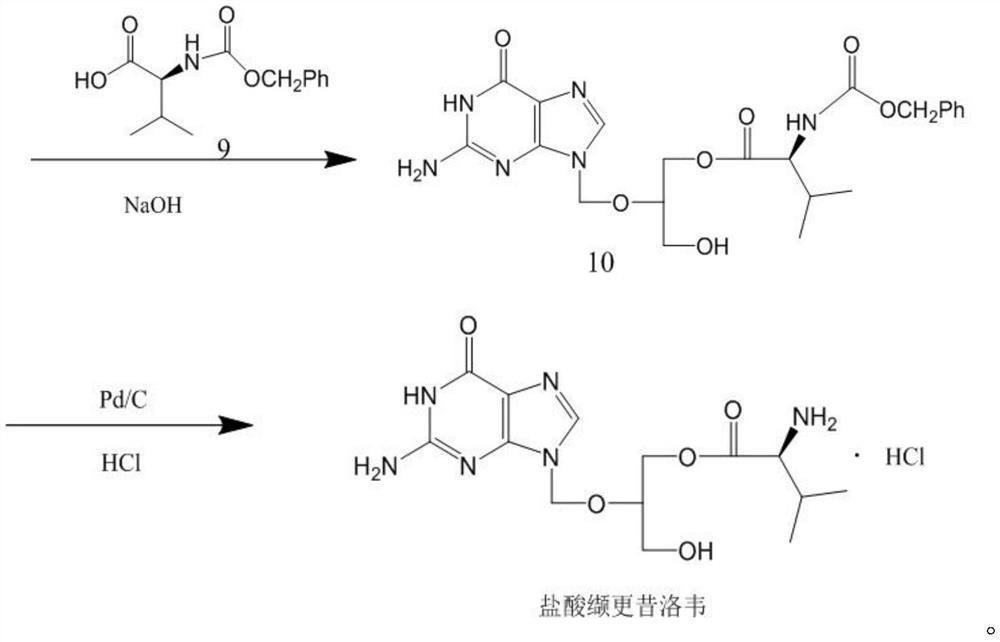
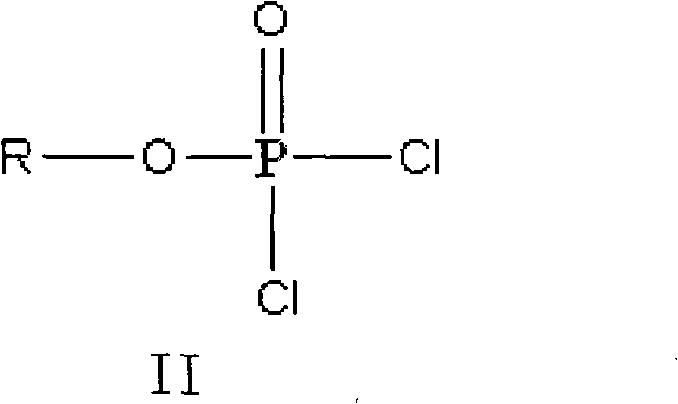Patents
Literature
33 results about "Valganciclovir Hydrochloride" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
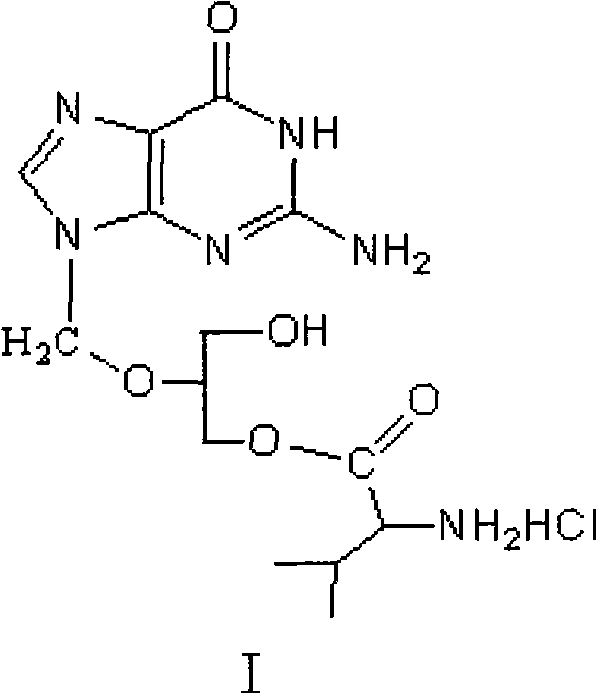
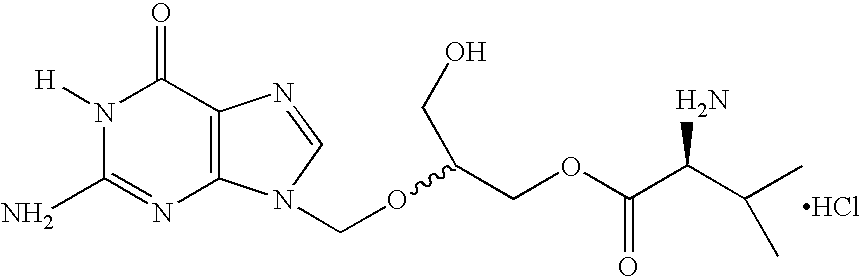
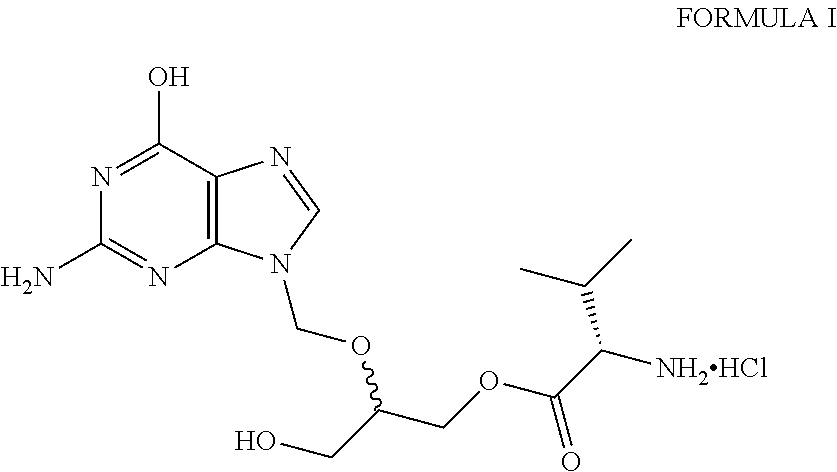
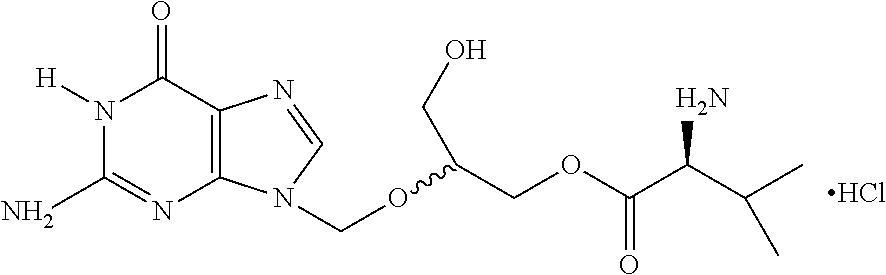
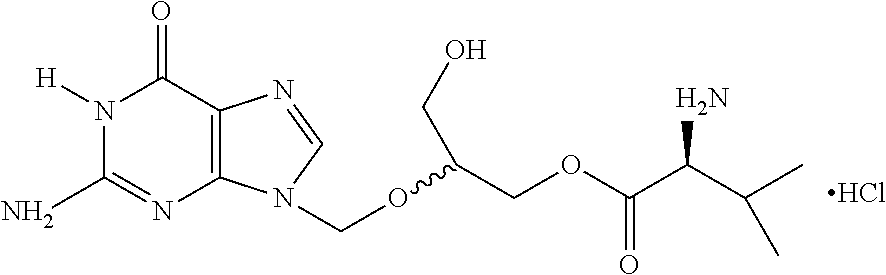
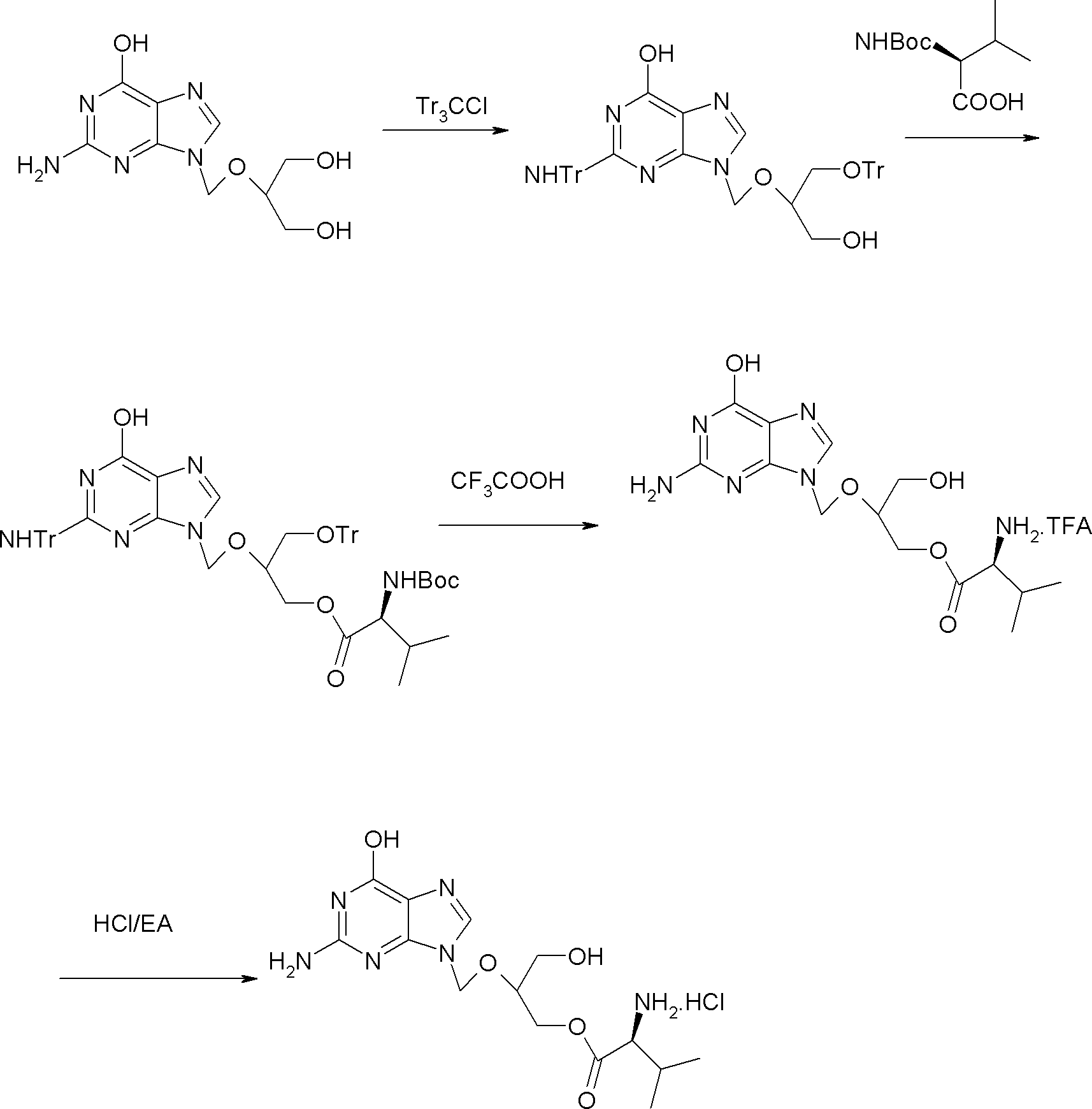
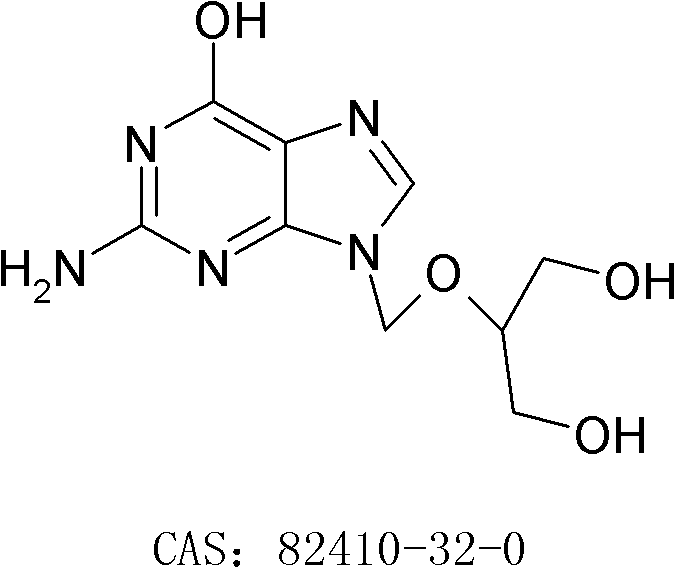
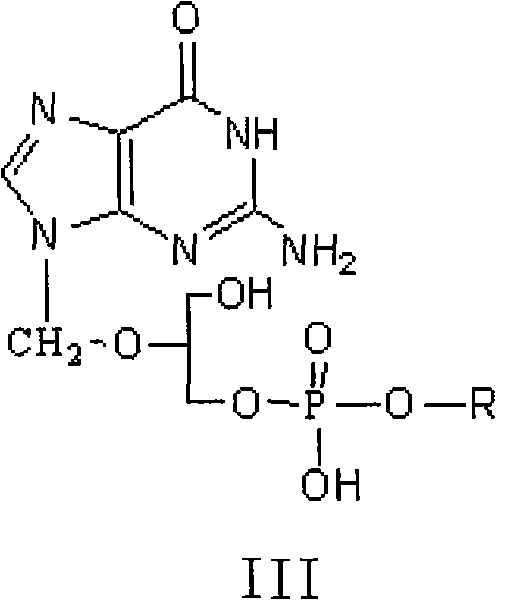
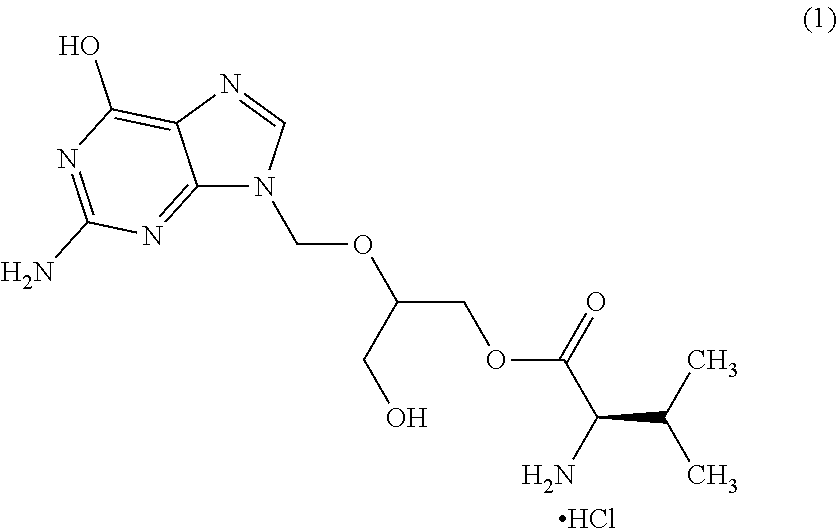
A hydrochloride salt form of valganciclovir, a prodrug form of ganciclovir, a nucleoside analog of 2'-deoxyguanosine, with antiviral activity. After phosphorylation, valganciclovir is incorporated into DNA, resulting in inhibition of viral DNA polymerase, and viral replication.
