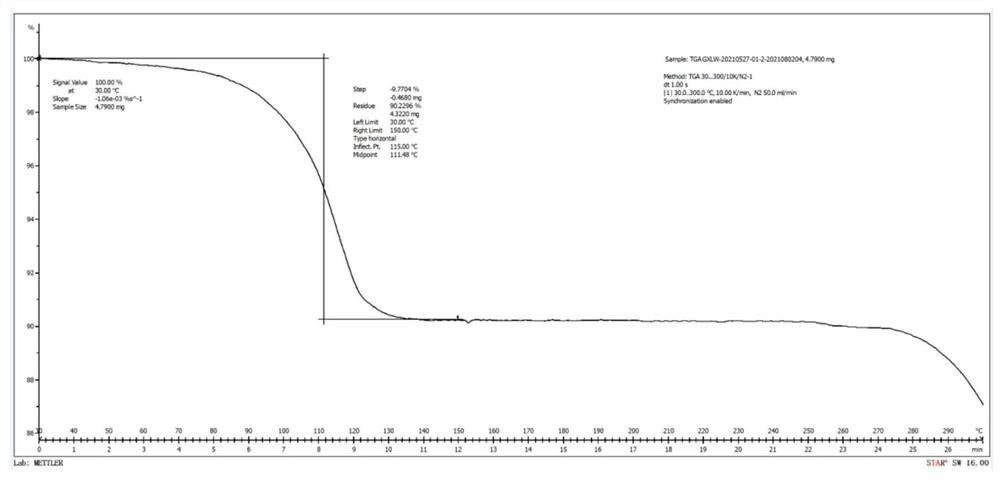
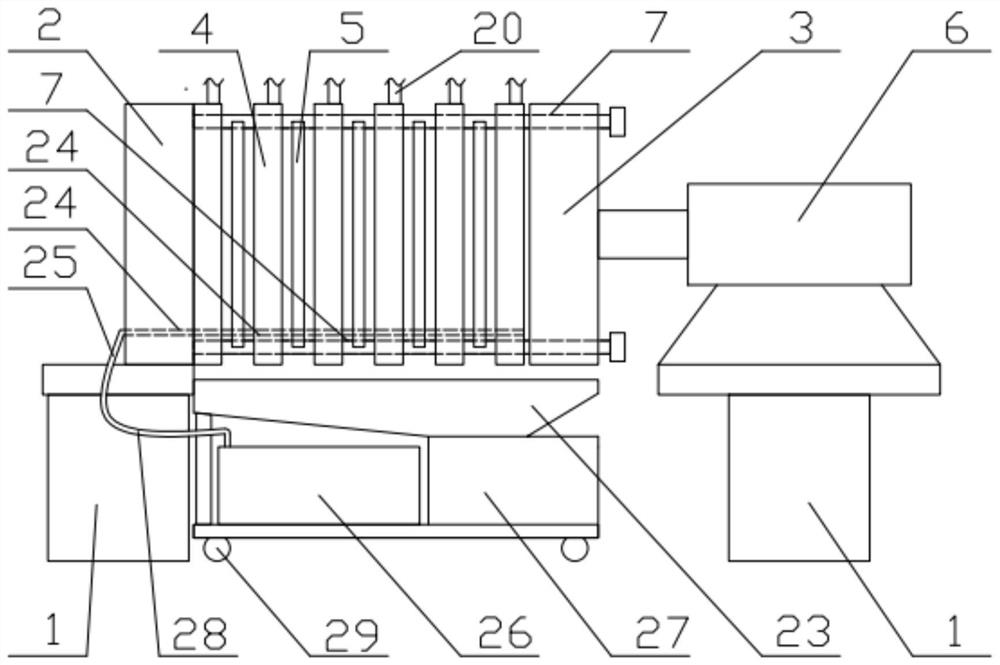
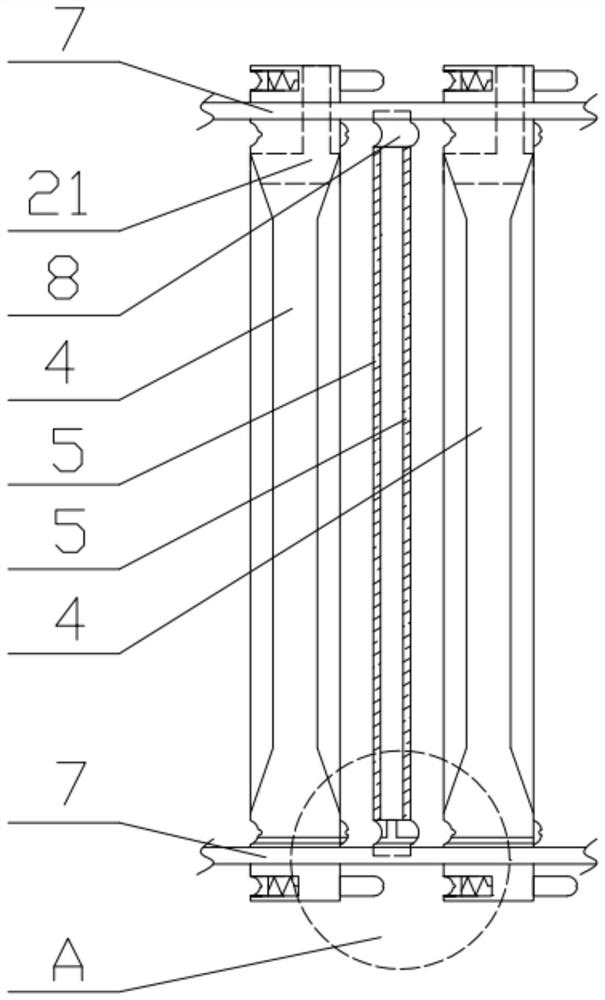
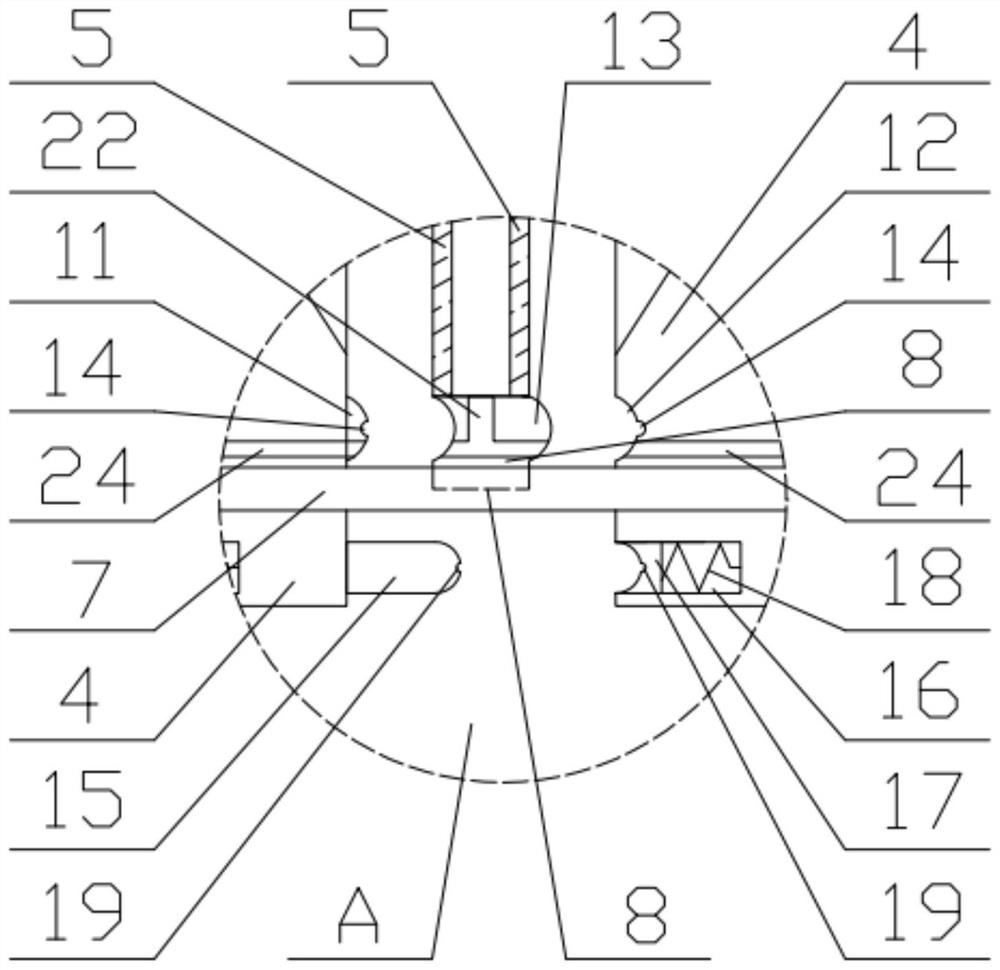
Patents
Literature
31 results about "Ganciclovirum" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
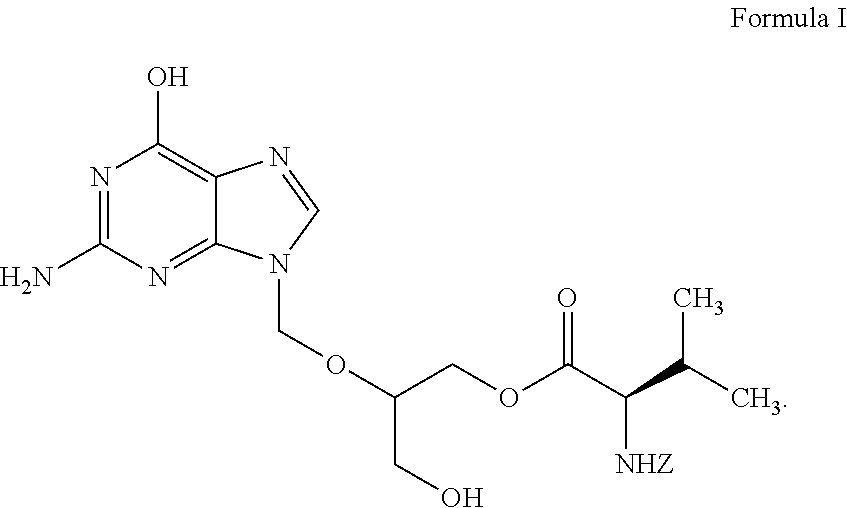
Stereochemically defined dipeptide esters of antiviral agents for enhanced ocular treatment
InactiveUS20090149482A1Enhanced enzymatic stabilitySufficient hydrophilicityBiocideSenses disorderDipeptideMedicine
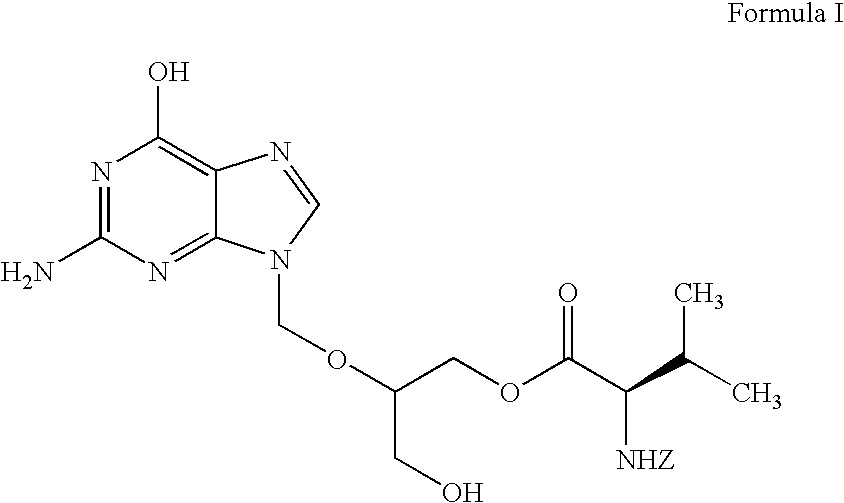
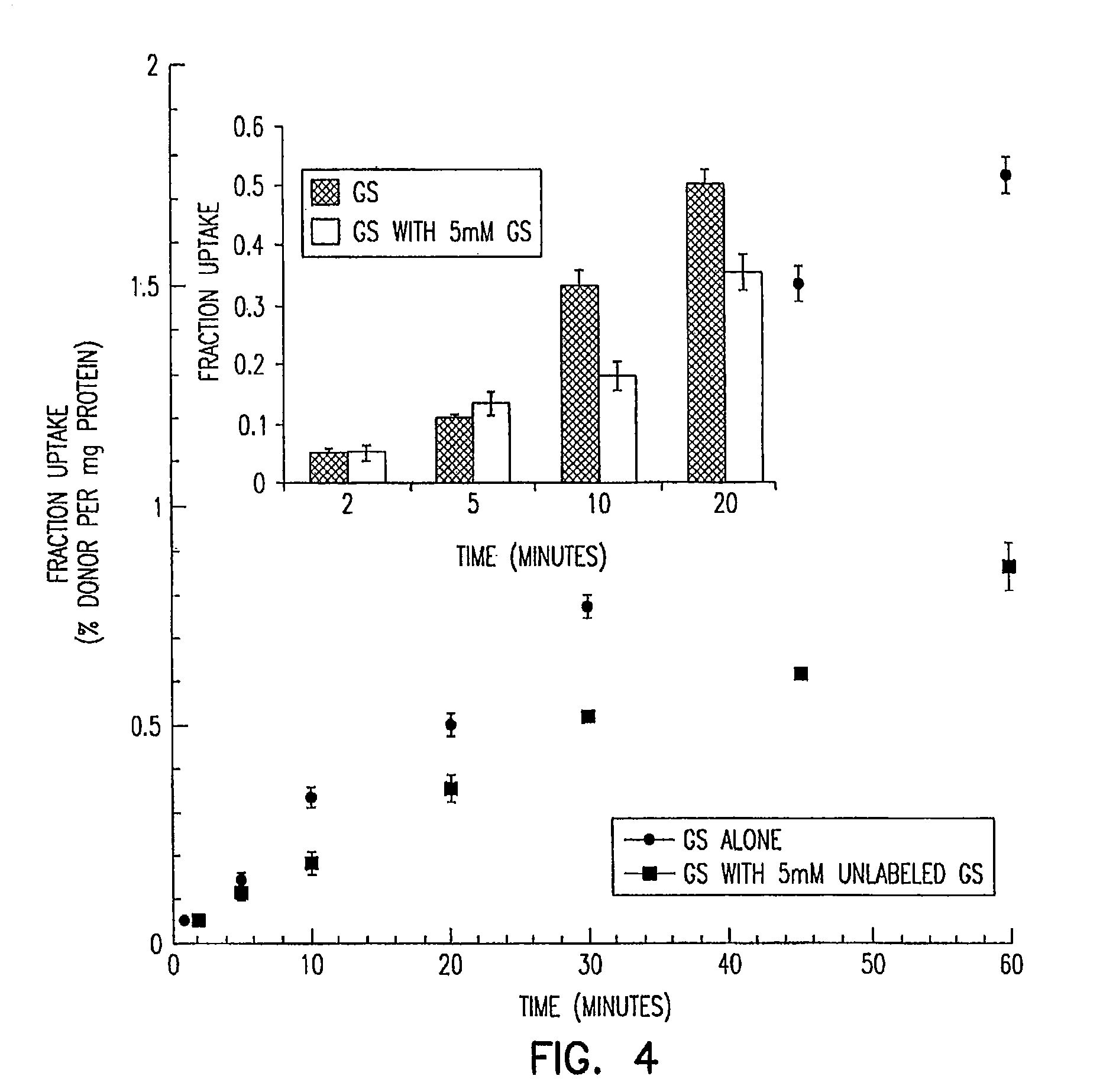
Stereochemically defined dipeptide esters of nucleoside-analogous antiviral agents including acyclovir and ganciclovir are provided. Certain of these stereochemically defined dipeptide esters are found to have unexpectedly enhanced delivery to and uptake by ocular tissues, crossing the blood-ocular barrier more effectively than other stereochemically defined dipeptide esters. For example, (L-Val)-(D-Val)-acyclovir was found to be taken up more effectively into corneal tissue than were underivatized acyclovir, monoesters (L-Val)-acyclovir or (D-Val)-acyclovir, or diester (L-Val)-(L-Val)-acyclovir.
Owner:UNIVERSITY OF MISSOURI
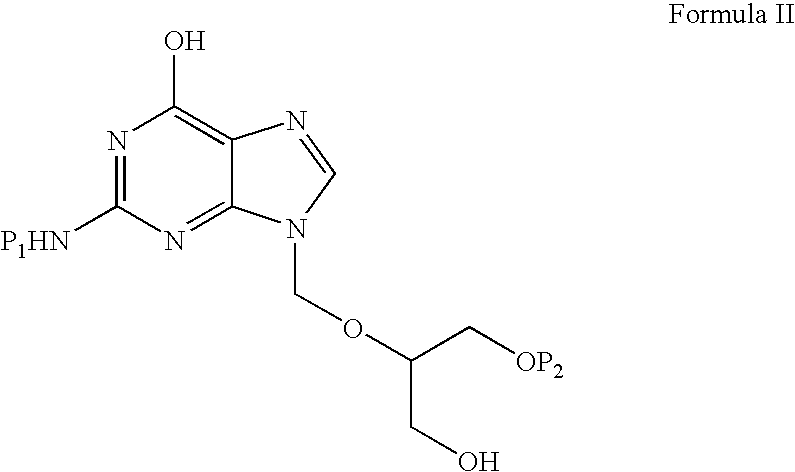
