Synthetic method of valganciclovir hydrochloride
The technology of a kind of valganciclovir hydrochloride and synthetic method is applied in the field of synthesis of valganciclovir hydrochloride, which can solve the problems of unfavorable industrial production, cumbersome separation process, and influence on process yield, and achieve mild conditions and simple process operation , The effect of simplifying the process steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
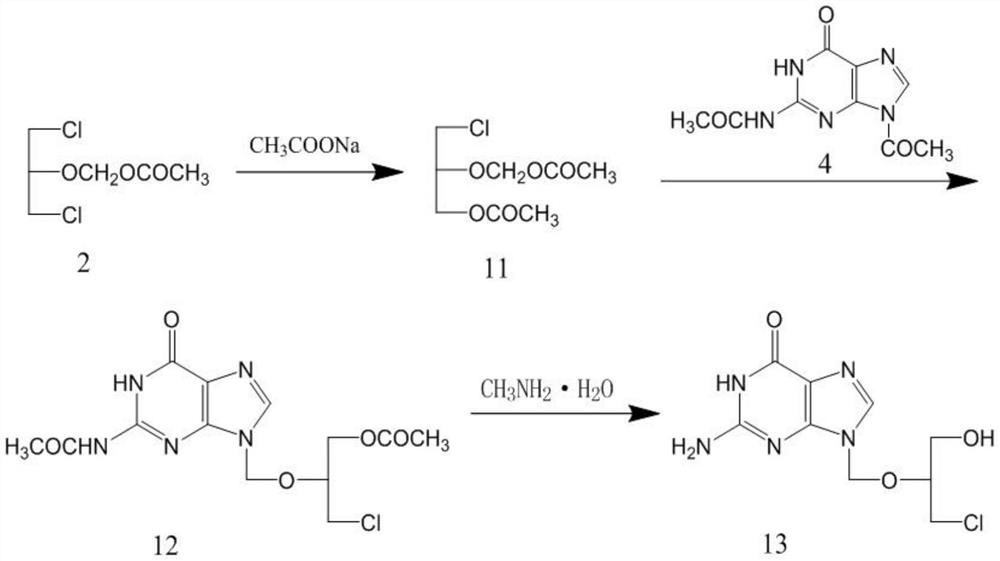
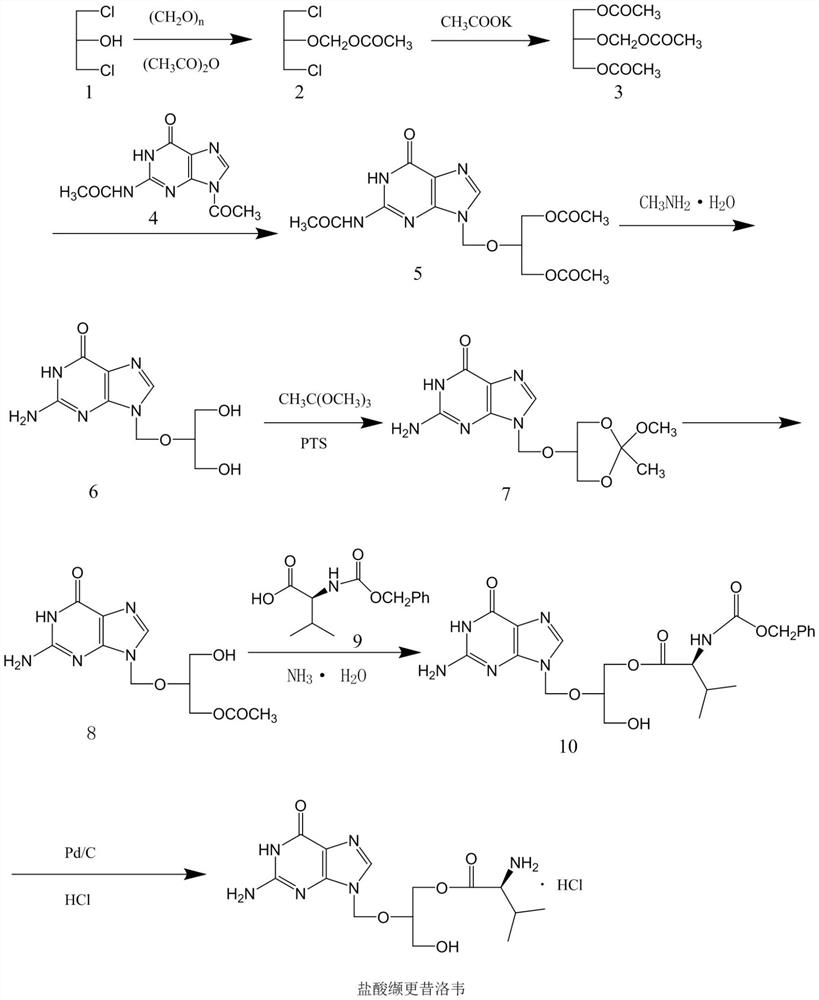
[0031] Embodiment 1, the preparation of compound 11
[0032] Add 120g of 1,3-dichloro-2-acetoxymethoxypropane, 300ml of N,N-dimethylformamide, 70g of anhydrous potassium acetate, 5g of benzyltriethylammonium chloride into the reaction flask, and control the temperature Stir and react at 20-30°C for 4 hours. After the reaction, distill off N,N-dimethylformamide under reduced pressure, add 100ml of dichloromethane and 100ml of purified water, stir and wash, separate the organic phase, and distill off the solvent to obtain a residue Compound 103g, namely compound 11.