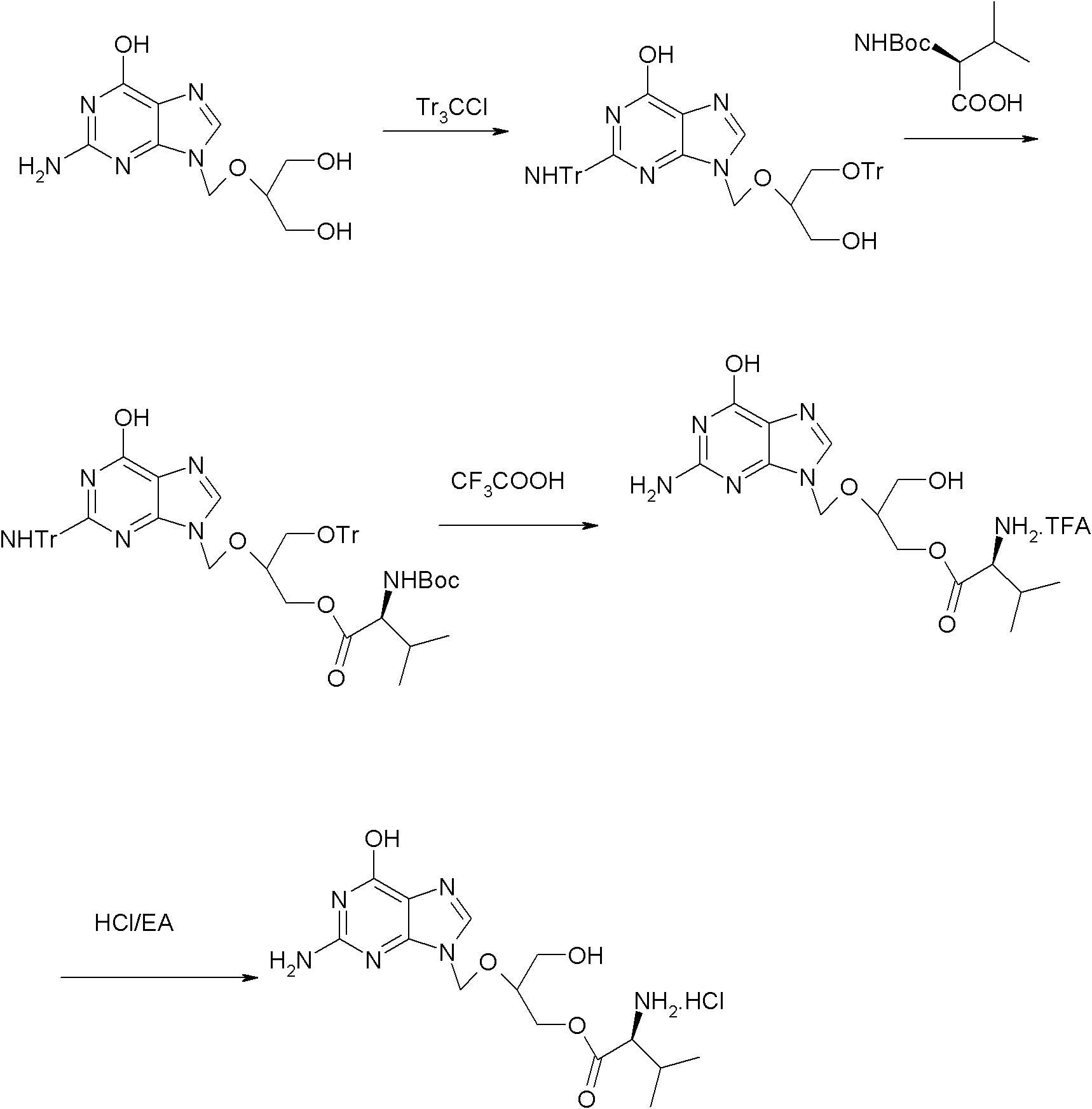
Method for preparing valganciclovir hydrochloride
InactiveCN101955481AEnhanced steric effectHigh yieldOrganic chemistryValganciclovir HydrochlorideHydrogenation reaction
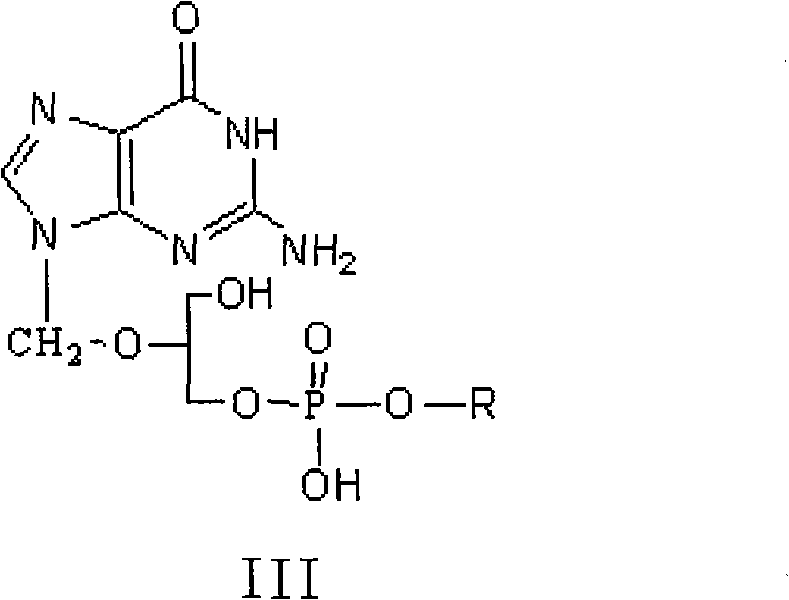
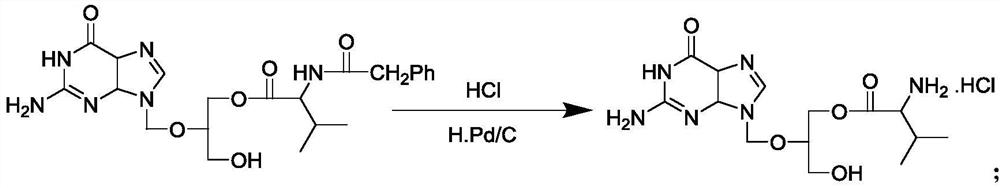
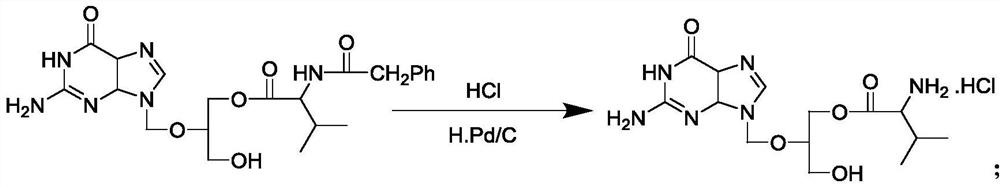
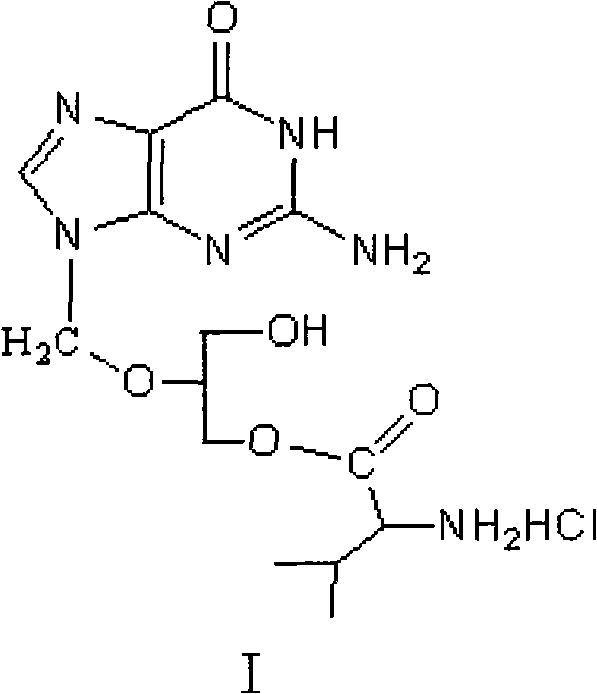
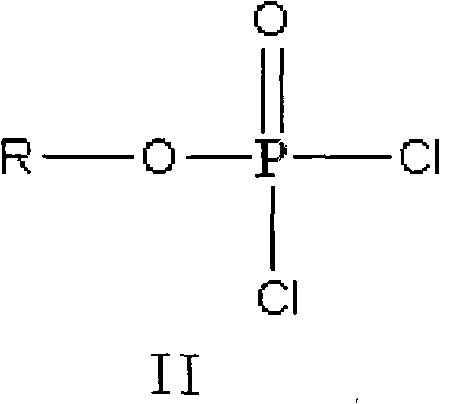
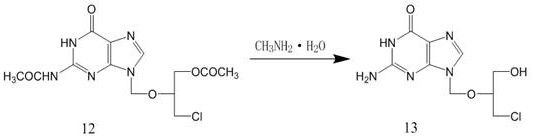
The invention discloses a method for preparing valganciclovir hydrochloride I. The method comprises the following steps of: 1, dissolving phosphorus oxychloride into an inert solvent, and performing a reaction of the mixture and the alcoholic liquor to obtain phosphoryl halide II; 2, performing a reaction of ganciclovir and the phosphoryl halide II obtained in the step to obtain ganciclovir monoester III; 3, esterfying the ganciclovir monoester III in the step 2 and N-carbobenzoxy-L-valine to obtain ganciclovir diester IV; 4, acidizing the ganciclovir diester IV in the step 3 for dephosphorylation to obtain N-carbobenzoxy-L-valine ganciclovir monoester V; and 5, performing a hydrogenation reaction on the product in the step 4 to prepare the valganciclovir hydrochloride I. By the method, the ganciclovir monoester with high purity and yield can be produced, the post-processing is easy, and the post-processing difficulty is reduced.
Owner:STAR LAKE BIOSCI CO INC ZHAOQING GUANGDONG
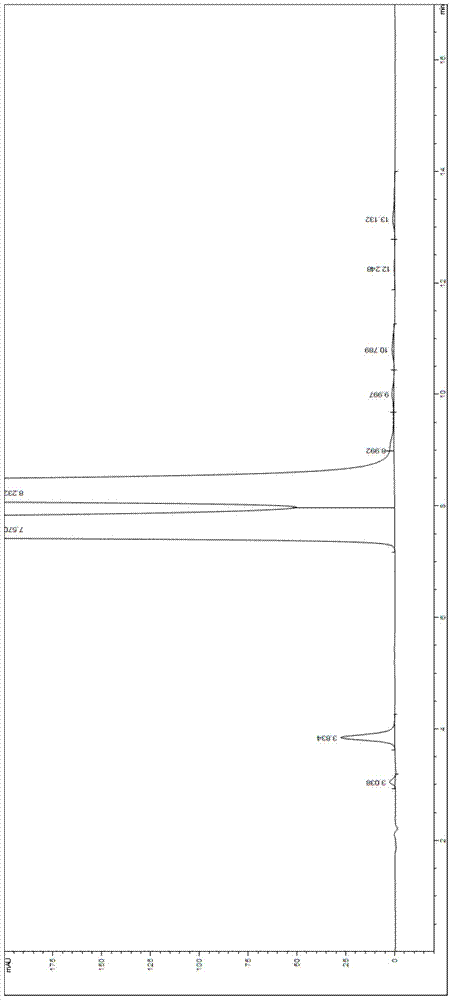
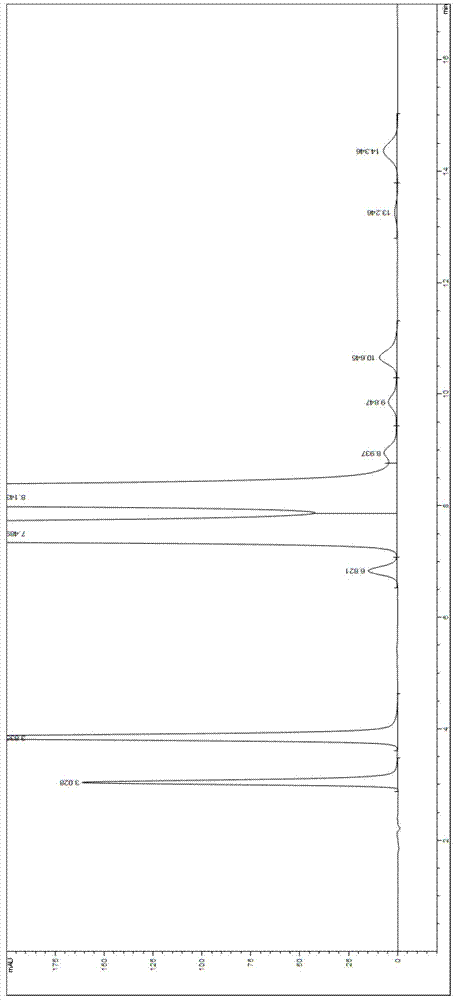
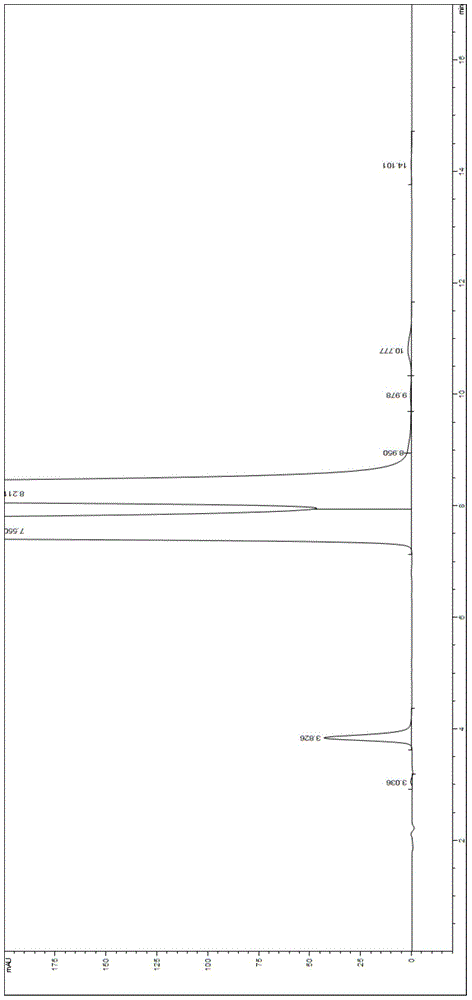
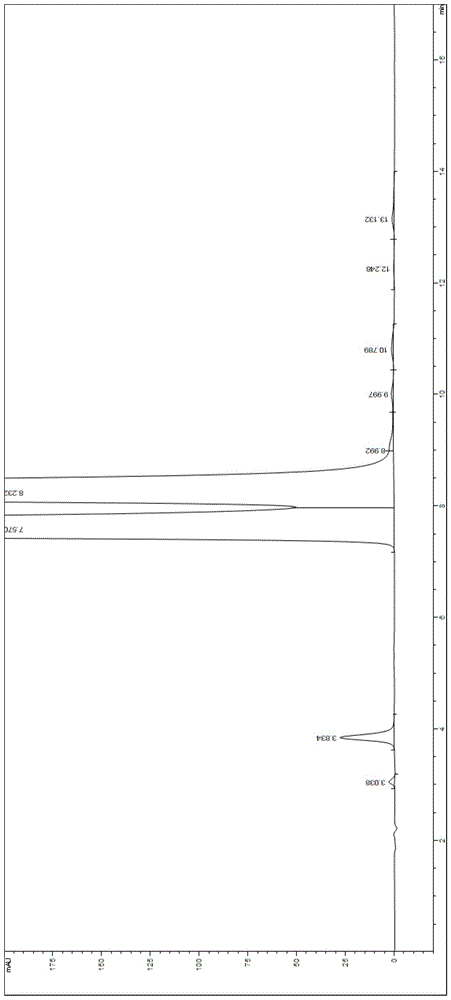
Valganciclovir hydrochloride impurity analytical detecting method
ActiveCN104749286ATroubleshooting Separation DifficultiesSimple and fast operationComponent separationValganciclovir HydrochlorideSilica gel
The invention belongs to the technical field of the analytical chemistry and in particular discloses a valganciclovir hydrochloride impurity HPLC (High Performance Liquid Chromatography) analytical detecting method, and more specifically, the invention relates to a method for analytically detecting guanine, ganciclovir, methoxymethylguanine, ganciclovir 1-N-methyl-valine, mono acetoxyl ganciclovir, mono chloro ganciclovir or a medicine or a preparation containing the above six impurities. A solution to be detected is filled in a high performance liquid chromatographic column taking phenylsilane bonded silica gel as a filling agent, a moving phase prepared by an amine-containing aqueous inorganic salt solution and a chromatographically pure organic solvent is adopted to wash and separate, and then ultraviolet analysis and detection is carried out. According to the method, ammonium acetate or ammonium formate with certain concentration is firstly added in the moving phase creatively, and difficult problems that the main peak and the impurity peak and the adjacent impurity peaks are separated difficultly are thoroughly solved, the operation is simple, the separation degree is high, the sensitivity is high, the accuracy is good, and no similar literature is reported through literature retrieval.