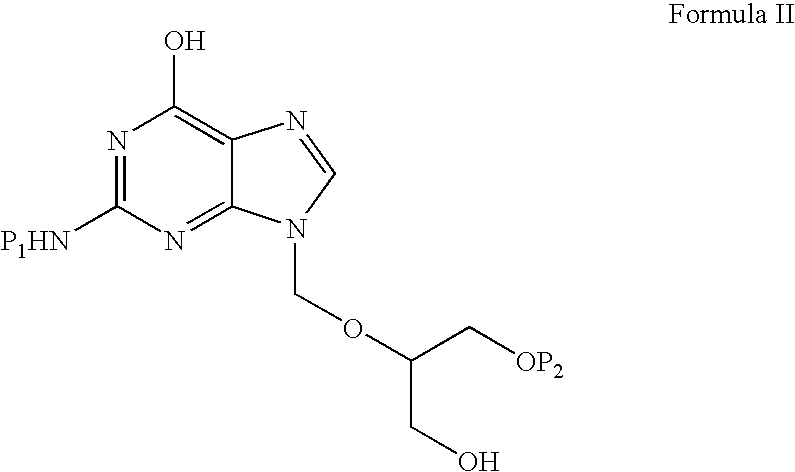
Preparation of ester of purine derivatives
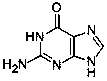
A process for the preparation of valganciclovir with triacetyl ganciclovir (V) as a starting material, comprising the following steps: selective hydrolysis, reacting with a coupling agent and a solvent, followed by hydrolysis under basic conditions and hydrogenolysis in the presence of a catalyst.
Owner:CIPLA LTD
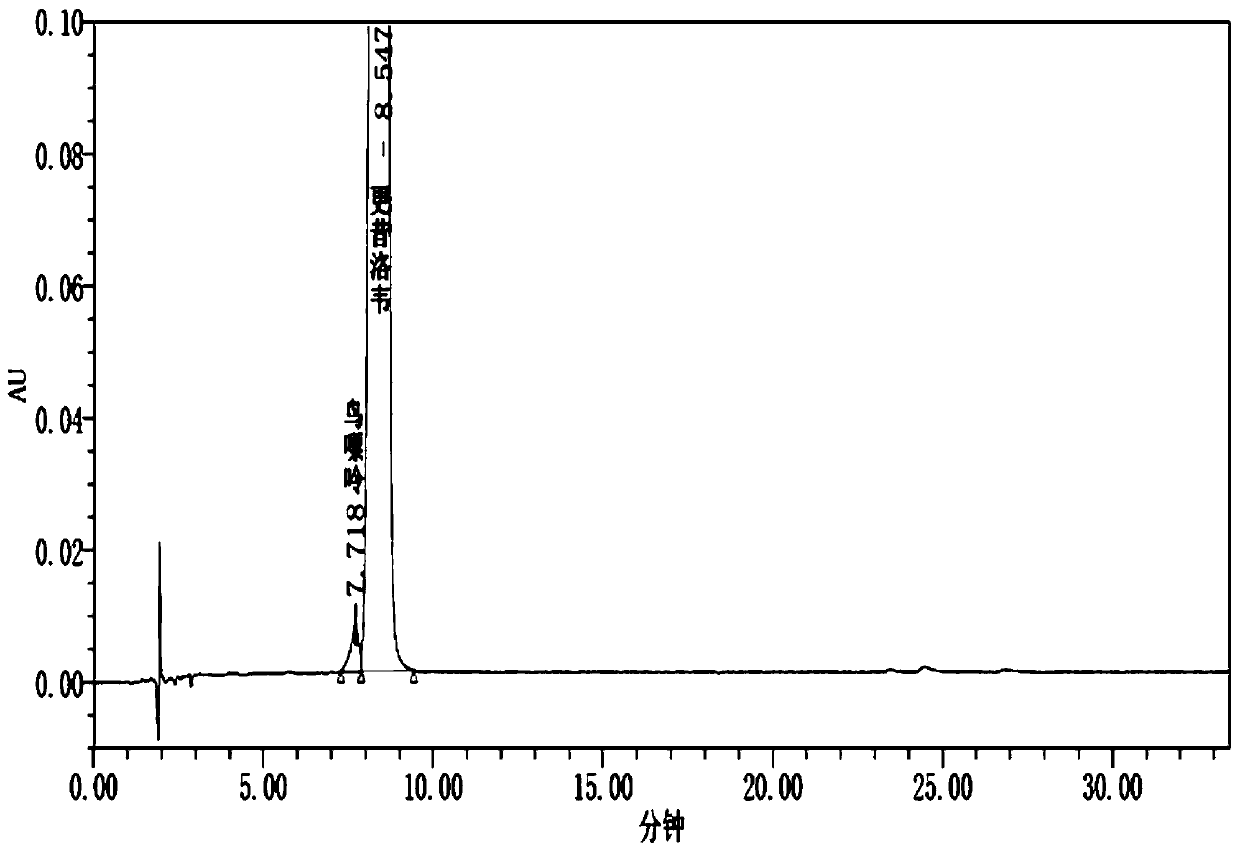
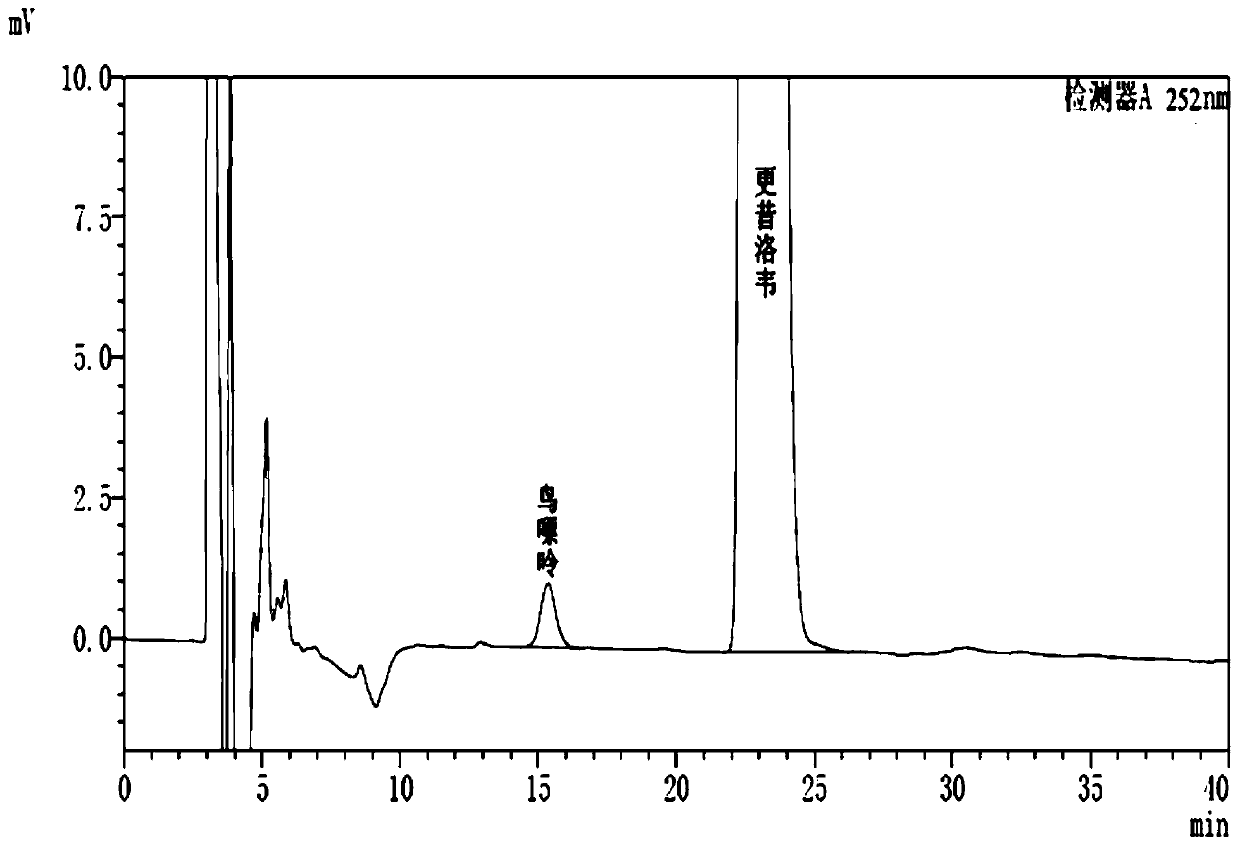
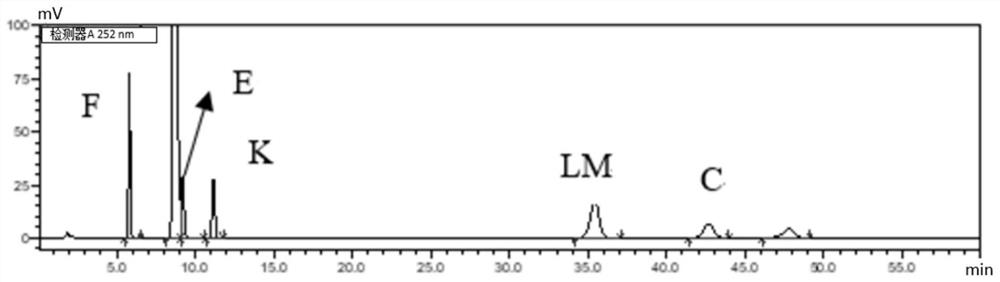
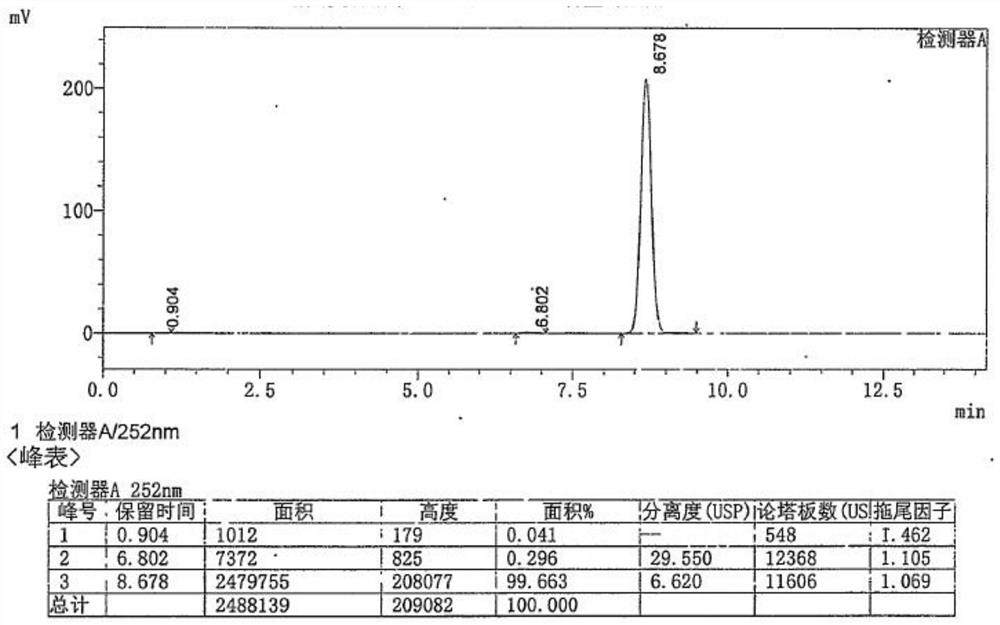
Method for determining content of guanine in ganciclovir
The invention discloses a method for determining content of guanine in ganciclovir, which belongs to the technical field of drug analysis and detection, and adopts a high-performance liquid chromatography as the detection method. The invention provides a normal phase detection method, which enables the guanine to have good peak shapes in a liquid chromatographic column and being able to be completely separated from ganciclovir for facilitating quantitative analysis. The method is characterized by comprising the steps of: taking n-hexane-isopropanol as a mobile phase; adopting cellulose-tris (4-methylphenyl formate) silica gel as a