High performance liquid chromatography (HPLC) detection method for valganciclovir hydrochloride intermediate condensation product isomer
A technology of valganciclovir hydrochloride and detection method, applied in the field of drug analysis, can solve problems such as unfavorable enterprise product quality control, and achieve the effects of simple, reliable analysis and detection method and high sensitivity in research and development and production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
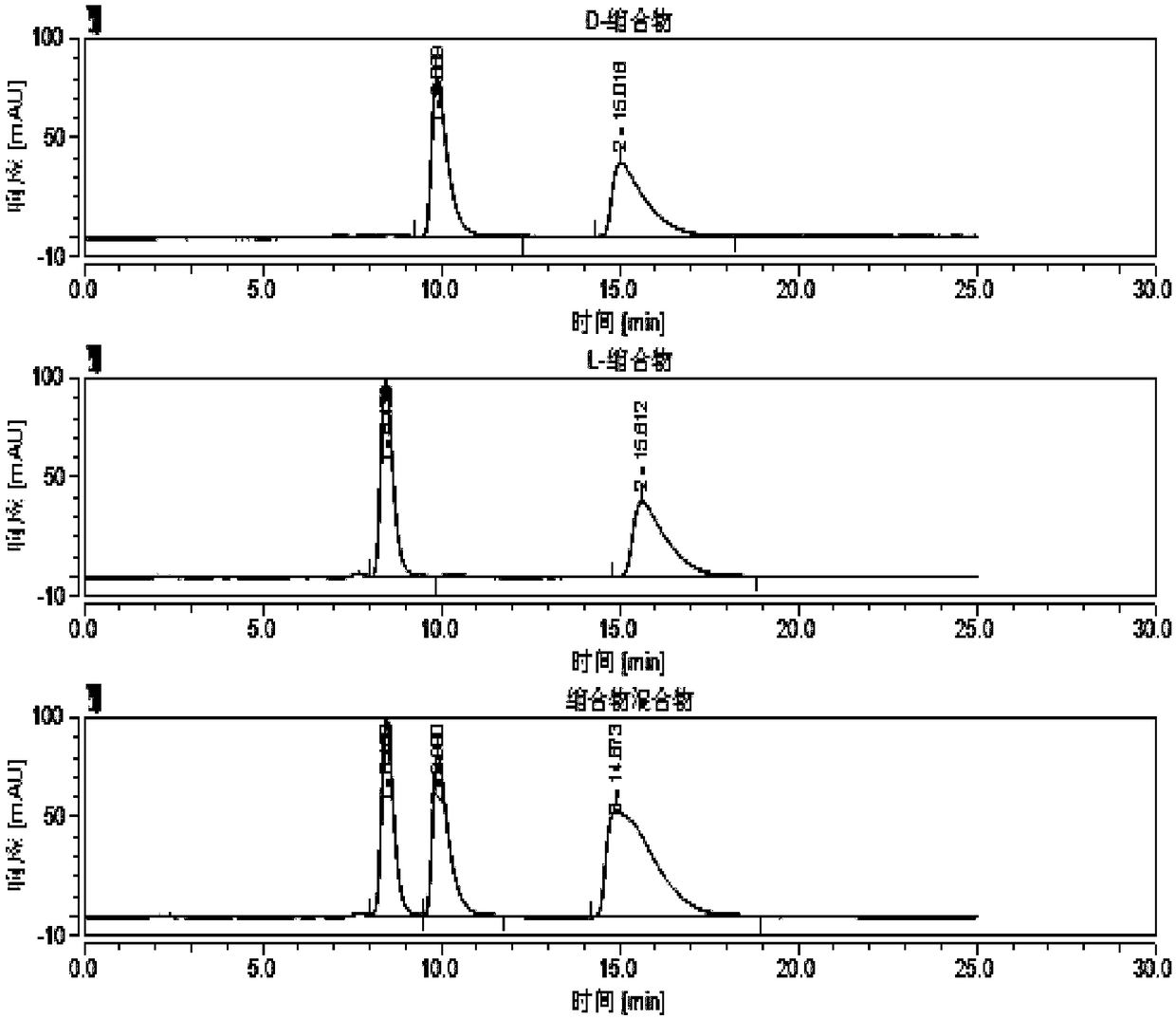
[0051] Example 1 Separation and analysis of different chiral columns Four chiral configurations of valganciclovir hydrochloride intermediate condensate, the steps are as follows:
[0052] (1) Stationary phase: cellulose-tris(3,5-dichlorophenylcarbamate) bonded silica gel;
[0053] Column: IC 4.6*250mm, 5um;
[0054] Chromatographic instrument: Agilent 1200 high performance liquid chromatograph;
[0055] Detector and wavelength: UV-254nm;
[0056] Mobile phase: n-hexane-absolute ethanol, (50:50, v / v);
[0057] Flow rate: 1.5ml / min;
[0058] Injection volume: 5ul;
[0059] Column temperature: 35°C;
[0060] Preparation of the test solution:
[0061] D-condensate reference substance solution: Accurately weigh about 25 mg of D-condensate reference substance, put it in a 25ml measuring bottle, add mobile phase to dissolve and dilute to the mark, and shake well.