Owner:HUBEI LIYI PHARM TECH CO LTD +1
Novel pharmaceutical dosage forms comprising valganciclovir hydrochloride
The present invention provides novel solid pharmaceutical dosage forms for oral administration, after being constituted in water. The solid dosage forms comprise a therapeutically effective amount of valganciclovir hydrochloride and a non-hygroscopic organic acid present in an amount sufficient to stabilize the valganciclovir hydrochloride in a predetermined amount of water. The present invention also provides novel liquid pharmaceutical dosage forms for oral administration after constituting the solid pharmaceutical dosage form with water. A non-hygroscopic bulking agent may optionally be included in the above dosage form. These novel pharmaceutical dosage forms are useful in the treatment or control of viruses such as herpes simplex virus and cytomegalovirus. The present invention also provides a method for treating these diseases employing the solid and liquid pharmaceutical dosage forms and a method for preparing these pharmaceutical dosage forms.
Owner:BACHYNSKY MARIA OKSANA +2
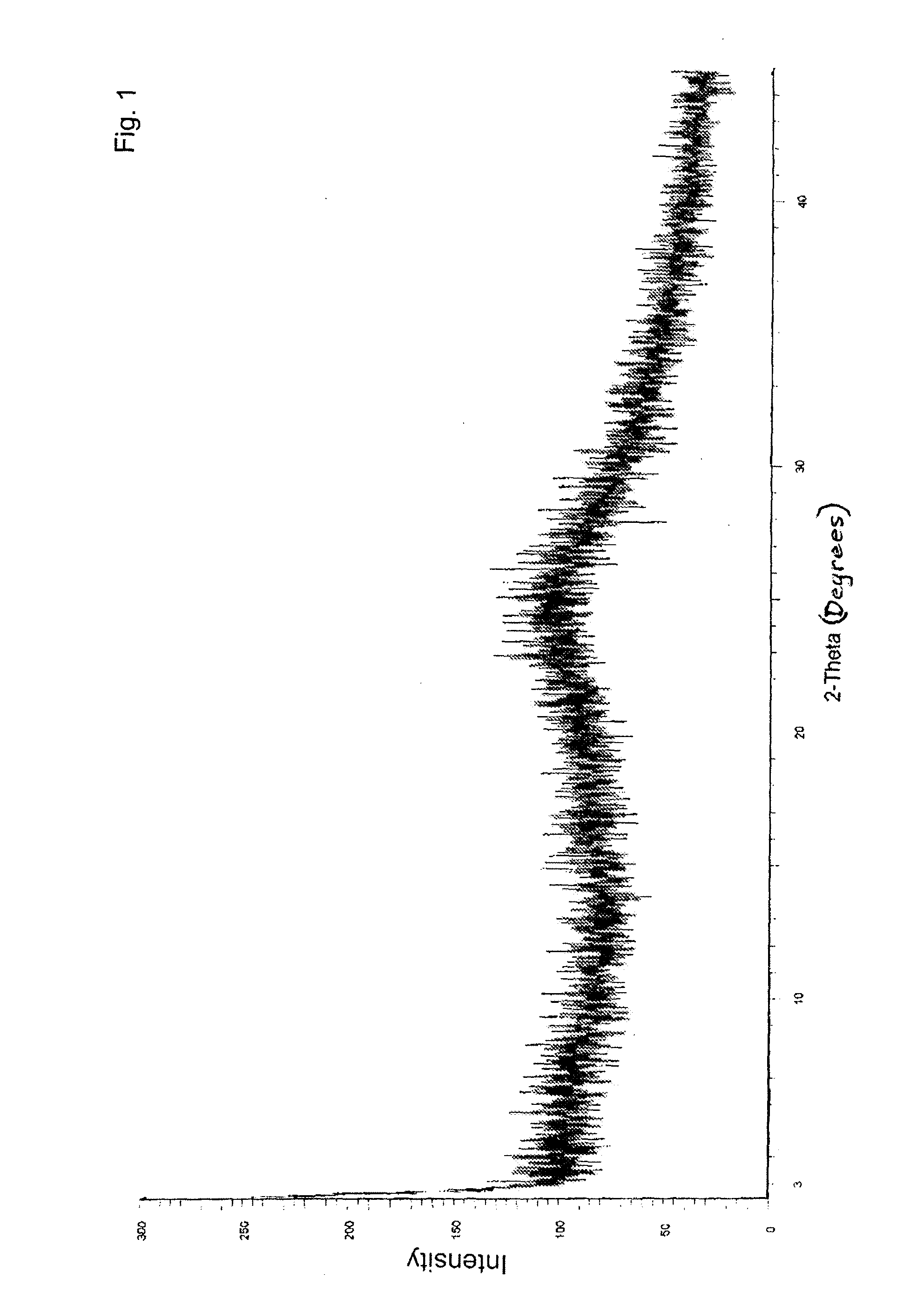
Amorphous valganciclovir hydrochloride
InactiveUS20070129385A1Improved profileBiocideOrganic chemistryValganciclovir HydrochlorideViral infection
The present invention relates to an amorphous form of valganciclovir hydrochloride and the pharmaceutical compositions thereof. The amorphous form can be directly prepared by spray-drying or azeotropic distillation of reaction mass. The amorphous form is useful in treating viral infections, for example, herpes simplex and cytomegalovirus.
Owner:RANBAXY LAB LTD
Preparation technology of (S)-3-carbobenzoxy-4-isopropyl-2,5-oxazolidinedione
ActiveCN110204505AHigh yieldLow yieldOrganic chemistry methodsBulk chemical productionOxazolidinedioneValganciclovir Hydrochloride
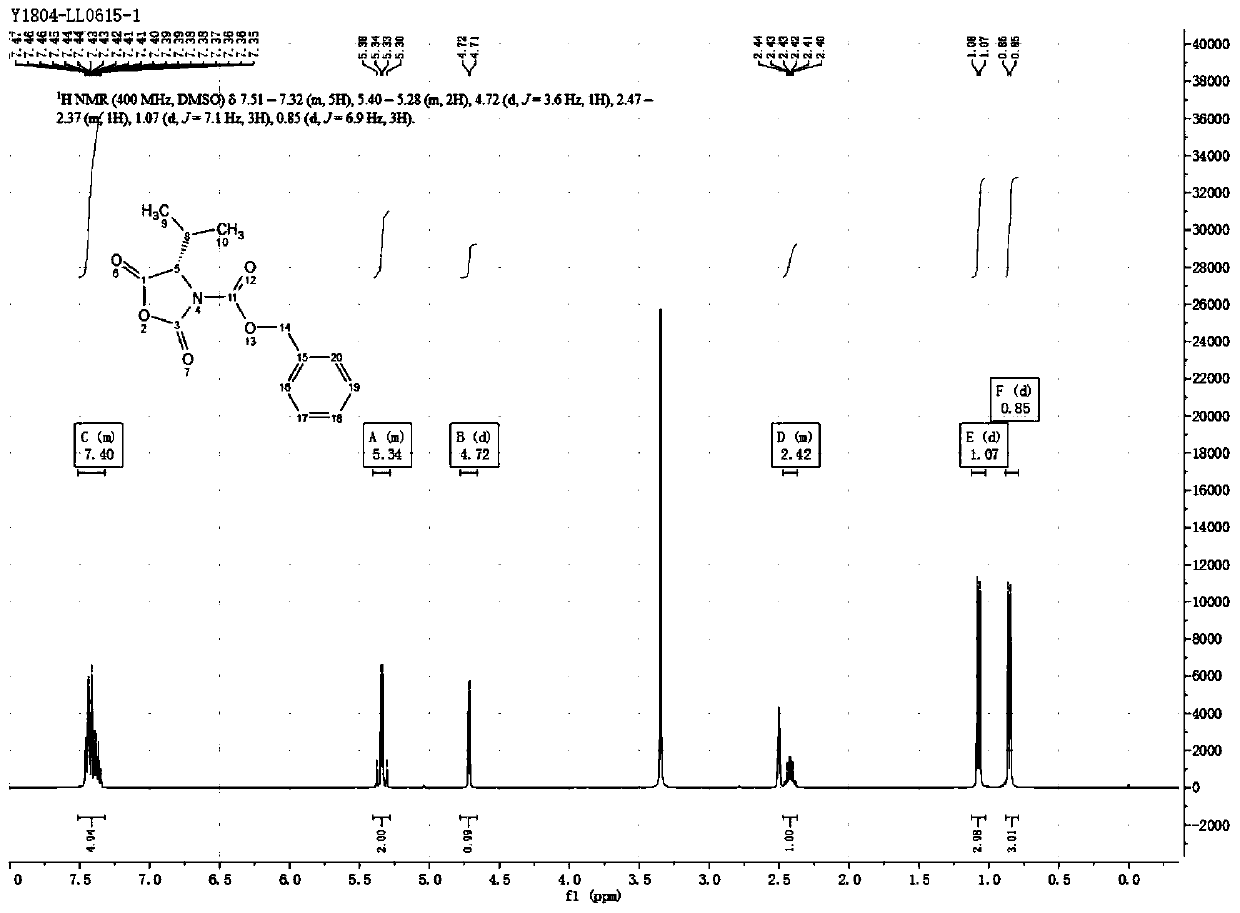
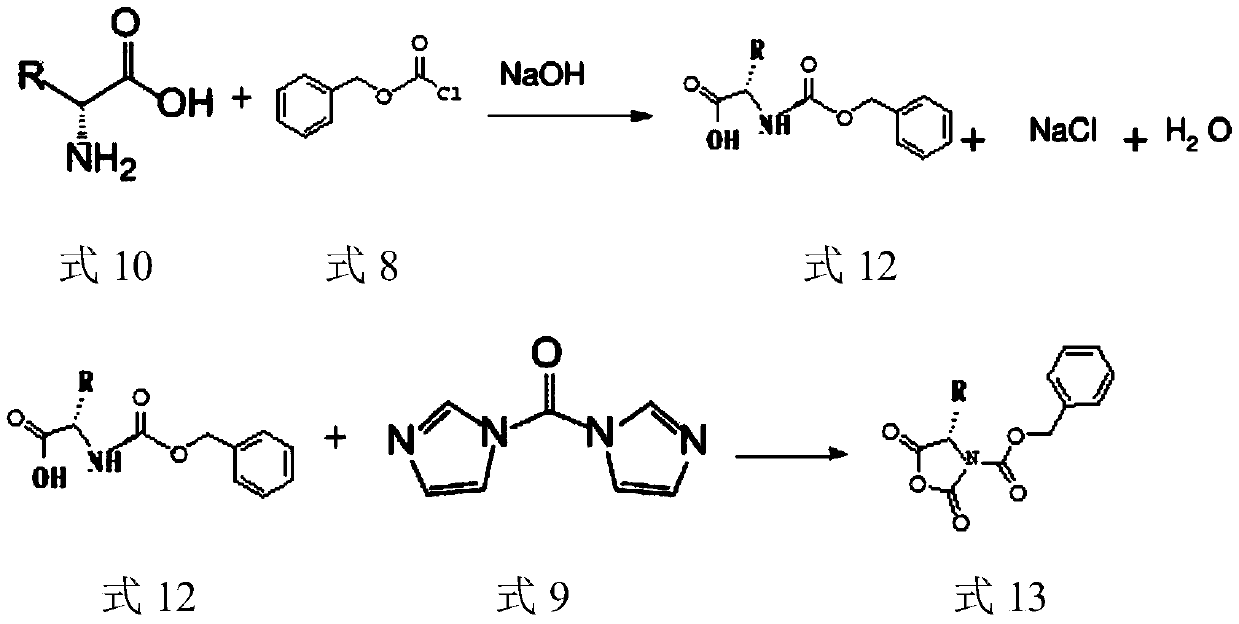
The invention provides a preparation technology of (S)-3-carbobenzoxy-4-isopropyl-2,5-oxazolidinedione. The (S)-3-carbobenzoxy-4-isopropyl-2,5-oxazolidinedione can serve as a valganciclovir hydrochloride intermediate. The technology includes the following operations that an L-valine starting material reacts with benzyl chloroformate to generate N-carbobenzoxy-L-valine; the N-carbobenzoxy-L-valinereacts with N,N-carbonyl diimidazole (CDI) to generate the (S)-3-carbobenzoxy-4-isopropyl-2,5-oxazolidinedione. The preparation technology is simple in method, purification is easy, the process is stable, the quality is controllable, the product yield is greatly improved, environmental pollution is not caused, and the technology is suitable for industrial mass production.