filler; taking the n-hexane- isopropanol (80:20) as the mobile phase, wherein the flow rate is 0.8-1.5 ml / min, the column temperature is 25-35 DEG C, the detection wavelength is 252 nm and a sample injection quantity is 10 mu L; and waiting for elution. The method of the invention is simple, reliable and high in sensitivity, has good resolution and reproducibility, is precise and reliable in result, and can be used for controlling the content of guanine in ganciclovir.
Owner:武汉长联来福制药股份有限公司
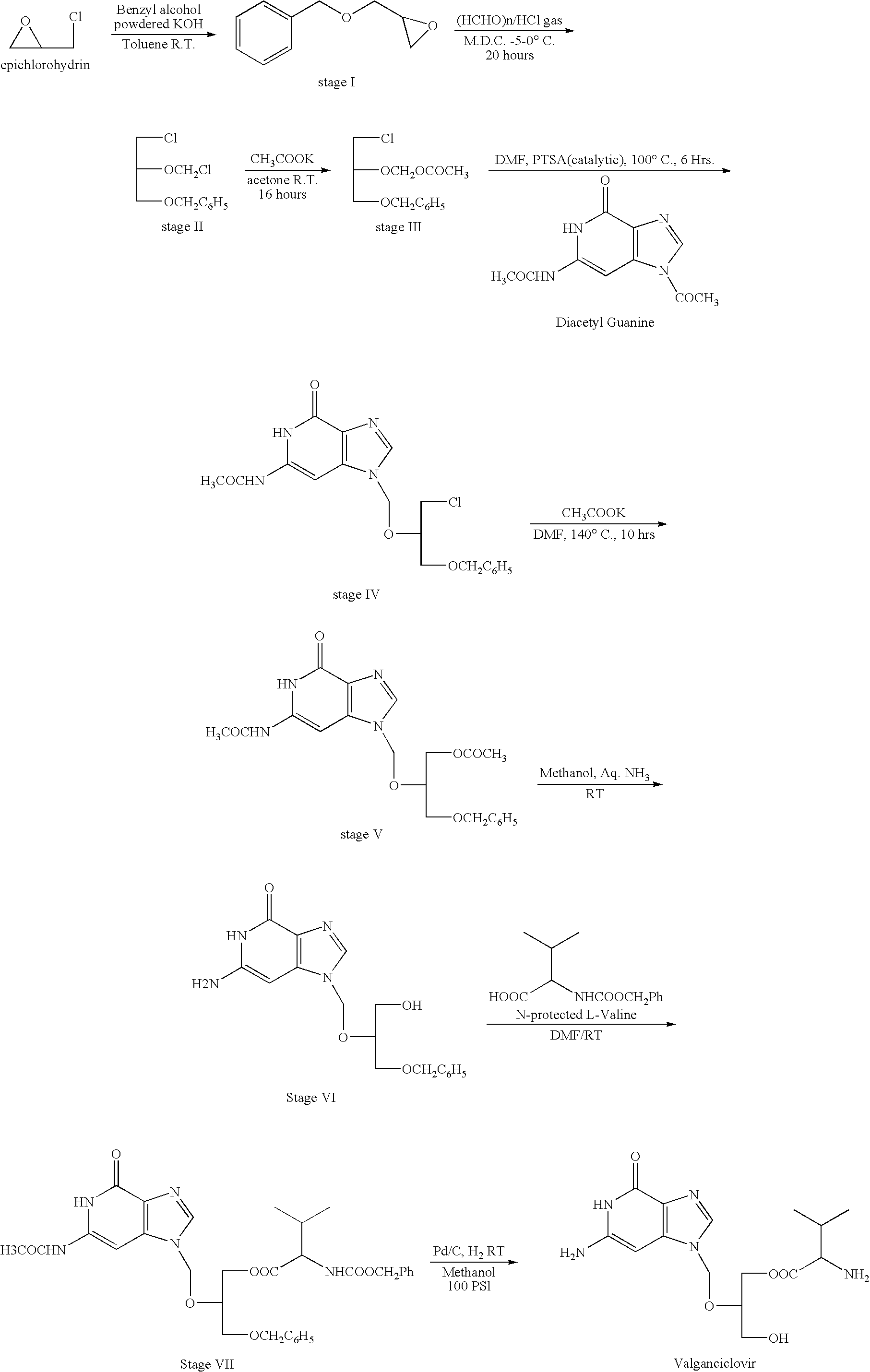
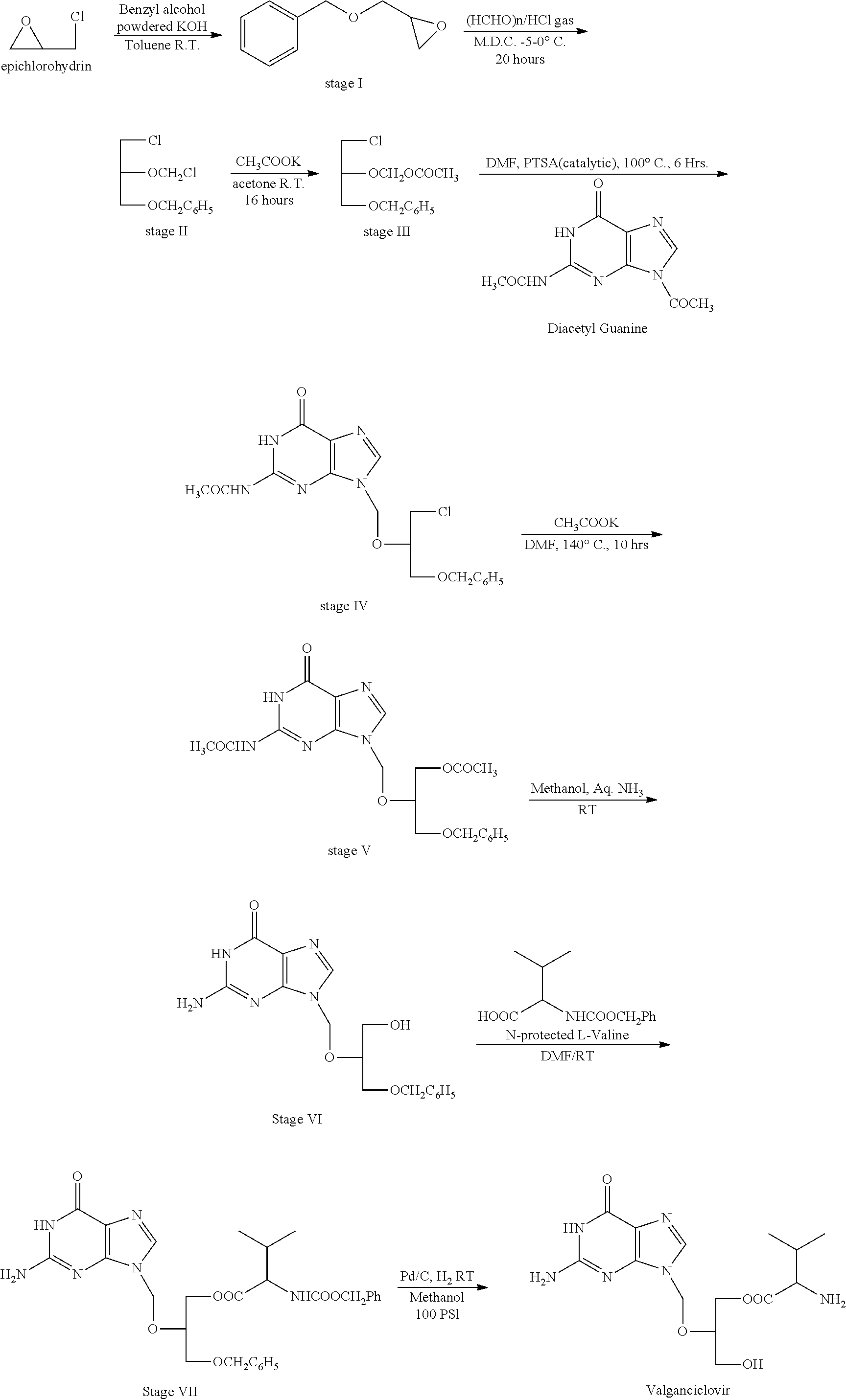
Novel valganciclovir hydrochloride synthesis method
ActiveCN107163050AStandards compliantEasy to operateOrganic chemistrySynthesis methodsValganciclovir Hydrochloride
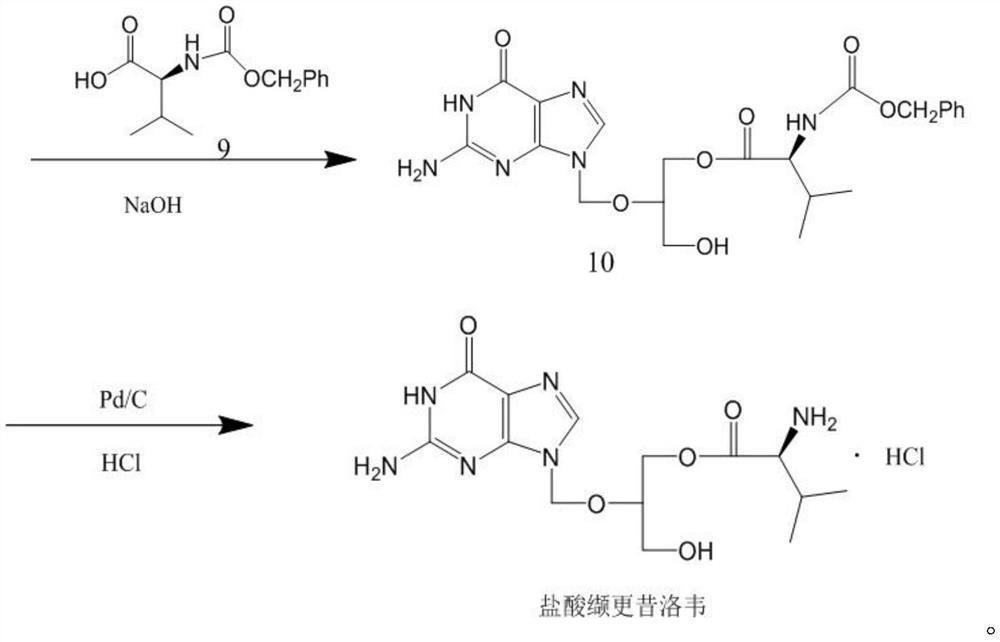
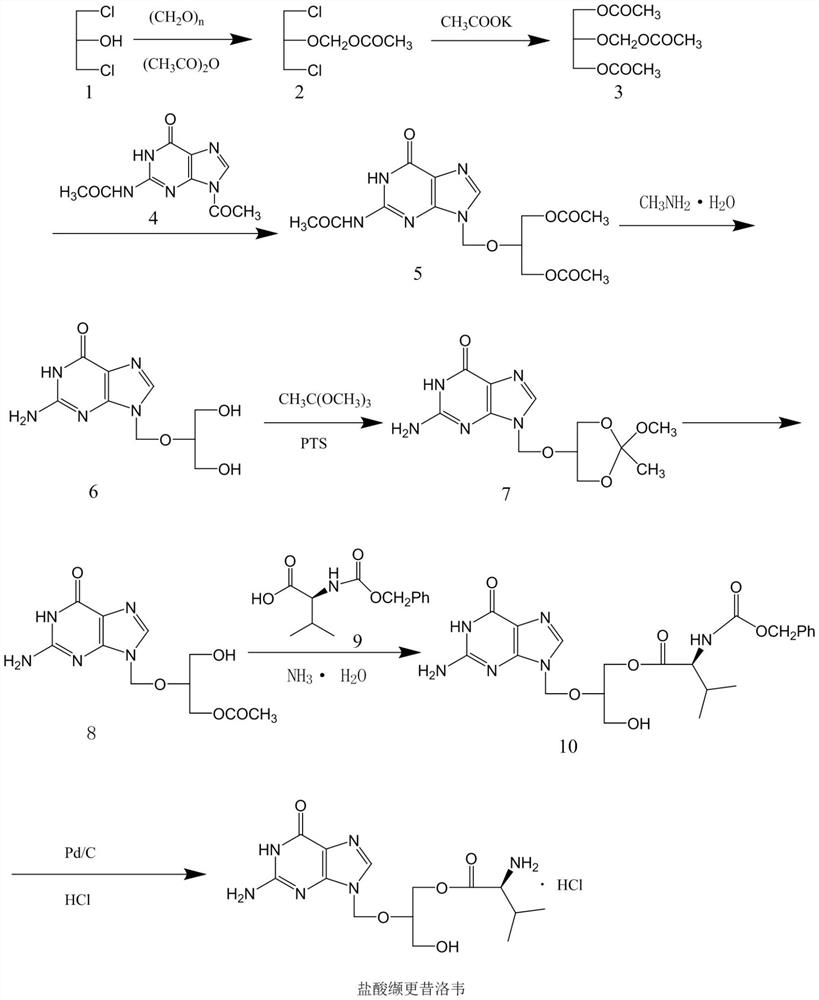
The invention discloses a novel valganciclovir hydrochloride synthesis method. The method includes: taking ganciclovir as a raw material, adopting ortho-ester for protecting a hydroxyl radical, then subjecting to condensation with N-carbobenzoxy-L-valine, and performing hydrolysis reaction to obtain N-carbobenzoxy valganciclovir; performing hydrogenation reduction reaction to obtain valganciclovir hydrochloride. The product purity reaches 99.0% or above and accords with United States Pharmacopeia standards.