[0062] L-condensate reference substance solution: Accurately weigh about 25 mg of L-condensate reference substanc...
Embodiment 2
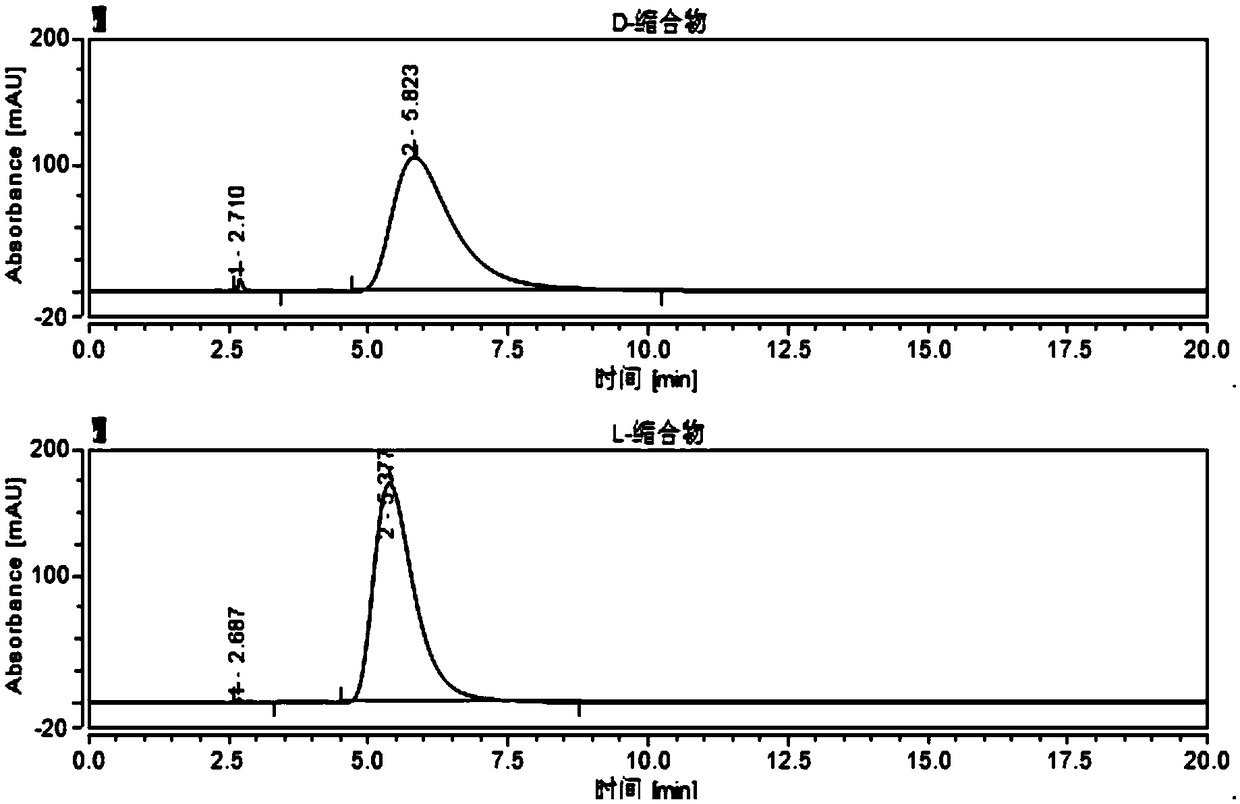
[0110] Example 2 Different mobile phases and ratio separation and analysis of four chiral configurations of valganciclovir hydrochloride intermediate condensate, the steps are as follows:
[0111] Chromatographic conditions:
[0112] (1) Mobile phase: water-acetonitrile (70:30, v / v);
[0113] (2) Mobile phase: water-acetonitrile (63:37, v / v);
[0114] (3) Mobile phase: water-acetonitrile (60:40, v / v);
[0115] Stationary phase: cellulose-tris(4-chloro-3-methylphenylcarbamate) coated silica gel;
[0116] Chromatographic instrument: Agilent 1260 high performance liquid chromatograph;
[0117] Column: OX-3R 4.6*150mm, 3um;
[0118] Detector and wavelength: UV-254nm;
[0119] Flow rate: 0.5ml / min;
[0120] Injection volume: 10ul;
[0121] Column temperature: 15°C;
[0122] Preparation of the test solution:
[0123] Diluent: water-acetonitrile (50:50, v / v).