Owner:荆门医药工业技术研究院 +1
Processes for the Preparation of Solid Dosage Forms of Amorphous Valganciclovir Hydrochloride
The present invention relates to a process for the preparation of solid dosage forms of amorphous valganciclovir hydrochloride by dry method.
Owner:RANBAXY LAB LTD
Novel valganciclovir hydrochloride synthesis method
ActiveCN107163050AStandards compliantEasy to operateOrganic chemistrySynthesis methodsValganciclovir Hydrochloride
The invention discloses a novel valganciclovir hydrochloride synthesis method. The method includes: taking ganciclovir as a raw material, adopting ortho-ester for protecting a hydroxyl radical, then subjecting to condensation with N-carbobenzoxy-L-valine, and performing hydrolysis reaction to obtain N-carbobenzoxy valganciclovir; performing hydrogenation reduction reaction to obtain valganciclovir hydrochloride. The product purity reaches 99.0% or above and accords with United States Pharmacopeia standards.
Owner:湖北坦沐生物科技有限公司
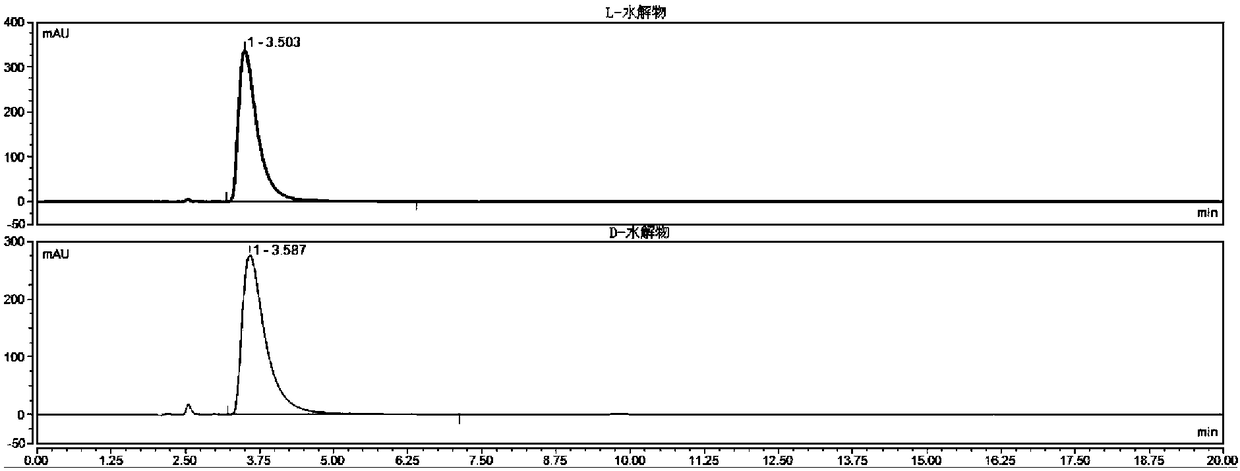
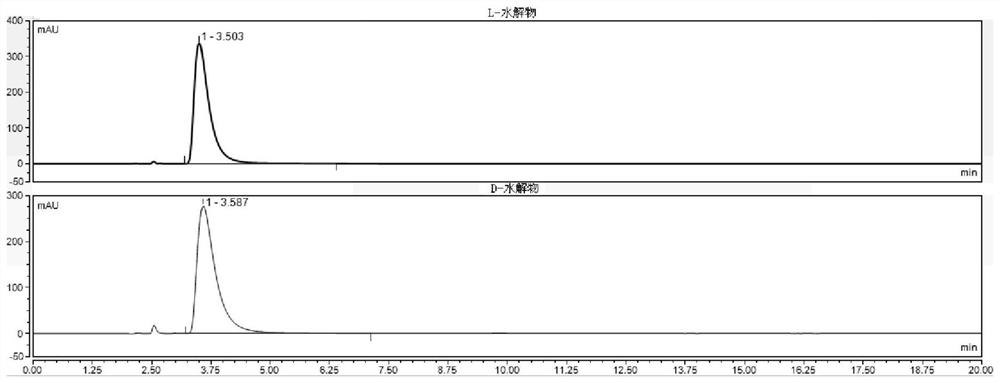
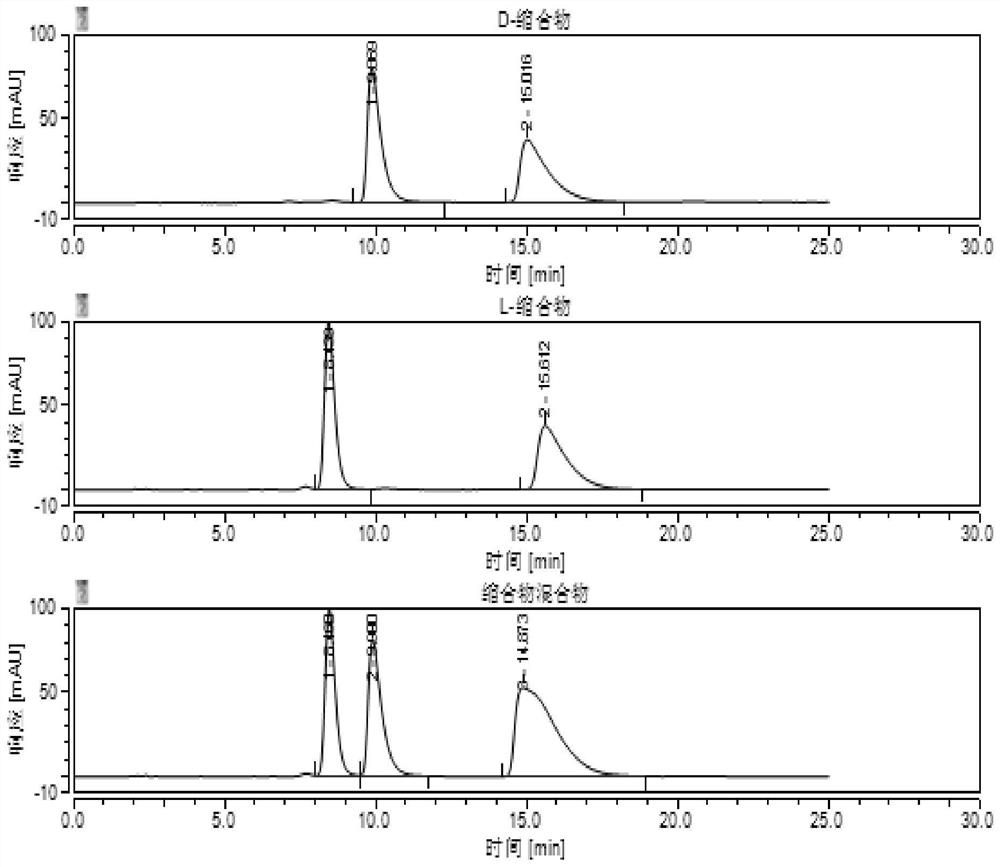
Method for detecting HPLC of valganciclovir hydrochloride intermediate hydrolysate isomers
ActiveCN108267519AAchieve quality controlImprove qualityComponent separationValganciclovir HydrochlorideHydrolysate
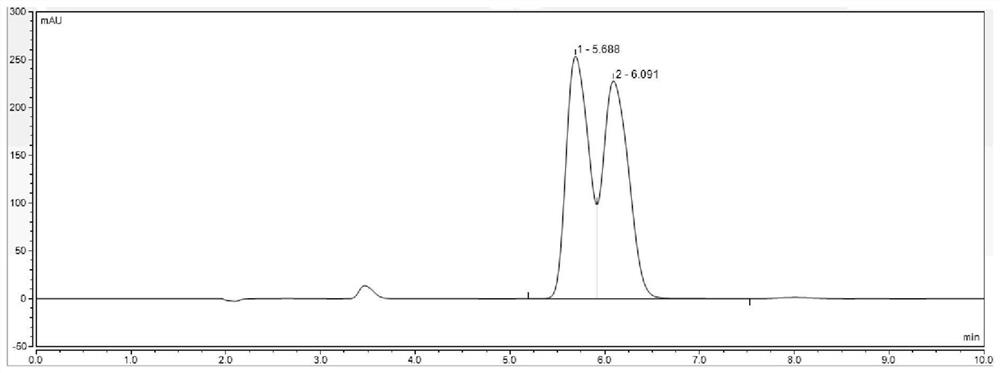
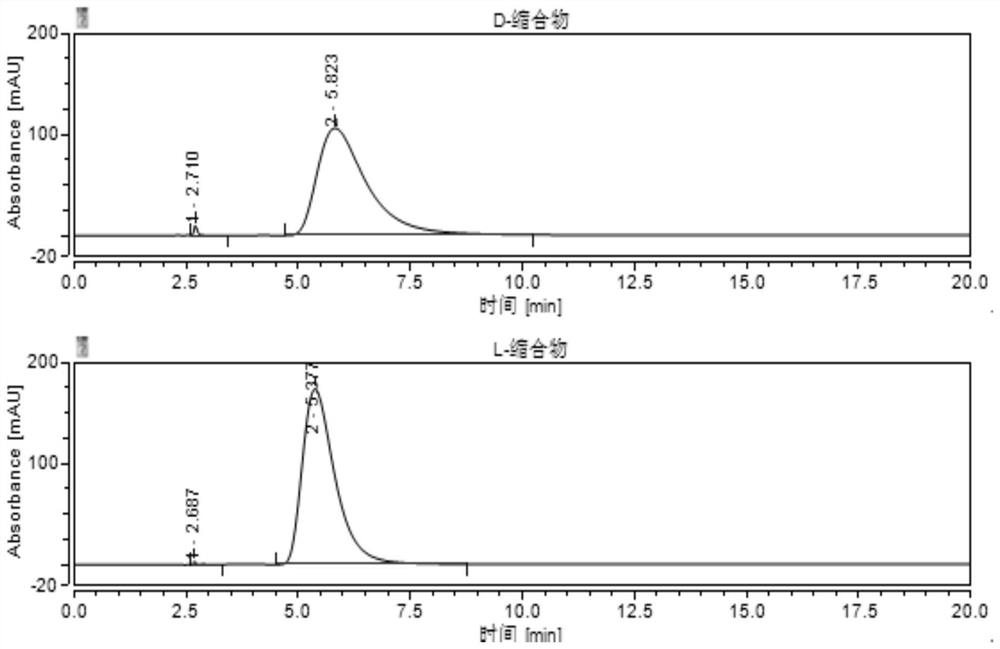
The invention belongs to the field of pharmaceutical analysis, and particularly discloses a method for detecting HPLC of valganciclovir hydrochloride intermediate hydrolysate isomers. The method useschiral HPLC columns taking cellulose-3(3,5-dichloro phenyl amino formic ether) bonded silica gel as a stuffing bulking agent, and takes a mixed solution of hydrocarbon alkyl and alcohols as mobile phase, the column temperature is 20-40 DEG C, the flow velocity is 1.0-1.5 milliliters per minute, and the wave length is 250-260 nanometers. The method for detecting the HPLC of valganciclovir hydrochloride intermediate hydrolysate isomer has the advantages that the effective segregation of four isomers of the valganciclovir hydrochloride intermediate hydrolysate is achieved, the accuracy of the detection result is good, the sensitivity is high, the operation is simple and convenient, the cost is low, the analysis time is short, and guarantee is provided for the quality control of the valganciclovir hydrochloride intermediate hydrolysate.
Owner:HUBEI LIYI PHARM TECH CO LTD
A kind of analytical detection method of valganciclovir hydrochloride impurity
ActiveCN104749286BTroubleshooting Separation DifficultiesSimple and fast operationComponent separationValganciclovir HydrochlorideSilica gel
The invention belongs to the technical field of analytical chemistry, and specifically discloses an HPLC analysis and detection method for valganciclovir hydrochloride impurities, more specifically, relates to an analysis and detection method for guanine, ganciclovir, and methoxymethylguanine Purine, ganciclovir 1‑N‑methylvaline ester, monoacetoxy ganciclovir, monochloroganciclovir, or drugs containing the impurities in the above 6 or their preparations. The solution to be tested is injected into a high-performance liquid chromatography column with phenylsilane-bonded silica gel as a filler, washed and separated with a mobile phase composed of an amine-containing inorganic salt solution and a chromatographically pure organic solvent, and then analyzed and detected by ultraviolet light. For the first time, this method creatively adds a certain concentration of ammonium acetate or ammonium formate into the mobile phase, which completely solves the difficult problem of separation between the main peak and impurity peaks and adjacent impurity peaks. It is easy to operate, high in resolution, high in sensitivity and good in accuracy. , after literature search, there is no similar literature report.