Owner:湖北坦沐生物科技有限公司
Synthetic method of valganciclovir hydrochloride
ActiveCN112661757AAvoiding the Separation Transformation ProblemAvoid yield lossOrganic chemistryCondensation processGanciclovirum
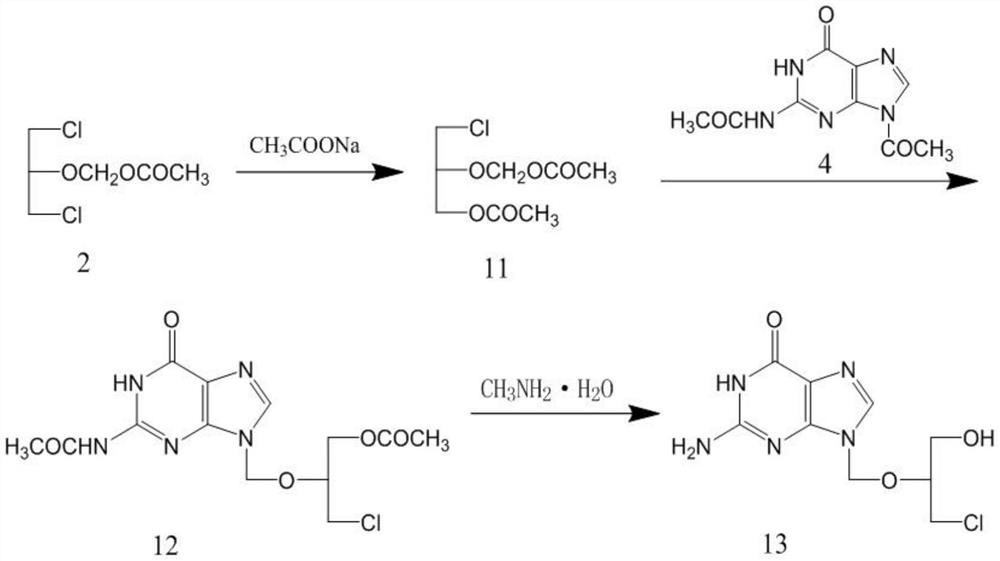
The invention discloses a method for synthesizing valganciclovir hydrochloride, which comprises the following steps: with 1,3-dichloro-2-acetoxymethoxypropane as an initial raw material, preparing monochlorinated ganciclovir, and carrying out esterification, hydrolysis and deprotection salification to finally obtain the valganciclovir hydrochloride. The invention provides a synthesis method of siganciclovir hydrochloride, which avoids the problem of separation and conversion of N-7 and N-9 isomers in the diacetyl guanine condensation process. The method directly synthesizes monochlorinated ganciclovir without using ganciclovir, instead of the synthesis of monoacetyl ganciclovir in the prior art, so that the method avoids the problem of diester compound separation caused by ganciclovir residues, has the advantages of short process steps, simple operation, convenient purification and low cost, is beneficial to industrial production, and is suitable for synthesis of valganciclovir hydrochloride.