[0124] Using experiment (four) in Example 1 to use cellulose-three (4-chloro-3-methylphenylcarbamate) coated sil...
Embodiment 3
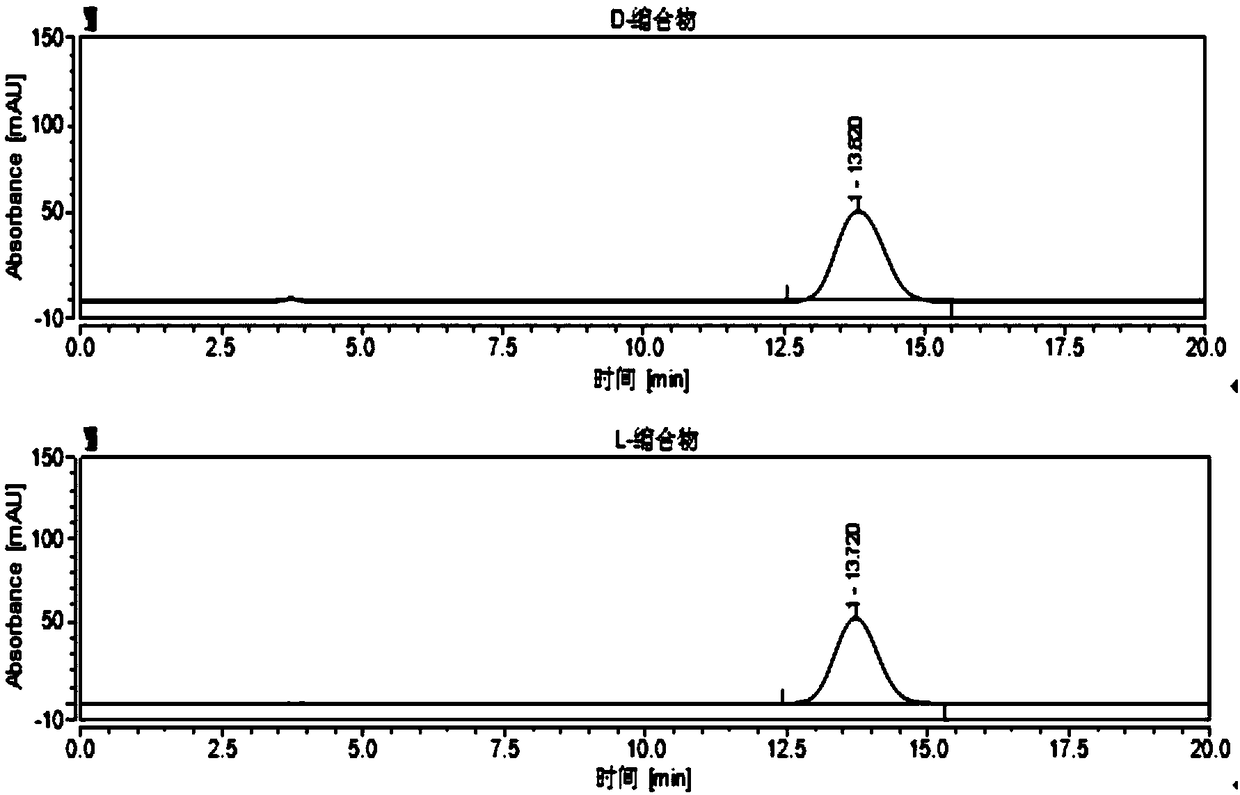
[0130] Example 3 Separate and analyze four chiral configurations of valganciclovir hydrochloride intermediate condensate at different column temperatures, the steps are as follows:
[0131] (1) Column temperature: 20°C;
[0132] (2) Column temperature: 10°C;
[0133] Stationary phase: cellulose-tris(4-chloro-3-methylphenylcarbamate) coated silica gel;
[0134] Chromatographic instrument: Agilent 1260 high performance liquid chromatograph;
[0135] Column: OX-3R 4.6*150mm, 3um;
[0136] Detector and wavelength: UV-254nm;
[0137] Mobile phase: water-acetonitrile (63:37, v / v);
[0138] Flow rate: 0.5ml / min;
[0139] Injection volume: 10ul;
[0140] Preparation of the test solution:
[0141] Diluent: water-acetonitrile (50:50, v / v).
[0142] Using experiment (four) in Example 1 to use cellulose-three (4-chloro-3-methylphenylcarbamate) to coat silica gel as the stationary phase, the L-condensate and D-condensate mixed control prepared The sample solution was used as the...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Wavelength | aaaaa | aaaaa |
| Wavelength | aaaaa | aaaaa |
| Wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com