Owner:HUBEI LIYI PHARM TECH CO LTD +1
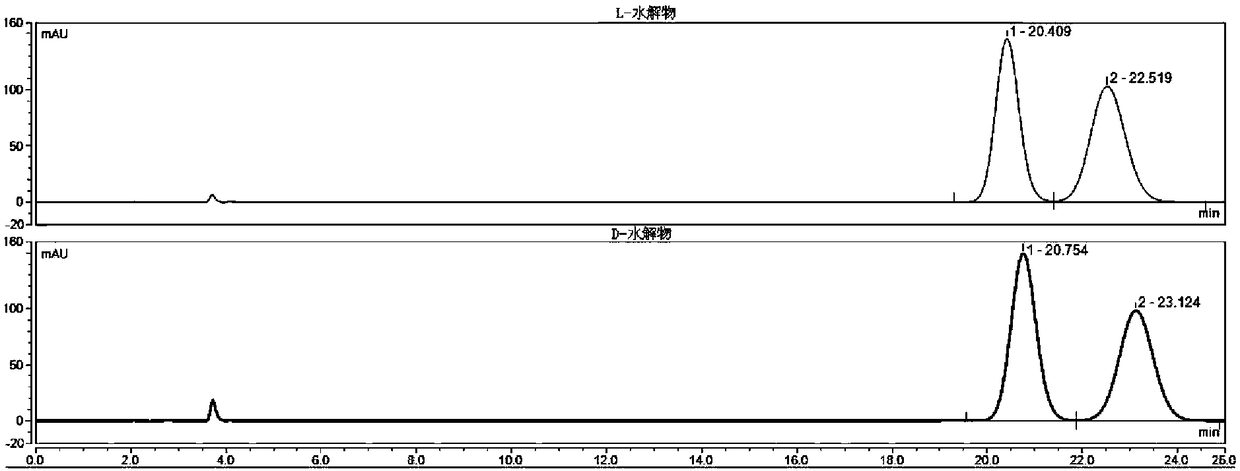
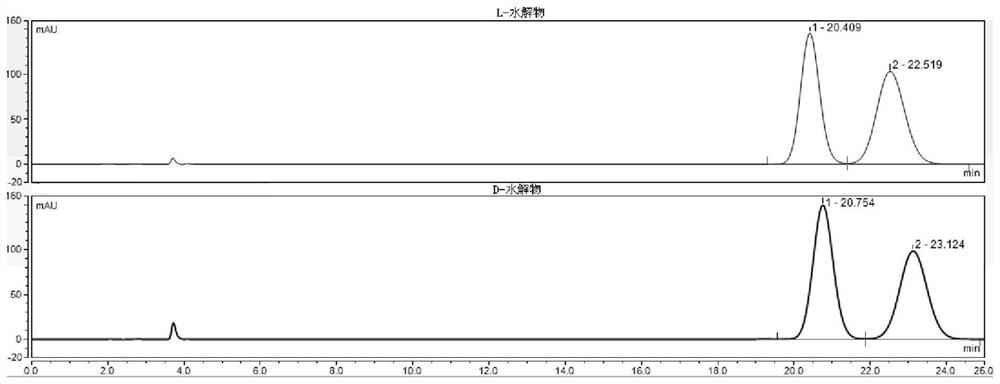
High performance liquid chromatography (HPLC) detection method for valganciclovir hydrochloride intermediate condensation product isomer
ActiveCN109212093AAchieve quality controlEffective quality controlComponent separationCarbamateValganciclovir Hydrochloride
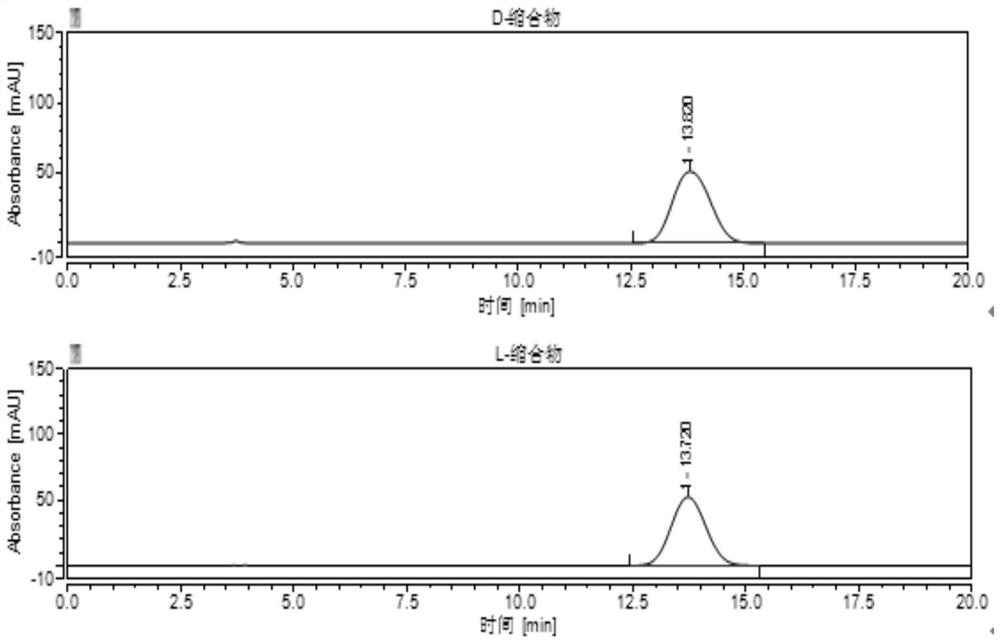
The invention belongs to the field of pharmaceutical analysis, and particularly discloses a high performance liquid chromatography (HPLC) detection method for a valganciclovir hydrochloride intermediate condensation product isomer. According to the method, cellulose-tri(4,-chlorine-3-methyl phenyl carbamate) coating silica gel is used as a chiral column of a filling agent, a mixed solution of water and nitrile is used as a mobile phase, the column temperature is 10-20 DEG C, the flowing speed is 0.4-0.6ml / min, and the wavelength is 250-260 nanometers. By the method, effective separation of four isomers of a valganciclovir hydrochloride intermediate condensation product is achieved, the method has the advantages of good detection result accuracy, high sensitivity, low cost and short analysis time and is simple and convenient to operate, and a guarantee is provided for quality control of the valganciclovir hydrochloride intermediate condensation product.
Owner:HUBEI LIYI PHARM TECH CO LTD +1
Method for preparing and purifying Valganciclovir hydrochloride
ActiveCN102718765AEasy post-processingSuitable for industrial productionOrganic chemistryValganciclovir HydrochlorideGanciclovir
The invention provides a method for preparing and purifying Valganciclovir hydrochloride, characterized by using ganciclovir as a raw material and carrying out N,O triphenylmethyl protection, esterification, deprotection, salt transformation and recrystallization to obtain Valganciclovir hydrochloride. According to the invention, by using mixed anhydride method and avoiding using DCC, the posttreatment is simple; by using BOC protection to replace the CBZ protection used in the prior art, the reaction procedures are simplified, and the need for using noble metal Pd is avoided. The invention is suitable for industrial production.
Owner:SICHUAN KELUN PHARMA RES INST CO LTD
Preparation of amorphous valganciclovir hydrochloride
The present application relates to processes for the preparation amorphous valganciclovir hydrochloride, comprising combining a solution of valganciclovir with an anti-solvent.
Owner:DR REDDYS LAB LTD +1
Pharmaceutical dosage forms comprising valganciclovir hydrochloride
The present invention provides novel solid pharmaceutical dosage forms for oral administration, after being constituted in water. The solid dosage forms comprise a therapeutically effective amount of valganciclovir hydrochloride and a non-hygroscopic organic acid present in an amount sufficient to stabilize the valganciclovir hydrochloride in a predetermined amount of water. The present invention also provides novel liquid pharmaceutical dosage forms for oral administration after constituting the solid pharmaceutical dosage form with water. A non-hygroscopic bulking agent may optionally be included in the above dosage form. These novel pharmaceutical dosage forms are useful in the treatment or control of viruses such as herpes simplex virus and cytomegalovirus. The present invention also provides a method for treating these diseases employing the solid and liquid pharmaceutical dosage forms and a method for preparing these pharmaceutical dosage forms.
Owner:CHEPLAPHARM ARZNEIMITTEL GMBH
Process for the preparation and purification of valgancyclovir hydrochloride
A process for the preparation of valgancyclovir which comprises:a) reacting a compound of formula 7,in an aprotic solvent, in the presence of a condensing agent, with a compound of formula 8,wherein R1, and R2 may be, each independently, hydrogen, an halogen atom or an hydroxyl group; the double bond may either be in the E or Z configuration or a mixture thereof to yield a compound of formula 9b) mild hydrolysis of compound obtained in a) to give valgancyclovir.
Owner:OLON
Synthetic method of valganciclovir hydrochloride
ActiveCN112661757AAvoiding the Separation Transformation ProblemAvoid yield lossOrganic chemistryCondensation processGanciclovirum
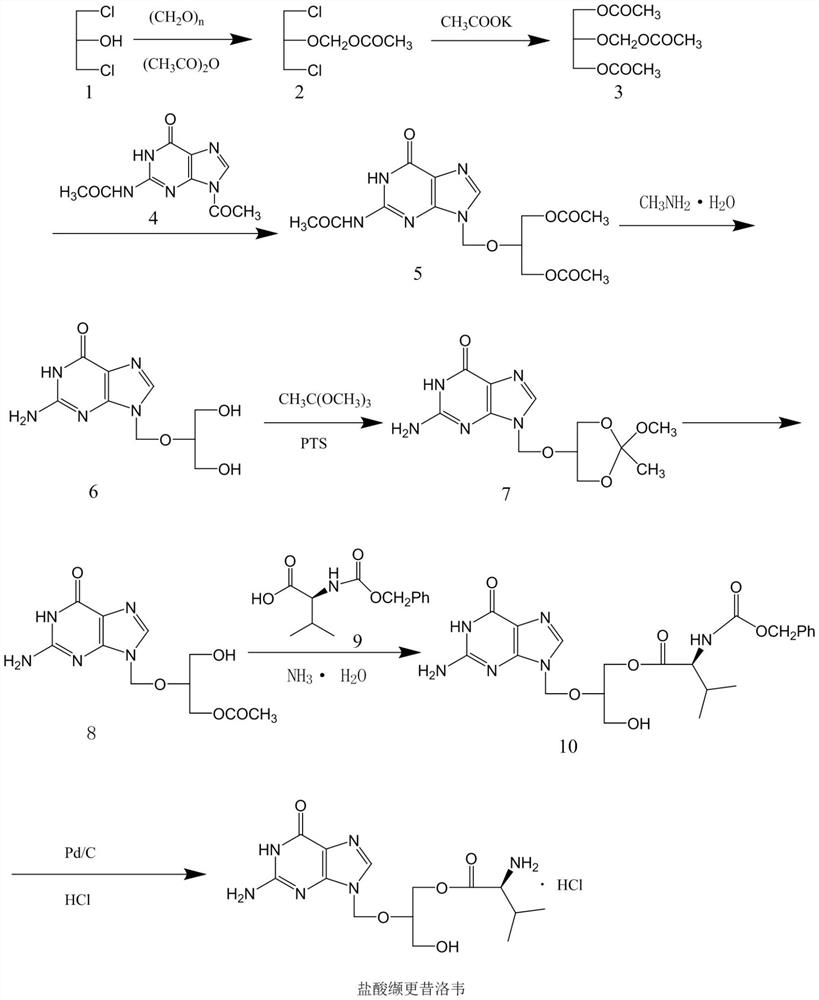
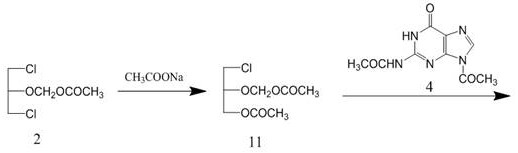
The invention discloses a method for synthesizing valganciclovir hydrochloride, which comprises the following steps: with 1,3-dichloro-2-acetoxymethoxypropane as an initial raw material, preparing monochlorinated ganciclovir, and carrying out esterification, hydrolysis and deprotection salification to finally obtain the valganciclovir hydrochloride. The invention provides a synthesis method of siganciclovir hydrochloride, which avoids the problem of separation and conversion of N-7 and N-9 isomers in the diacetyl guanine condensation process. The method directly synthesizes monochlorinated ganciclovir without using ganciclovir, instead of the synthesis of monoacetyl ganciclovir in the prior art, so that the method avoids the problem of diester compound separation caused by ganciclovir residues, has the advantages of short process steps, simple operation, convenient purification and low cost, is beneficial to industrial production, and is suitable for synthesis of valganciclovir hydrochloride.