Owner:河北合佳医药科技集团股份有限公司
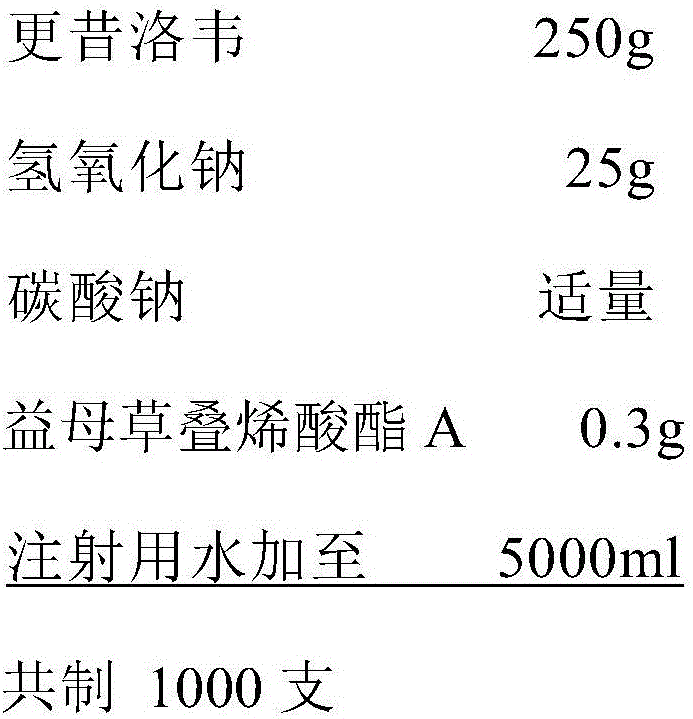
Ganciclovir injection and preparation process thereof
ActiveCN106176595AGood compatibilityPharmaceutical containersPharmaceutical delivery mechanismForeign matterGanciclovir
The invention belongs to the technical field of medicine preparation, and particularly relates to a ganciclovir injection and a preparation process thereof. The ganciclovir injection comprises ganciclovir and a pH modifier. According to the ganciclovir injection, water for injection is adopted as a solvent, and a plastic ampoule is adopted as an inner packaging container in direct contact with the ganciclovir injection. The mechanism that the ganciclovir injection is prone to generate muddy sediment is deeply analyzed and studied, and the essence reason for easily generated unqualified visible foreign matter and increase of insoluble particles is found. Compared with the prior art, when the ganciclovir injection is prepared, a plastic container packaging material is selected, medicine liquid and the packaging material both have good compatibility, and therefore the visible foreign matter and the insoluble particles are remarkably reduced.
Owner:CISEN PHARMA
A kind of preparation method of ganciclovir
ActiveCN110938075BImprove product qualityRaw materials are easy to obtainOrganic chemistryGanciclovirumTrifluoroacetic acid
Owner:武汉华龙生物制药有限公司
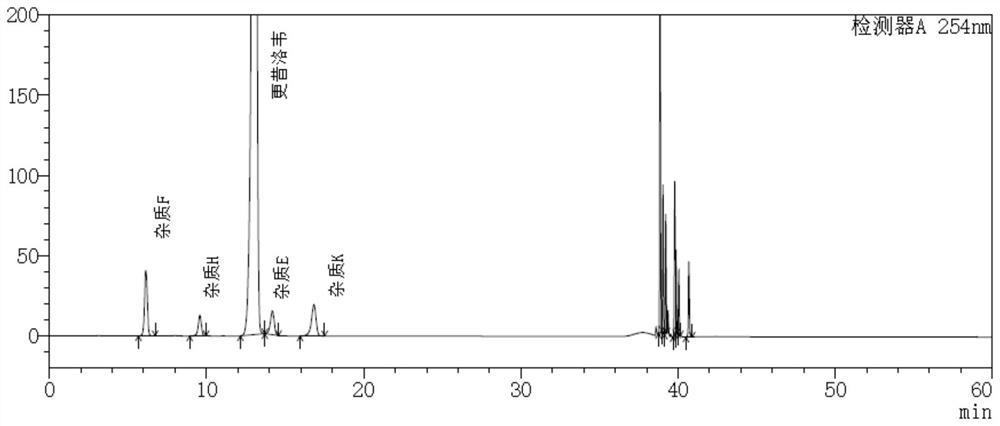
Method for detecting impurity K in ganciclovir and method for separating impurity
PendingCN113933413AComplete elutionGuaranteed separation effectComponent separationO-Phosphoric AcidHplc mass spectrometry
The invention discloses a method for detecting an impurity K in ganciclovir and a method for separating the impurity. The method comprises the following steps: detecting a test solution containing ganciclovir by adopting high performance liquid chromatography, wherein a chromatographic column adopted by the high performance liquid chromatography is a cation exchange chromatographic column, mobile phases comprise a mobile phase A and a mobile phase B, and the mobile phase A is a phosphoric acid aqueous solution containing 0.012 M ammonium dihydrogen phosphate, in the phosphoric acid aqueous solution, the volume percent of phosphoric acid is 0.12%, the mobile phase B is acetonitrile. In combination with a specific elution mode, the method not only can realize effective separation and detection of the impurity K, but also can completely elute other known impurities, and is beneficial to the service life of a chromatographic column.
Owner:武汉九州钰民医药科技有限公司
Preparation of ester of purine derivatives
A process for the preparation of valganciclovir with triacetyl ganciclovir (V) as a starting material, comprising the following steps: selective hydrolysis, reacting with a coupling agent and CBZ valine and a solvent, followed by hydrolysis under basic conditions and hydrogenolysis in the presence of a catalyst.
Owner:CIPLA LTD
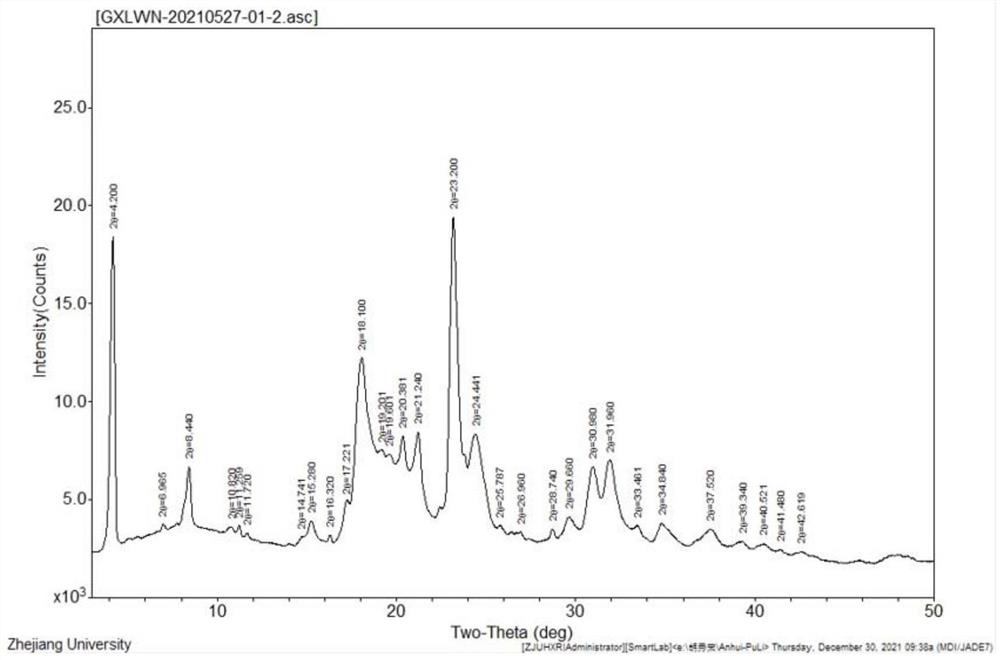
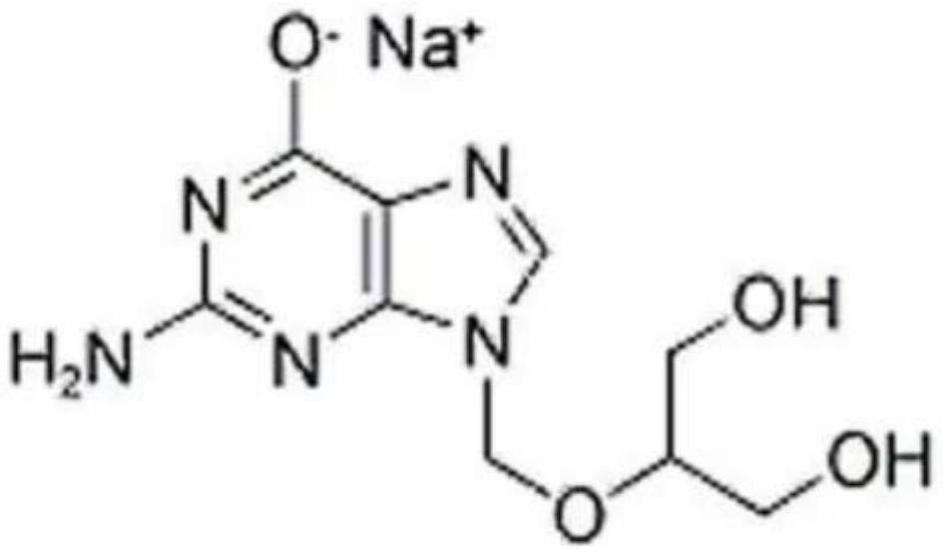
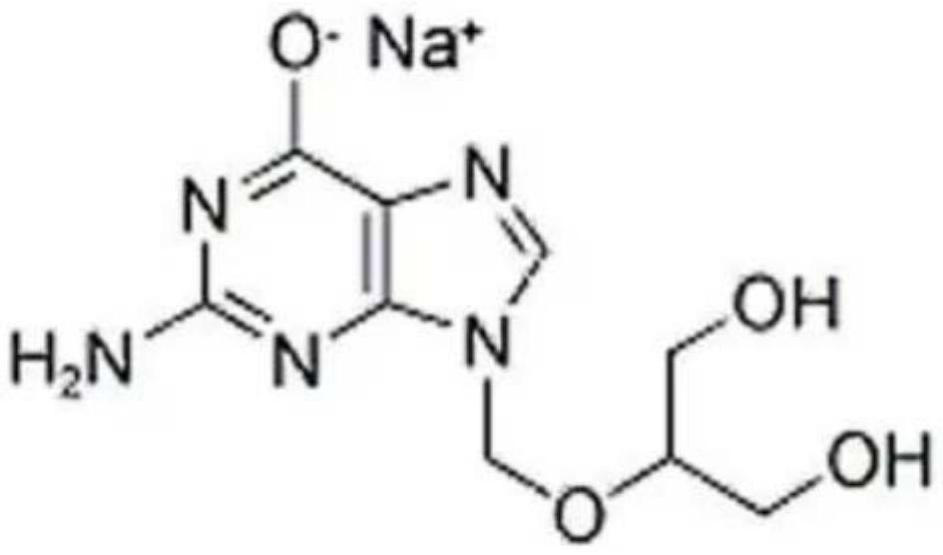
Ganciclovir sodium monohydrate and preparation method thereof
PendingCN114380831AEasy to operateMild reaction conditionsOrganic chemistry methodsHydration reactionGanciclovirum
The invention provides ganciclovir sodium monohydrate, which is radiated by Cu-K alpha, and an X-ray powder diffraction pattern of the ganciclovir sodium monohydrate comprises the following diffraction peaks expressed by angle 2 theta: 4.2 degrees + / -0.1 degrees, 18.1 degrees + / -0.1 degrees, 23.2 degrees + / -0.1 degrees, 8.44 degrees + / -0.1 degrees, 15.28 degrees + / -0.1 degrees, 20.381 degrees + / -0.1 degrees, 21.24 degrees + / -0.1 degrees, 24.441 degrees + / -0.1 degrees, 30.98 degrees + / -0.1 degrees and 31.96 degrees + / -0.1 degrees. The X-ray powder diffraction pattern of the ganciclovir sodium monohydrate is basically as shown in Figure 1. According to the preparation method provided by the invention, water and ganciclovir react, 95% ethanol is added for crystallization after the reaction is finished, and the monohydrate crystal form of ganciclovir sodium is prepared; operation is simple, and reaction conditions are mild and easy to control.