Embodiment 2
[0033] Embodiment 2, the preparation of compound 11
[0034] Add 120g of 1,3-dichloro-2-acetoxymethoxypropane, 200ml of N,N-dimethylformamide, 54g of anhydrous sodium acetate, 4g of tetrabutylammonium bromide, and control the temperature for 30- Stir and react at 40°C for 4 hours. After the reaction, distill off N,N-dimethylformamide under reduced pressure, add 100ml of dichloromethane and 100ml of purified water, stir and wash, separate the organic phase, and distill off the solvent to obtain a residue of 108g , namely compound 11.
Embodiment 3
[0035] Embodiment 3, the preparation of compound 13
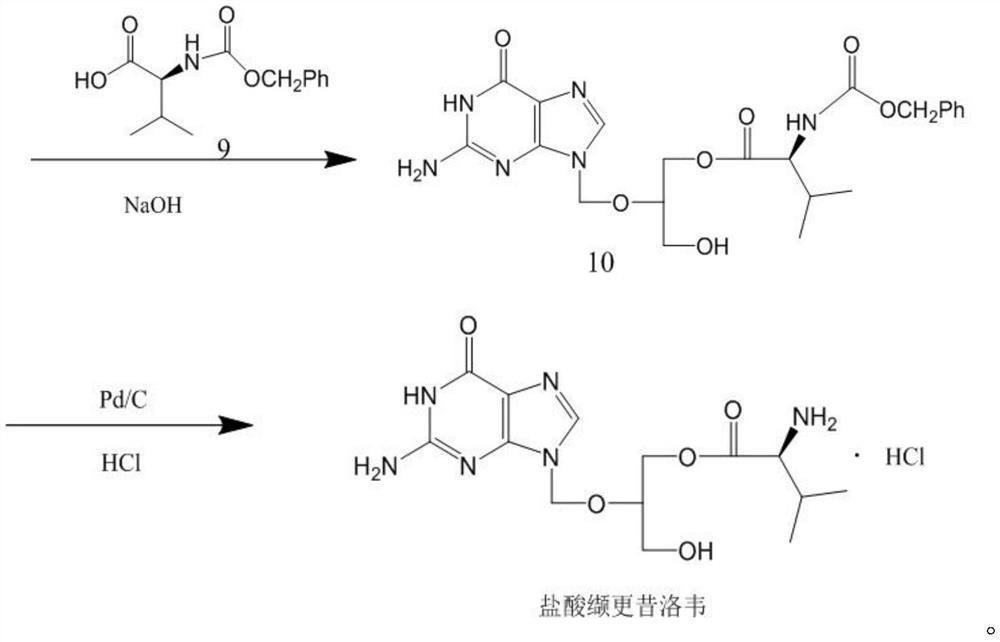
[0036] Add 75g of diacetylguanine, 4g of p-toluenesulfonic acid, 200ml of N,N-dimethylformamide into the reaction flask, raise the temperature to 115-120°C, slowly add 100g of the compound 11 prepared in Example 1, continue React at this temperature for 24 hours. After the reaction, cool the system down to 50°C, slowly add 100ml of 40% methylammonia aqueous solution, stir and react at 50-60°C for 4h, then use concentrated hydrochloric acid to adjust the pH value to 6-7, and cool down to 5- At 10°C, the solid was filtered out, and the filter cake was recrystallized with 1200 ml of purified water to obtain 56.5 g of compound 13.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com