Owner:河北合佳医药科技集团股份有限公司
Valganciclovir hydrochloride oral solution and preparation method thereof
ActiveCN110934823ALittle degree of degradationUniform qualityDispersion deliveryAntiviralsValganciclovir HydrochlorideSolvent
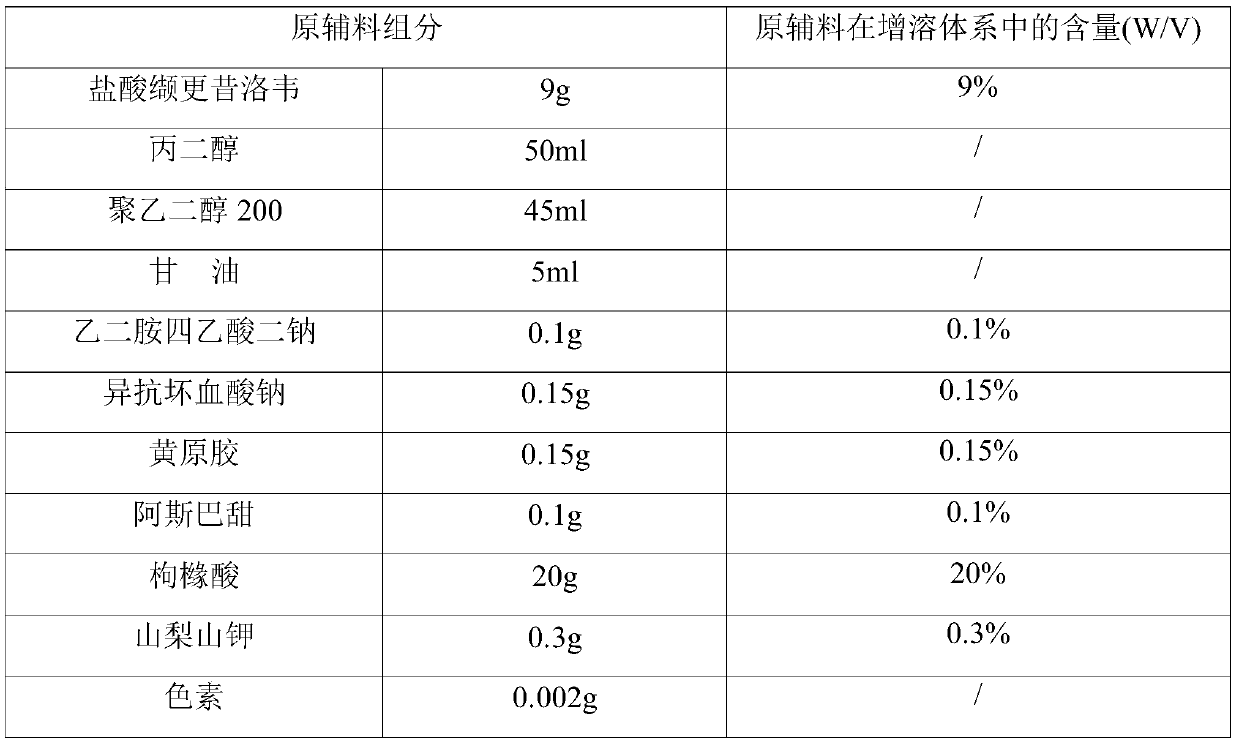
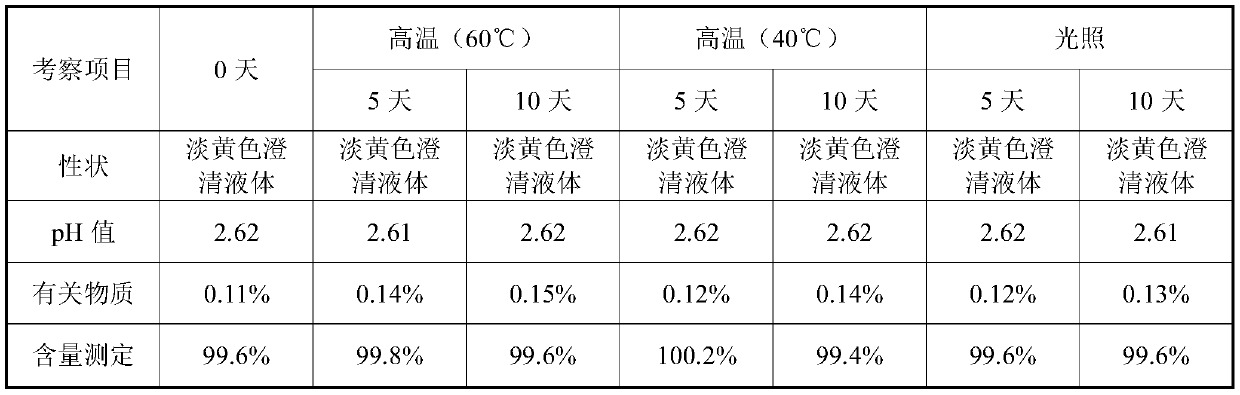
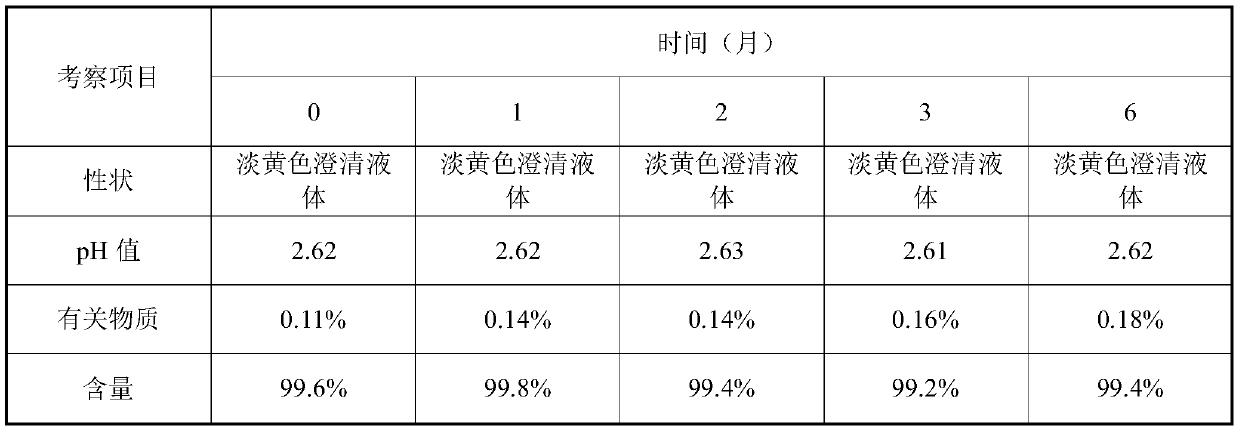
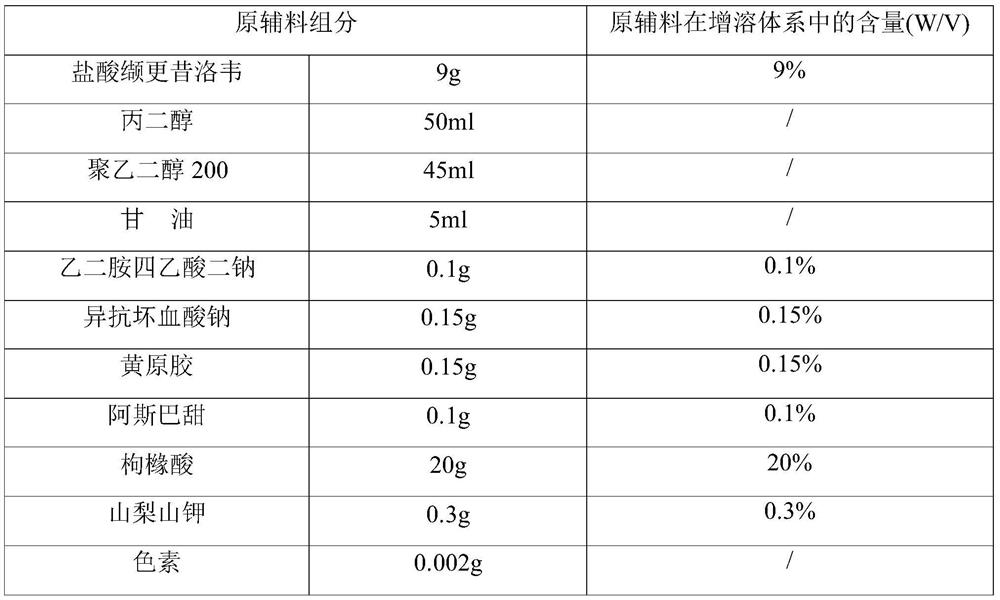
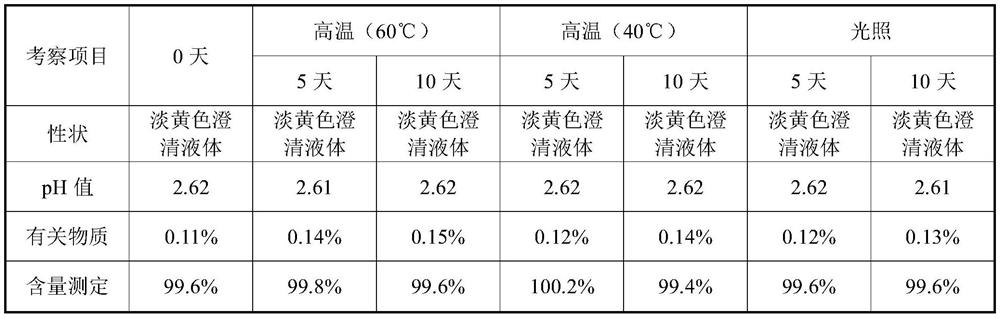
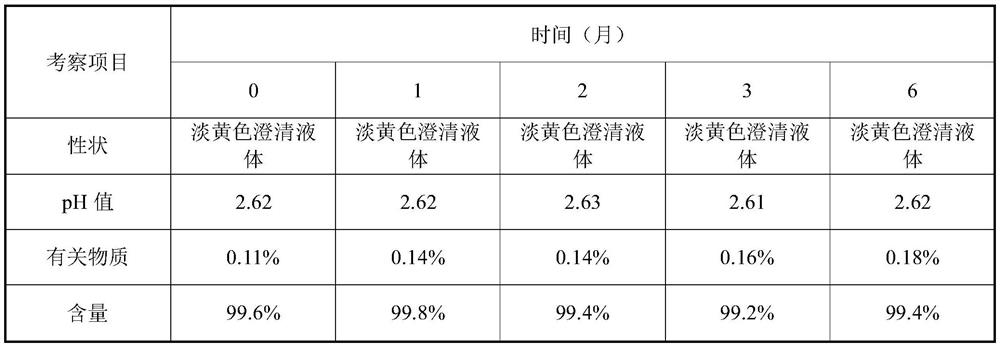
The invention provides a valganciclovir hydrochloride oral solution and a preparation method thereof, the valganciclovir hydrochloride oral solution is mainly composed of valganciclovir hydrochloride,a solubilizing system, a stabilizing system, a sweetener, an acidity regulator and a preservative; wherein the stabilizing system consists of a complexing agent, an antioxidant and a thickening agent; the contents of valganciclovir hydrochloride, the complexing agent, the antioxidant, the thickener, the sweetener, the acidity regulator and the preservative in the solubilizing system are respectively 7 to 12 percent (W / V), 0.08 to 0.12 percent (W / V), 0.12 to 0.18 percent (W / V), 0.15 to 0.25 percent (W / V), 0.06 to 0.12 percent (W / V), 18 to 22 percent (W / V) and 0.20 to 0.35 percent (W / V). According to the valganciclovir hydrochloride oral solution disclosed by the invention, the raw material medicines can be uniformly dispersed in the liquid solvent; according to the present invention, and are not hydrolyzed, such that the uniform and stable quality is provided, the xanthan gum is added to the acid solubilizing system so as to effectively reduce the degradation degree of valganciclovir hydrochloride, the valganciclovir hydrochloride degradation is less than 1.0% after the long-term sample reserving for 24 months, and the content uniformity RSD is less than 2%.
Owner:HUBEI KANGYUAN PHARMA
Valganciclovir hydrochloride preparation method
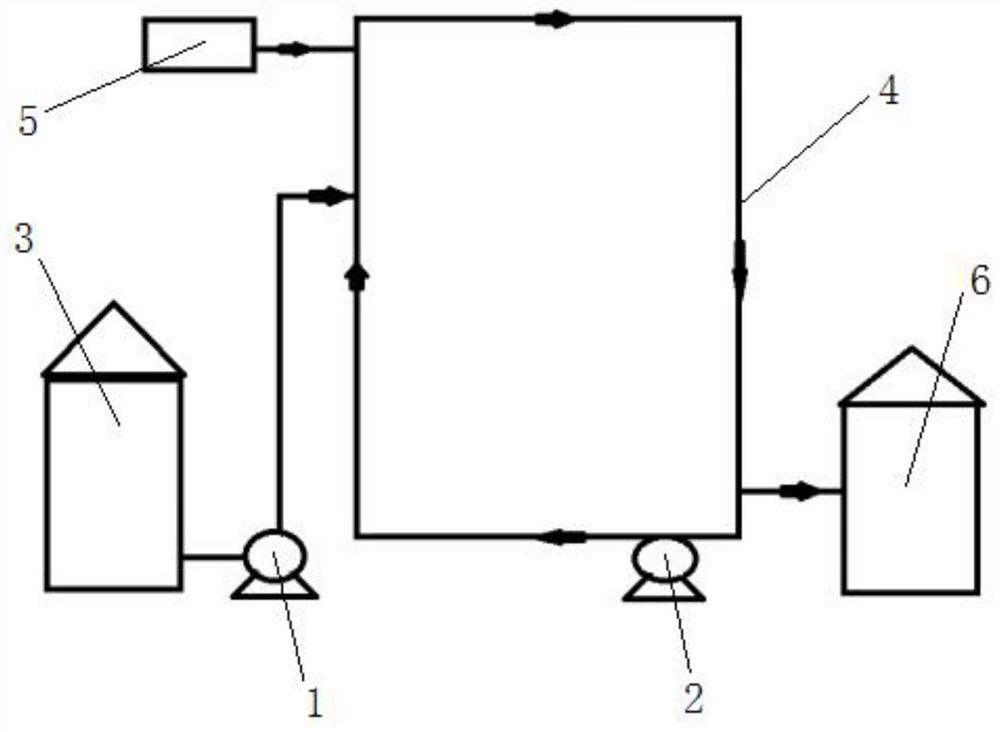
InactiveCN112409359AIncrease reaction rateImprove conversion efficiencyOrganic chemistryPtru catalystValganciclovir Hydrochloride
The invention discloses a valganciclovir hydrochloride preparation method, and belongs to the technical field of drug preparation. The preparation method comprises the steps: mixing a monoester substance, methanol and hydrochloric acid, dissolving, introducing into a material tank, introducing the dissolved substance in the material tank into a tubular reactor pre-filled with a catalyst through apump body, and enabling the material to circularly move in the tubular reactor through the other pump body, starting the circular reaction after compressed hydrogen is introduced, introducing nitrogeninto the tubular reactor after the reaction is finished, discharging the material into the receiving tank from the tubular reactor, and then impurity removal, decoloration and crystallization treatment is performed on the material to obtain a finished product. The tubular reactor is used as a main reaction place so that the reaction time can be greatly shortened, and the conversion efficiency isimproved; the catalyst filling in the reactor is easy to recover so that the cost can be saved; the automation degree is high, and the industrial continuous production requirement is met.
Owner:沃德药业(安徽)股份有限公司
A kind of HPLC detection method of valganciclovir hydrochloride intermediate hydrolyzate isomer
The invention belongs to the field of drug analysis, and specifically discloses an HPLC detection method for an enantiomer of a valganciclovir hydrochloride intermediate hydrolyzate. The method uses cellulose-tri(3,5-dichlorophenylamino Formic acid ester) bonded silica gel as a chiral chromatographic column with a mixed solution of alkanes and alcohols as the mobile phase, the column temperature is 20-40°C, the flow rate is 1.0-1.5ml / min, and the wavelength is 250-260nm . The invention realizes the effective separation of four isomers of valganciclovir hydrochloride intermediate hydrolyzate, has good detection result accuracy, high sensitivity, simple operation, low cost and short analysis time, and is valganciclovir hydrochloride The quality control of intermediate hydrolyzate provides guarantee.
Owner:HUBEI LIYI PHARM TECH CO LTD
Novel pharmaceutical dosage forms comprising valganciclovir hydrochloride
The present invention provides novel solid pharmaceutical dosage forms for oral administration, after being constituted in water. The solid dosage forms comprise a therapeutically effective amount of valganciclovir hydrochloride and a non-hygroscopic organic acid present in an amount sufficient to stabilize the valganciclovir hydrochloride in a predetermined amount of water. The present invention also provides novel liquid pharmaceutical dosage forms for oral administration after constituting the solid pharmaceutical dosage form with water. A non-hygroscopic bulking agent may optionally be included in the above dosage form. These novel pharmaceutical dosage forms are useful in the treatment or control of viruses such as herpes simplex virus and cytomegalovirus. The present invention also provides a method for treating these diseases employing the solid and liquid pharmaceutical dosage forms and a method for preparing these pharmaceutical dosage forms.