Owner:ANHUI POLY PHARM CO LTD
A kind of preparation method of ganciclovir powder injection and filter press used therefor
ActiveCN110051635BLow impurity contentImprove efficacyPowder deliveryAntiviralsGanciclovirumFreeze-drying
The invention discloses a preparation method of ganciclovir powder injection and a filter press used therefor, belonging to the technical field of pharmaceutical production. The sodium chloride aqueous solution is mixed evenly, and finally ganciclovir powder injection is obtained by freeze-drying; the filter press includes a bracket, a thrust plate, a pressure plate, a filter plate, a filter cloth and a driving part, and the filter cloth and the filter plate are separated and installed in the On the frame body, the material of the frame body is polypropylene, and the upper and lower edges of the frame body are provided with deep clamping grooves and shallow clamping grooves corresponding to the upper and lower guide rods, which can be easily installed and disassembled on the upper and lower guide rods. The filter plates are respectively provided with the first sealing components, and the opposite surfaces of the two adjacent filter plates are respectively provided with the second sealing components. The impurity content of the ganciclovir powder injection prepared by the method provided by the present invention is relatively small, and the effect of the ganciclovir powder injection can be improved; Clean and replace.
Owner:南京瑄宇医药科技有限公司
Use of ganciclovir in preparation of drugs for treating or preventing diabetic-induced stroke
Owner:BINZHOU MEDICAL COLLEGE
Human cytomegalovirus containing foreign antigen
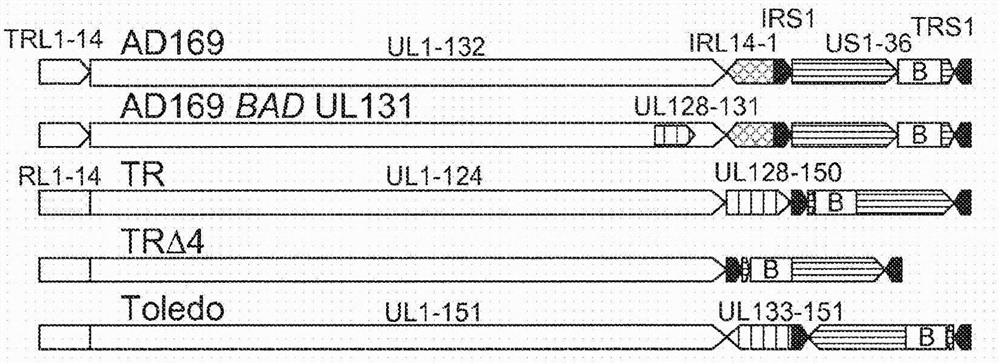
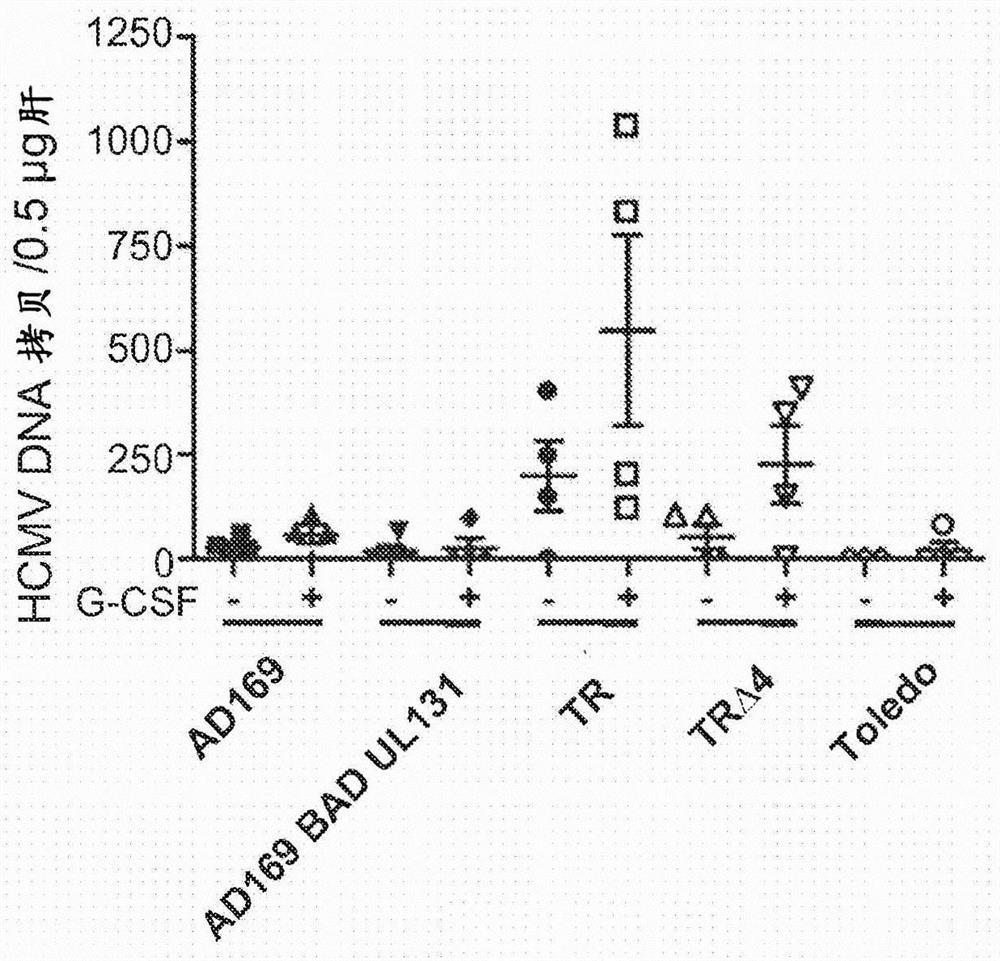
Human cytomegalovirus vectors comprising heterologous antigens are disclosed. The vector derived from the TR strain is ganciclovir-sensitive, contains active US2, US3, US6, US7, and UL131A genes, and has a deleterious or inactivating mutation in the UL82 gene that prevents expression of pp71.
Owner:OREGON HEALTH & SCI UNIV
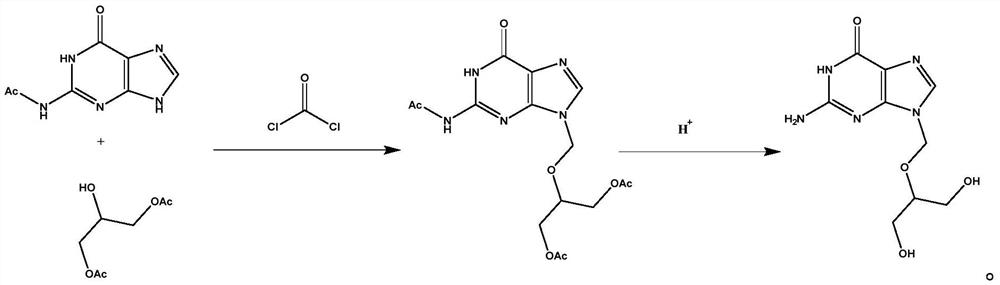
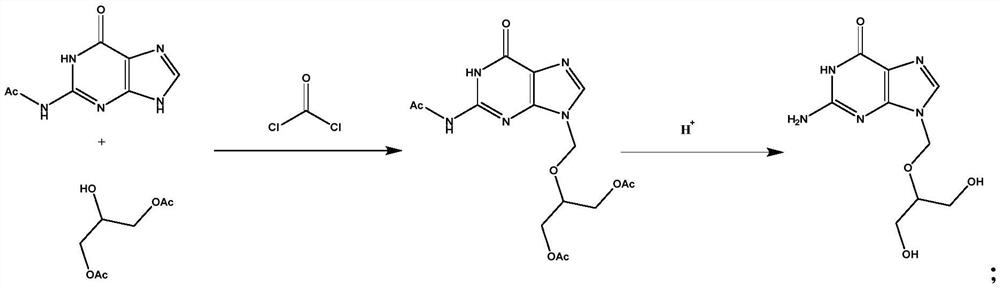
Preparation method and application of ganciclovir
ActiveCN113149988AIncrease profitAvoid instabilityOrganic chemistryPharmaceutical delivery mechanismPurineEngineering
The invention belongs to the field of medicine synthesis, and discloses a preparation method and application of ganciclovir. According to the preparation method, N-(6-carbonyl-6, 9-dihydro-1H-purine-2-yl) acetamide and 2-hydroxy-1, 3-propylene diacetate are subjected to condensation with chloroformyl chloride at the same time, an obtained intermediate is subjected to hydrolysis, and ganciclovir is obtained. The preparation method of ganciclovir can effectively improve the utilization rate of raw materials, reduce the use cost of the raw materials in the production process, shorten the process flow, and reduce the occurrence of side reactions and the use amount of solvents. The preparation method is suitable for preparing ganciclovir, and the prepared ganciclovir is used for preparing ganciclovir for injection.
Owner:HAINAN JINRUI PHARMA
Method for detecting ganciclovir and related substances in ganciclovir ophthalmic gel
The invention belongs to the field of medicines, and relates to a method for detecting a main component (ganciclovir) and / or related substances in ganciclovir ophthalmic gel. Specifically, the method comprises a step of removing carbomer in ganciclovir ophthalmic gel and a step of performing HPLC (High Performance Liquid Chromatography) analysis, and the detection method disclosed by the invention is high in specificity and stable in retention time of a chromatographic peak; according to the method for removing the carbomer from the sample, ganciclovir is good in solubility, the solution is stable, the carbomer can be effectively removed, a chromatographic column is not damaged, and the ganciclovir has a good application prospect.
Owner:SHENYANG XINGQI PHARM CO LTD
Stereochemically defined dipeptide esters of antiviral agents for enhanced ocular treatment
InactiveUS7951774B2Sufficient hydrophilicityEffectively transported into ocular tissueBiocideSenses disorderGanciclovirumDipeptide
Stereochemically defined dipeptide esters of nucleoside-analogous antiviral agents including acyclovir and ganciclovir are provided. Certain of these stereochemically defined dipeptide esters are found to have unexpectedly enhanced delivery to and uptake by ocular tissues, crossing the blood-ocular barrier more effectively than other stereochemically defined dipeptide esters. For example, (L-Val)-(D-Val)-acyclovir was found to be taken up more effectively into corneal tissue than were underivatized acyclovir, monoesters (L-Val)-acyclovir or (D-Val)-acyclovir, or diester (L-Val)-(L-Val)-acyclovir.
Owner:UNIVERSITY OF MISSOURI
A kind of preparation method of ganciclovir sodium freeze-dried powder for injection
ActiveCN113476413BGuaranteed purityGood lookingPowder deliveryPharmaceutical non-active ingredientsCelluloseGanciclovirum
Owner:HAINAN HAILING CHEMIPHARMA CORP
Gancilorvir dispersable tablet and its preparation
InactiveCN1287797CShort disintegration timeGood dispersionAntiviralsPill deliveryPyrrolidinonesMagnesium stearate
The invention provides a Gancilorvir dispersable tablet and its preparation, wherein the dispersion tablet comprises 62.5 wt% of Ganciclovir, 10-25% of crystalline cellulose, or calcium hydrogen phosphate and / or starch, 1-10% of sodium carboxymethylstarch, or low substituted methylcellulose propylene glycol ether and / or cross bonding polyvinylpyrrolidone or crosslinked sodium carboxymethylcellulose, 0.1-1.4% of polyvinylpyrrolidone, or methyl hydroxypropylcellulose, 5-15% of lactose, 0.5-1.4% of magnesium stearate. The preparing process comprises sieving, mixing, granulating, drying, and tabletting.
Owner:HUAZHONG NORMAL UNIV
Ganciclovir composition for injection and freeze drying process of ganciclovir composition
InactiveCN111973564AEnsure safetySimple prescriptionPowder deliveryDrying solid materials without heatDrug utilisationGanciclovirum
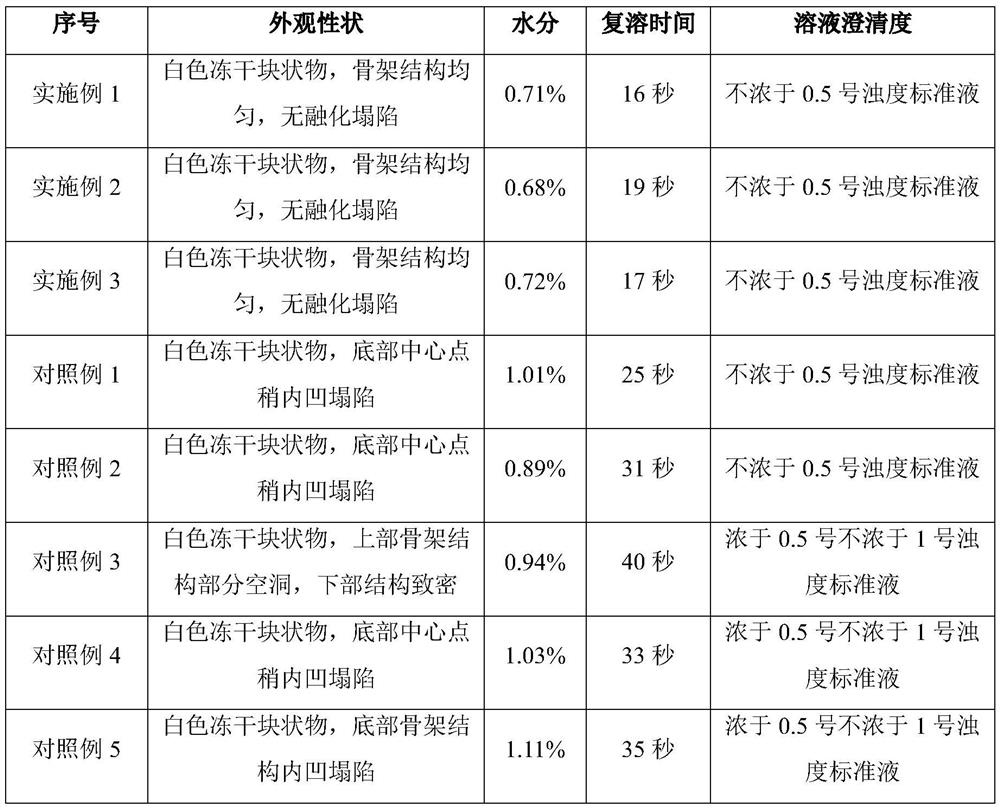
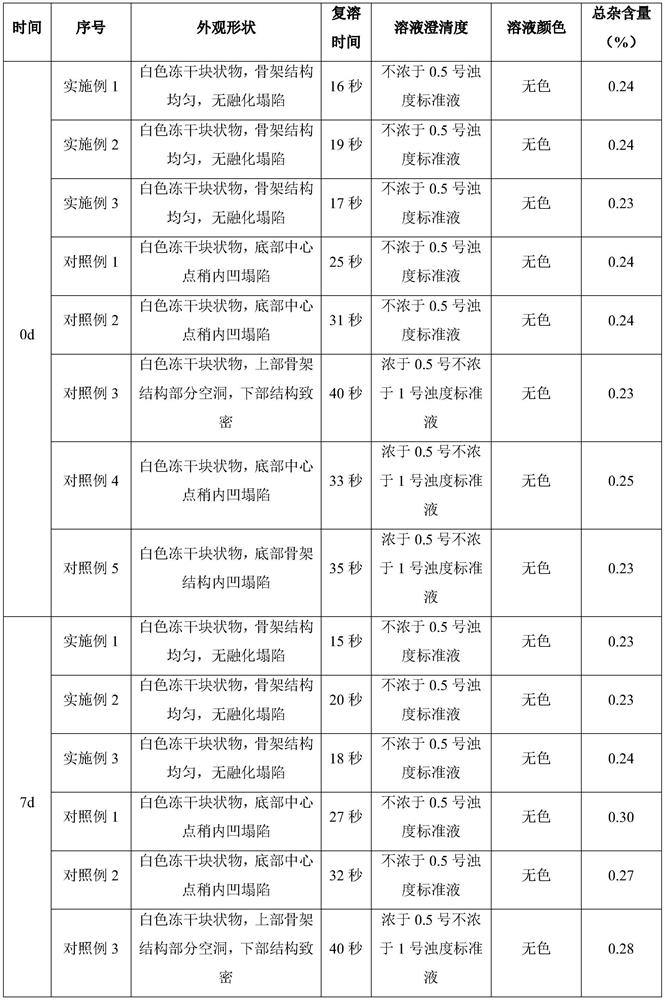
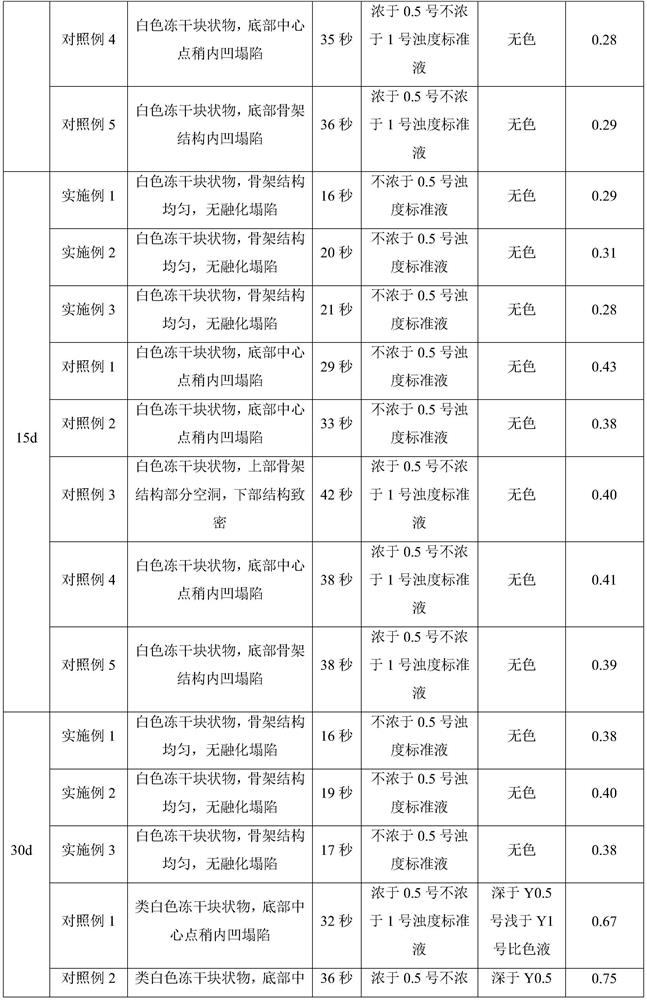
The invention discloses a ganciclovir composition for injection and a freeze drying process of the ganciclovir composition. The ganciclovir composition for injection comprises ganciclovir and sodium hydroxide, and is prepared by freeze drying of a liquid medicine containing 150-180 mg / mL of ganciclovir. The freeze drying process comprises the following steps: sequentially pre-freezing, sublimationdrying and desorption drying of the ganciclovir liquid medicine to obtain a ganciclovir powder injection. The prescription is simple and reasonable, the process is simple and easy to operate, the obtained product has excellent stability and redissolution performance, the problems that moisture is not easy to remove in the freeze drying process and the freeze-dried product structure is easy to collapse and shrink due to high solid content are solved on the premise of not greatly prolonging the freeze drying period, and the quality stability of the product and the safety of clinical medicationare fundamentally ensured.