Owner:CHEPLAPHARM ARZNEIMITTEL GMBH
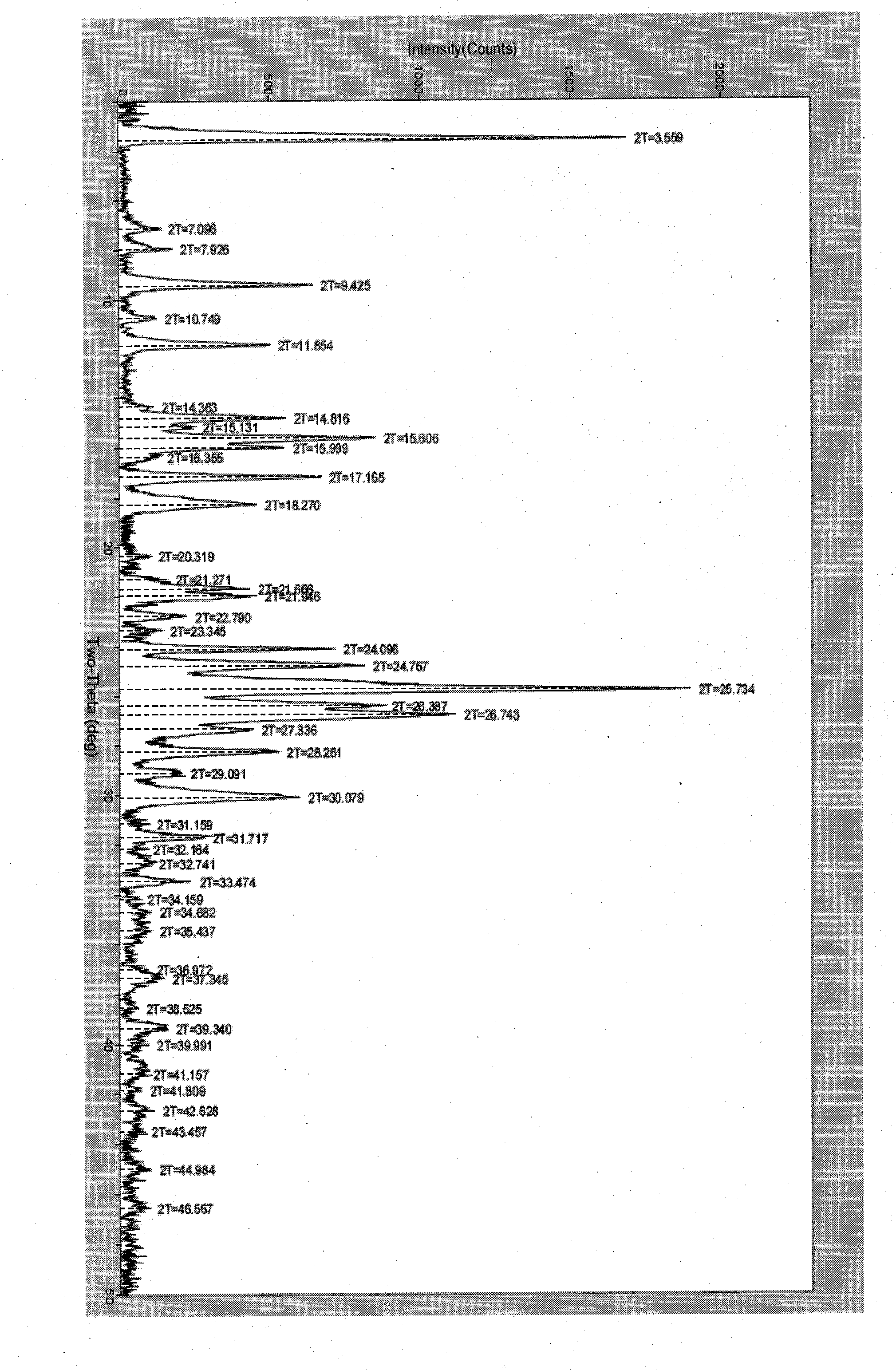
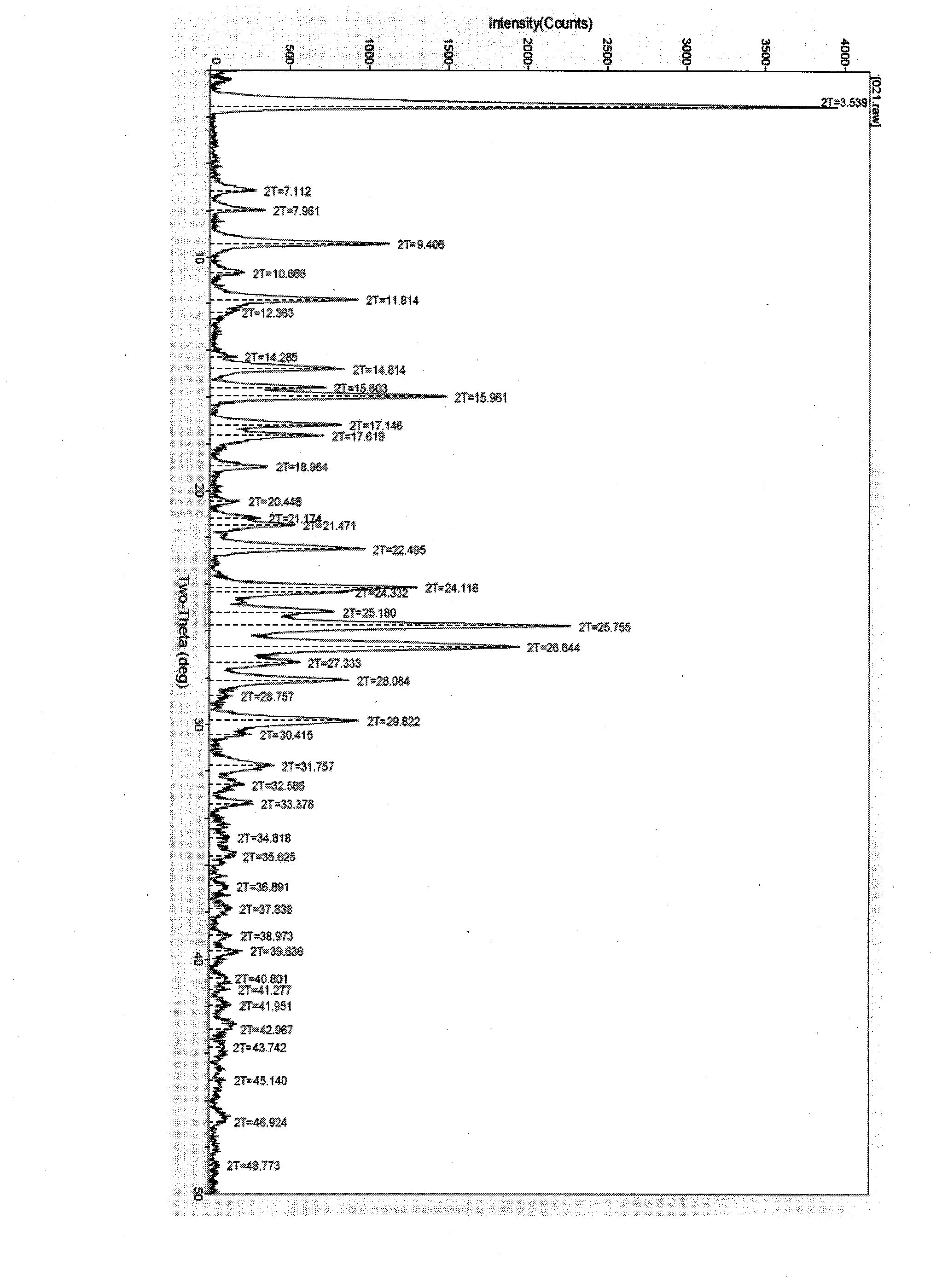
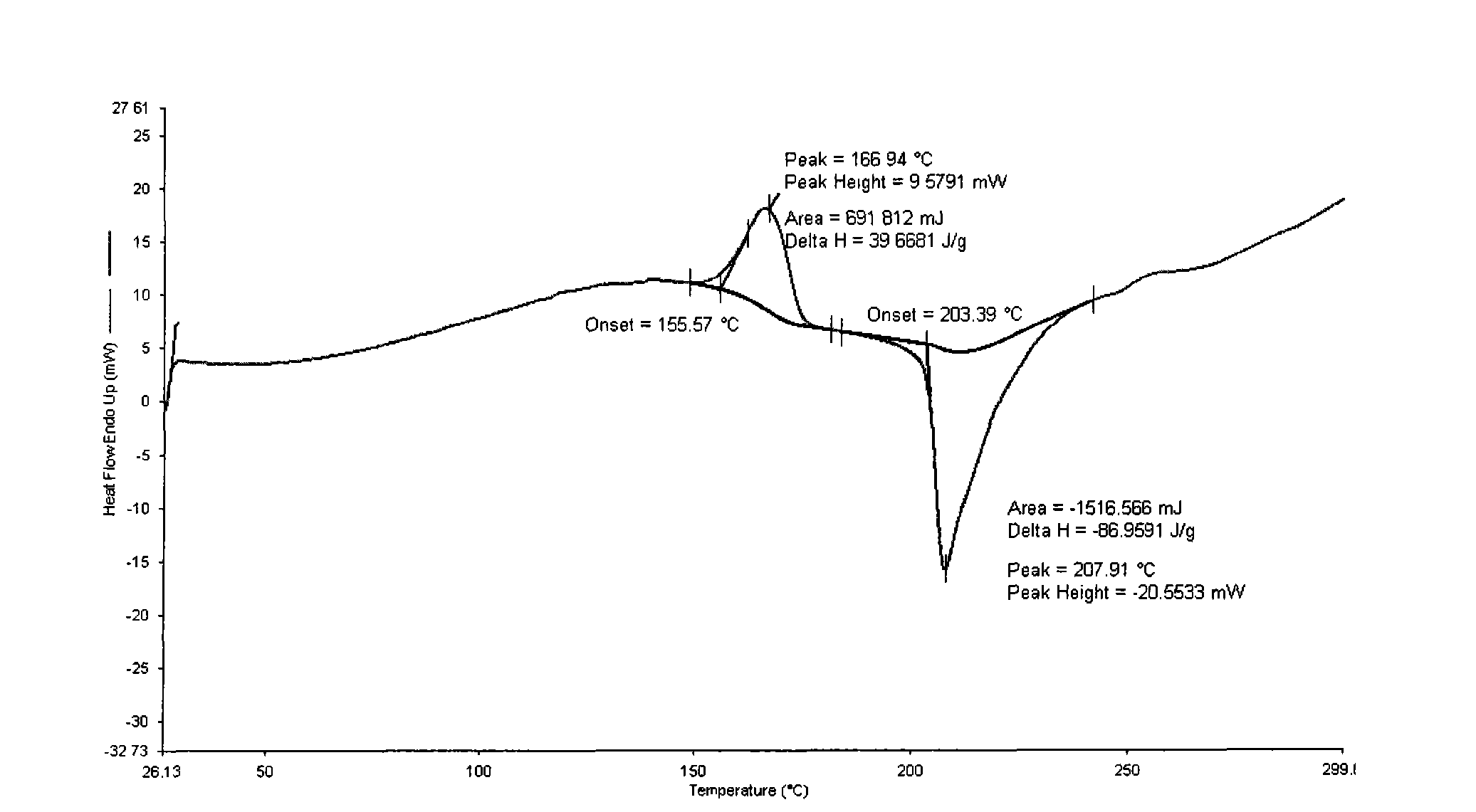
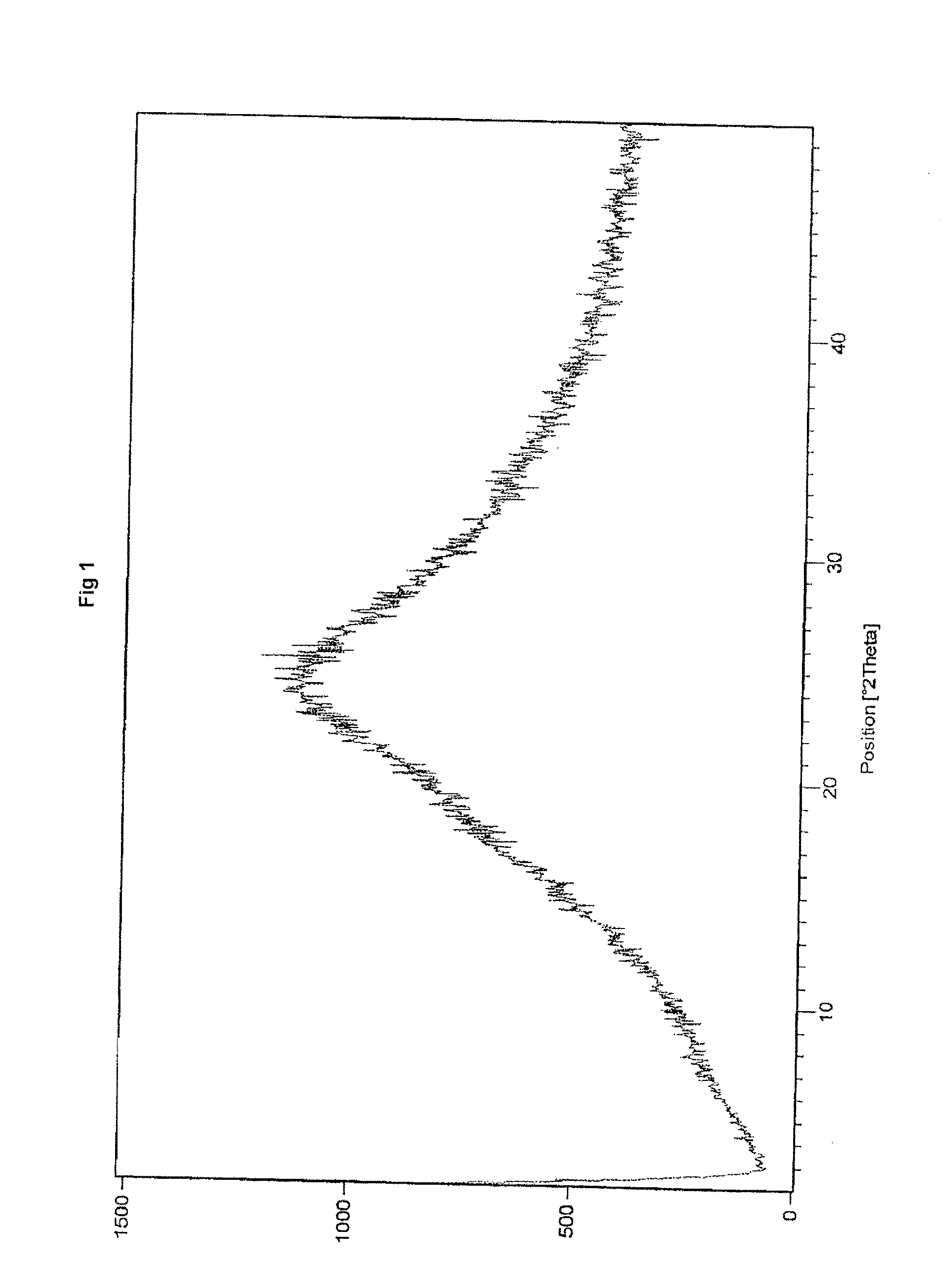
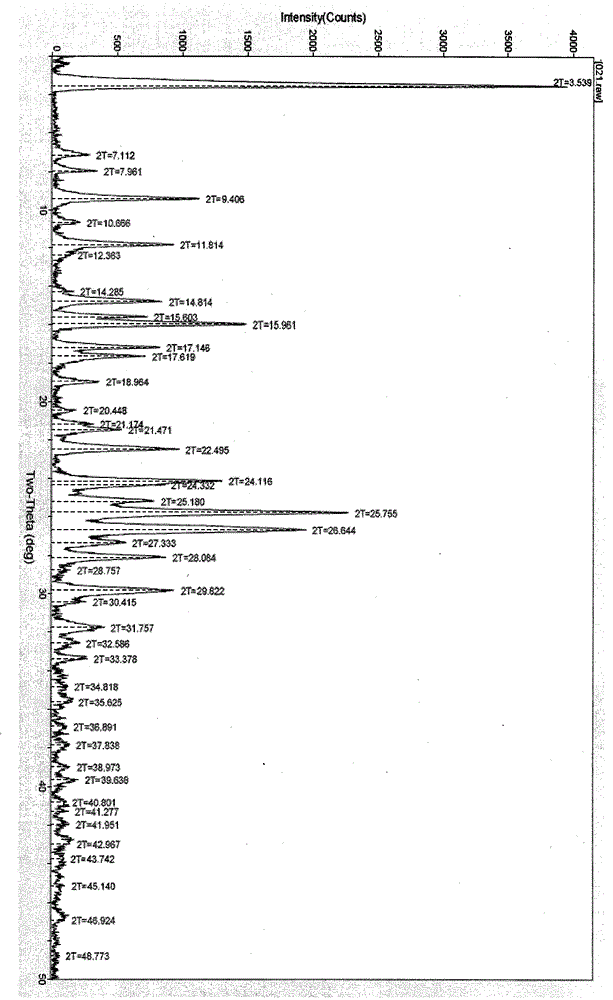
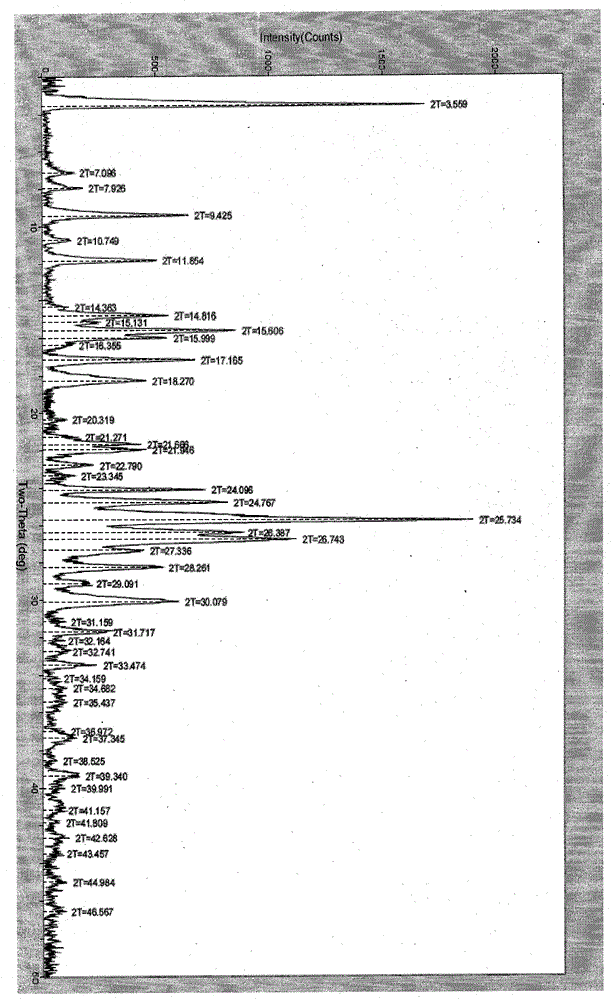
Polymorphic substance of valganciclovir hydrochloride and medical composition thereof
InactiveCN103012404AOrganic chemistryAntiviralsCytomegalovirus infectionsValganciclovir Hydrochloride
The invention provides a polymorphic substance of valganciclovir hydrochloride, and in particular relates to a crystal form A and a crystal form B. The invention further provides a preparation method of the polymorphic substance of valganciclovir hydrochloride, and an application of a medical composition containing the crystal form A and the crystal form B of polymorphic substance of valganciclovir hydrochloride in treating cytomegalovirus infection.
Owner:NINGBO BESTDRUG PHARMA CO LTD
Method for preparing and purifying Valganciclovir hydrochloride
ActiveCN102718765BEasy post-processingSuitable for industrial productionOrganic chemistryValganciclovir HydrochlorideGanciclovir
The invention provides a method for preparing and purifying Valganciclovir hydrochloride, characterized by using ganciclovir as a raw material and carrying out N,O triphenylmethyl protection, esterification, deprotection, salt transformation and recrystallization to obtain Valganciclovir hydrochloride. According to the invention, by using mixed anhydride method and avoiding using DCC, the posttreatment is simple; by using BOC protection to replace the CBZ protection used in the prior art, the reaction procedures are simplified, and the need for using noble metal Pd is avoided. The invention is suitable for industrial production.
Owner:SICHUAN KELUN PHARMA RES INST CO LTD
A kind of preparation method of valganciclovir hydrochloride
InactiveCN101955481BEnhanced steric effectHigh yieldOrganic chemistryValganciclovir HydrochlorideHydrogenation reaction
Owner:STAR LAKE BIOSCI CO INC ZHAOQING GUANGDONG
Process for the preparation of amorphus valgancyclovir hydrochloride
InactiveUS20130133289A1Maintain polymorphicMaintain chemical stabilityOrganic chemistrySynthetic resin layered productsValganciclovir HydrochlorideSolvent
Amorphous valgancyclovir hydrochloride has a median particle size of below 100 μm. A process for the preparation of the compound includes dissolving valgancyclovir hydrochloride in at least one solvent, removing the solvents under moisture controlled conditions, and drying the wet mass.
Owner:MYLAN LAB
A kind of HPLC detection method of valganciclovir hydrochloride intermediate condensate isomer
ActiveCN109212093BAchieve quality controlEffective quality controlComponent separationCelluloseCarbamate
The invention belongs to the field of drug analysis, and specifically discloses an HPLC detection method for valganciclovir hydrochloride intermediate condensate enantiomers. The method uses cellulose-tris(4-chloro-3-methylbenzene Base carbamate) coated silica gel as a chiral chromatographic column with a mixed solution of water and nitriles as the mobile phase, the column temperature is 10~20°C, the flow rate is 0.4~0.6ml / min, and the wavelength is 250 ~260nm. The invention realizes effective separation of four isomers of valganciclovir hydrochloride intermediate condensate, has good detection result accuracy, high sensitivity, simple operation, low cost and short analysis time, and is valganciclovir hydrochloride The quality control of intermediate condensate provides guarantee.
Owner:HUBEI LIYI PHARM TECH CO LTD +1
A kind of synthetic method of valganciclovir hydrochloride
ActiveCN107163050BStandards compliantEasy to operateOrganic chemistryGanciclovirumValganciclovir Hydrochloride
The invention discloses a novel valganciclovir hydrochloride synthesis method. The method includes: taking ganciclovir as a raw material, adopting ortho-ester for protecting a hydroxyl radical, then subjecting to condensation with N-carbobenzoxy-L-valine, and performing hydrolysis reaction to obtain N-carbobenzoxy valganciclovir; performing hydrogenation reduction reaction to obtain valganciclovir hydrochloride. The product purity reaches 99.0% or above and accords with United States Pharmacopeia standards.
Owner:湖北坦沐生物科技有限公司
Ophthalmic ointments comprising valganciclovir hydrochloride for treating infective eye diseases
InactiveUS20160095861A1Reduced systemic exposureImpairing their fertilityBiocideOintment deliverySide effectValganciclovir Hydrochloride
The present invention provides ophthalmic ointments for treating infective eye diseases containing valganciclovir hydrochloride as an active ingredient in the amount of from about 0.01% to about 10.0% w / w. These ophthalmic ointments are particularly effective for treating eye diseases caused by herpesviruses in the eye. Compared with oral administration, topical administration of valganciclovir hydrochloride is not accompanied by systemic side effects such as bone marrow suppression. Compared with topical administration of ganciclovir, topical administration of valganciclovir hydrochloride is much more effective in treating infective diseases in the eye and is not accompanied by toxicity to the surface of the eye.
Owner:SKIRON BIOSCI
A kind of synthetic method of valganciclovir hydrochloride
ActiveCN112661757BAvoiding the Separation Transformation ProblemAvoid yield lossOrganic chemistryGanciclovirumBiochemical engineering
The invention discloses a method for synthesizing valganciclovir hydrochloride. Using 1,3-dichloro-2-acetoxymethoxypropane as a starting material, monochloroganciclovir is prepared by esterification. , hydrolysis, deprotection into salt and finally obtain valganciclovir hydrochloride. The invention provides a method for synthesizing cganciclovir hydrochloride, which avoids the problem of separation and conversion of N-7 and N-9 isomers in the condensation process of diacetylguanine, and directly synthesizes monochlorovir without ganciclovir. Genanciclovir replaces the synthesis of monoacetyl ganciclovir in the prior art, and avoids the difficult problem of separation of diesters caused by residual ganciclovir. The method has short process steps, simple operation, easy purification and low cost. , conducive to industrial production, suitable for the synthesis of valganciclovir hydrochloride.
Owner:河北合佳医药科技集团股份有限公司
Valganciclovir composition
PendingCN110613718APromote proliferationWell mixedSenses disorderHydrocarbon active ingredientsValganciclovir HydrochlorideFiller Excipient
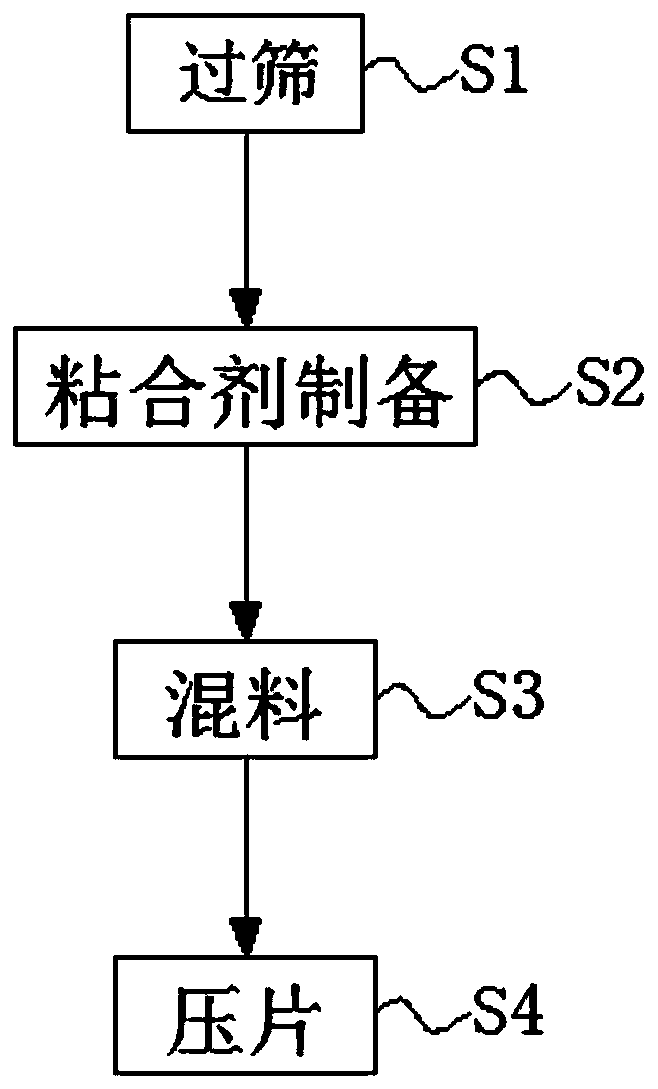
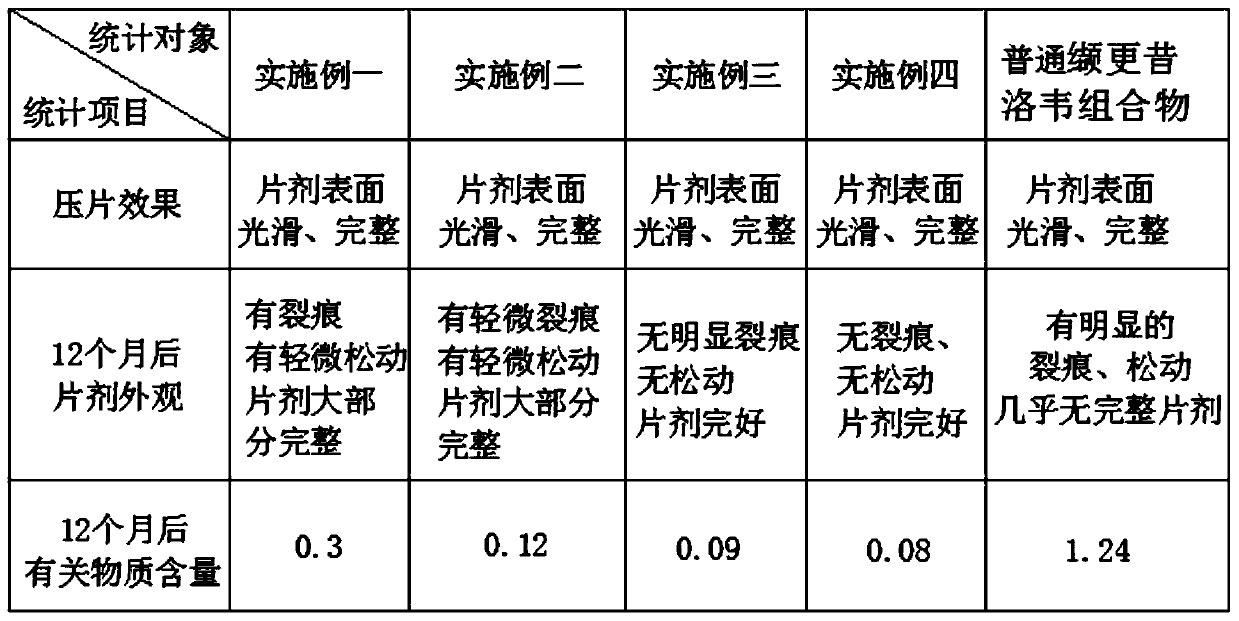
The invention discloses a valganciclovir composition which is prepared from the following raw materials in parts by weight:50 to 75 parts of valganciclovir hydrochlorides, 8 to 12 parts of sodium carboxymethyl starch, 5 to 8 parts of a filling agent, 3 to 6 parts of lactose, 15 to 25 parts of tomatin, 2 to 5 parts of a binding agent and 3 to 7 parts of a stabilizing agent. A preparation method ofthe valganciclovir composition comprises the following steps of Step 1, sieving, Step 2, bonding agent preparation, Step 3, material mixing, and Step 4, tableting. The valganciclovir composition relates to the technical field of pharmacy. A certain amount of tomatine is added into the valganciclovir composition; the tomatine can activate immune cells, can protect phagocytes from self oxidization injury, can promote the generation of some interleukin, can inhibit the generation of inflammatory mediators, and has the effect of improving the human body immunity; in addition, the tomatine is favorable for digestion; the absorption of the human body on taken medicine can be enhanced; and the utilization rate of active ingredients is improved. Through the addition of the bonding agent, the filling agent and the sodium carboxymethyl starch, the valganciclovir composition has a better tableting effect, cannot easily become loose, and is convenient to store.
Owner:湖北科益药业股份有限公司
A kind of valganciclovir hydrochloride oral solution and preparation method thereof
ActiveCN110934823BLittle degree of degradationUniform qualityDispersion deliveryAntiviralsValganciclovir HydrochlorideSolvent
The invention provides a valganciclovir hydrochloride oral solution and a preparation method thereof. The valganciclovir hydrochloride oral solution mainly consists of valganciclovir hydrochloride, a solubilizing system, a stabilizing system, a sweetener, and an acidity regulator. The valganciclovir hydrochloride, the complexing agent, the antioxidant, the thickening agent , the content of the sweetener, the acidity regulator, and the preservative in the solubilization system are respectively 7-12% (W / V), 0.08-0.12% (W / V), 0.12-0.12% 0.18% (W / V), 0.15‑0.25% (W / V), 0.06‑0.12% (W / V), 18‑22% (W / V), 0.20‑0.35% (W / V). Each crude drug in the valganciclovir hydrochloride oral solution of the present invention can be uniformly dispersed in the liquid solvent without being hydrolyzed, so that it has uniform and stable quality, and the addition of xanthan gum in the acid solubilizing system can effectively reduce the For the degree of degradation of valganciclovir hydrochloride, the degradation of valganciclovir hydrochloride was less than 1.0% and the RSD of content uniformity was less than 2% as measured by long-term retention of samples for 24 months.
Owner:HUBEI KANGYUAN PHARMA
Valganciclovir hydrochloride polymorph and pharmaceutical composition thereof
InactiveCN103012404BOrganic chemistryAntiviralsValganciclovir HydrochloridePharmaceutical Substances
The invention provides a polymorphic substance of valganciclovir hydrochloride, and in particular relates to a crystal form A and a crystal form B. The invention further provides a preparation method of the polymorphic substance of valganciclovir hydrochloride, and an application of a medical composition containing the crystal form A and the crystal form B of polymorphic substance of valganciclovir hydrochloride in treating cytomegalovirus infection.
Owner:NINGBO BESTDRUG PHARMA CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com