Owner:HAINAN LEVTEC PHARMA
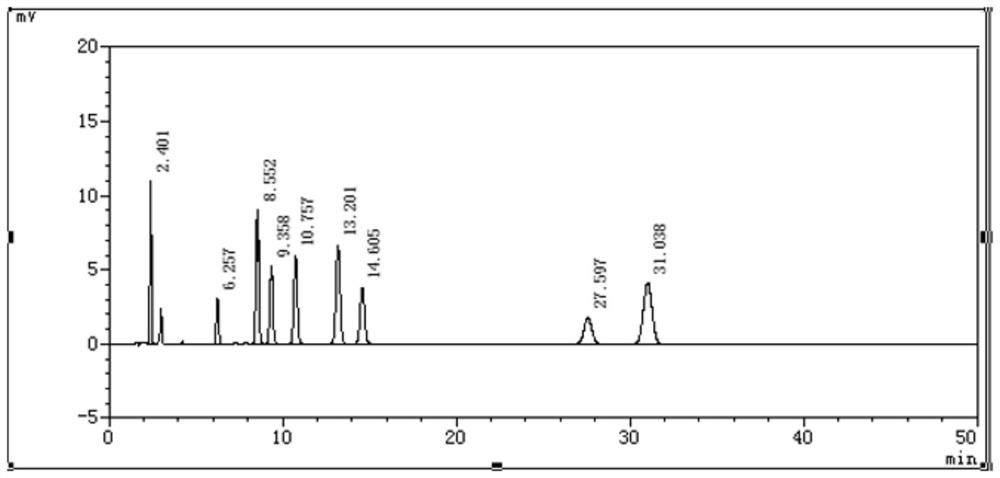
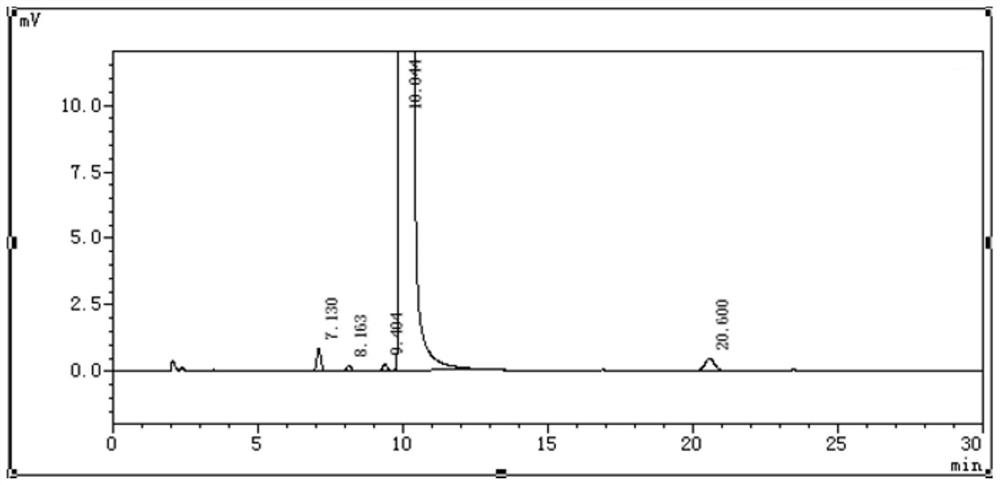
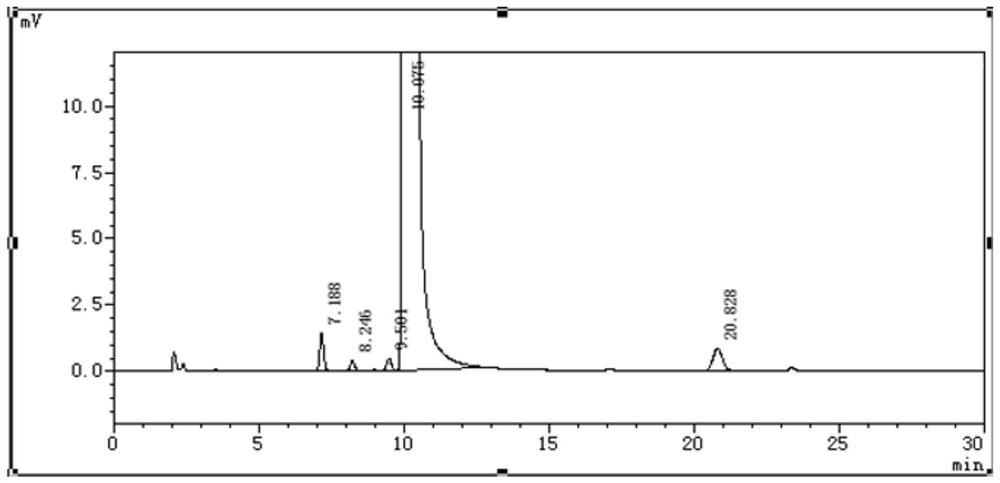
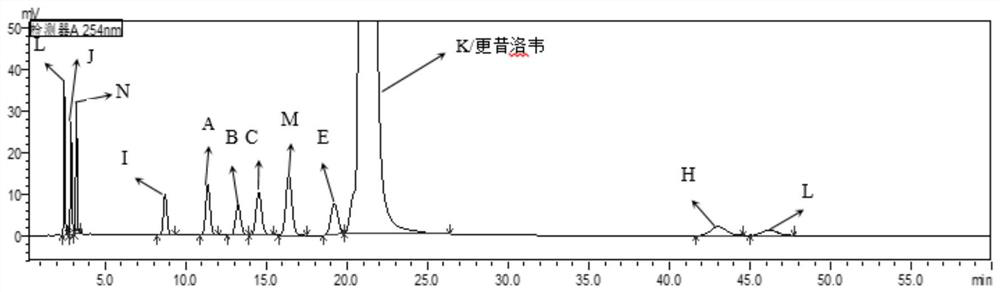
Ganciclovir related substance detection method
ActiveCN113834880ARapid Quality MonitoringEasy to separateComponent separationAgainst vector-borne diseasesO-Phosphoric AcidSilica gel
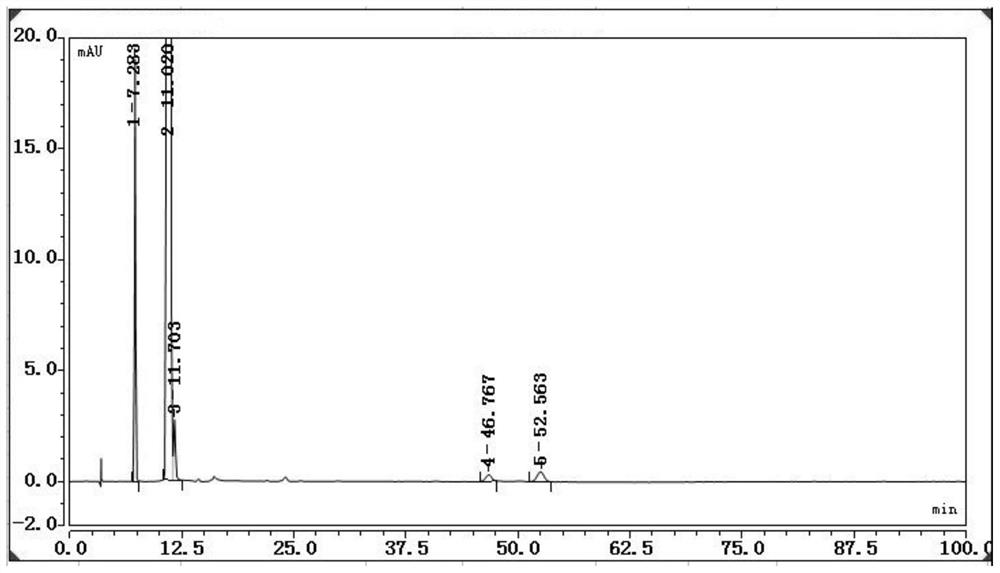
The invention discloses a ganciclovir related substance detection method, and relates to the field of pharmaceutical analysis. According to the ganciclovir related substance detection method, an octadecyl silane bonded silica gel chromatographic column is used, 0.012 mol / L ammonium dihydrogen phosphate solution-methanol (98-95: 2-5, V / V) is used as a mobile phase A, methanol is used as a mobile phase B, gradient elution is carried out, and the related substances in ganciclovir are detected. The pH value of the ammonium dihydrogen phosphate solution is adjusted to 2.5-3.5 by using a phosphoric acid solution. The method can be used for rapidly and effectively separating various potential trace related substances in ganciclovir raw material medicines, has the advantages of stable base line, attractive peak shape, good separation degree between a main peak and adjacent impurities and between the adjacent impurities, high durability and the like, and can be used for efficiently realizing qualitative and quantitative treatment of various related substances in ganciclovir. The method is suitable for rapid quality monitoring of ganciclovir in industrial mass production, and has important significance for product quality control.
Owner:HAINAN LEVTEC PHARMA
A kind of synthetic method of valganciclovir hydrochloride
ActiveCN107163050BStandards compliantEasy to operateOrganic chemistryGanciclovirumValganciclovir Hydrochloride
The invention discloses a novel valganciclovir hydrochloride synthesis method. The method includes: taking ganciclovir as a raw material, adopting ortho-ester for protecting a hydroxyl radical, then subjecting to condensation with N-carbobenzoxy-L-valine, and performing hydrolysis reaction to obtain N-carbobenzoxy valganciclovir; performing hydrogenation reduction reaction to obtain valganciclovir hydrochloride. The product purity reaches 99.0% or above and accords with United States Pharmacopeia standards.
Owner:湖北坦沐生物科技有限公司
External preparation of ganciclovir and application thereof
The invention relates to an external preparation of ganciclovir and application thereof. The external preparation is prepared from the following raw materials: ganciclovir, a polymer matrix and a pH regulator. Compared with acyclovir gel, the external preparation of ganciclovir disclosed by the invention has a better antiviral effect.
Owner:北京鼎实医药科技有限责任公司
A kind of synthetic method of valganciclovir hydrochloride
ActiveCN112661757BAvoiding the Separation Transformation ProblemAvoid yield lossOrganic chemistryGanciclovirumBiochemical engineering
The invention discloses a method for synthesizing valganciclovir hydrochloride. Using 1,3-dichloro-2-acetoxymethoxypropane as a starting material, monochloroganciclovir is prepared by esterification. , hydrolysis, deprotection into salt and finally obtain valganciclovir hydrochloride. The invention provides a method for synthesizing cganciclovir hydrochloride, which avoids the problem of separation and conversion of N-7 and N-9 isomers in the condensation process of diacetylguanine, and directly synthesizes monochlorovir without ganciclovir. Genanciclovir replaces the synthesis of monoacetyl ganciclovir in the prior art, and avoids the difficult problem of separation of diesters caused by residual ganciclovir. The method has short process steps, simple operation, easy purification and low cost. , conducive to industrial production, suitable for the synthesis of valganciclovir hydrochloride.
Owner:河北合佳医药科技集团股份有限公司
A kind of ophthalmic composition and its preparation method and application
ActiveCN112807275BGood anti-inflammatory and anti-viral effectReduce congestion rateSenses disorderAntipyreticConjunctivaPalpebral edema
Owner:湖北远大天天明制药有限公司
Pharmaceutical compositions of ganciclovir
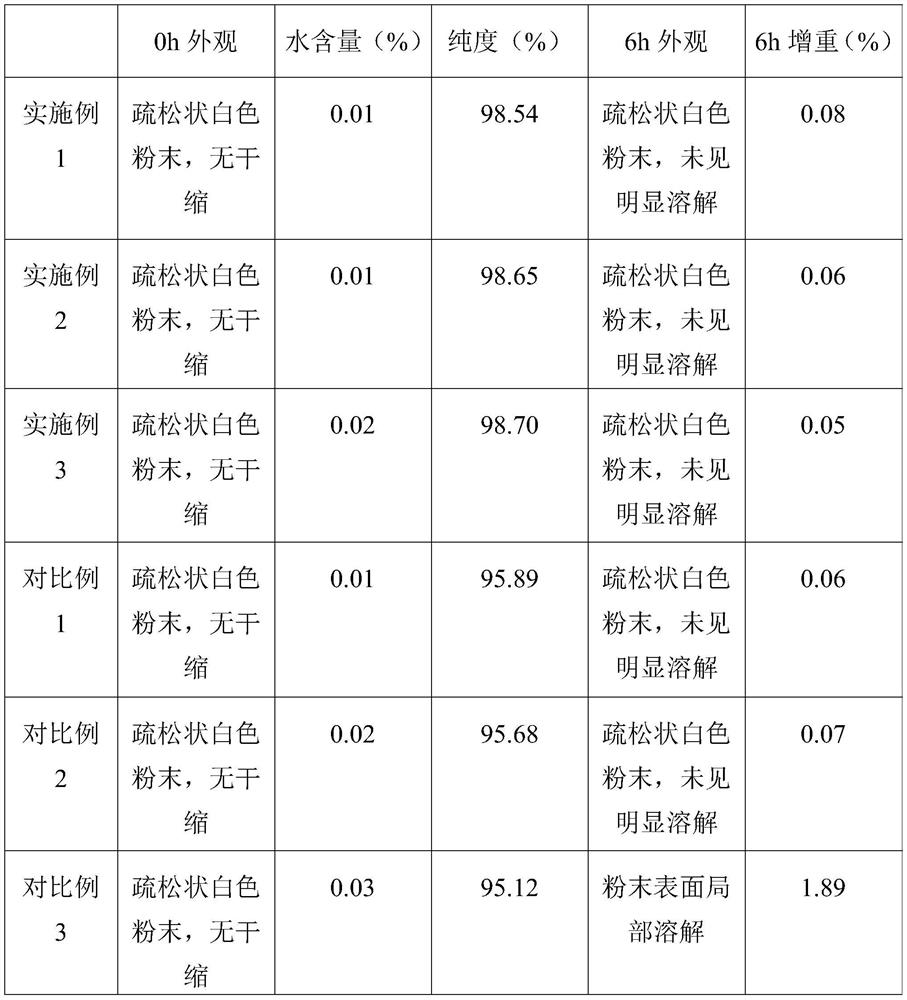
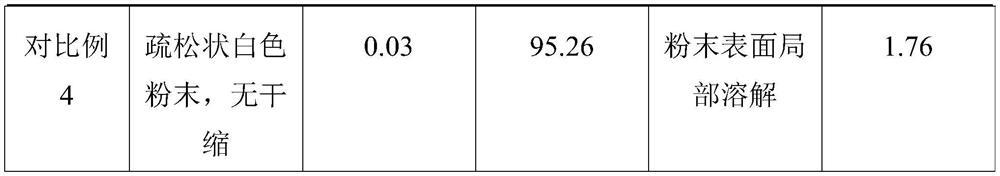
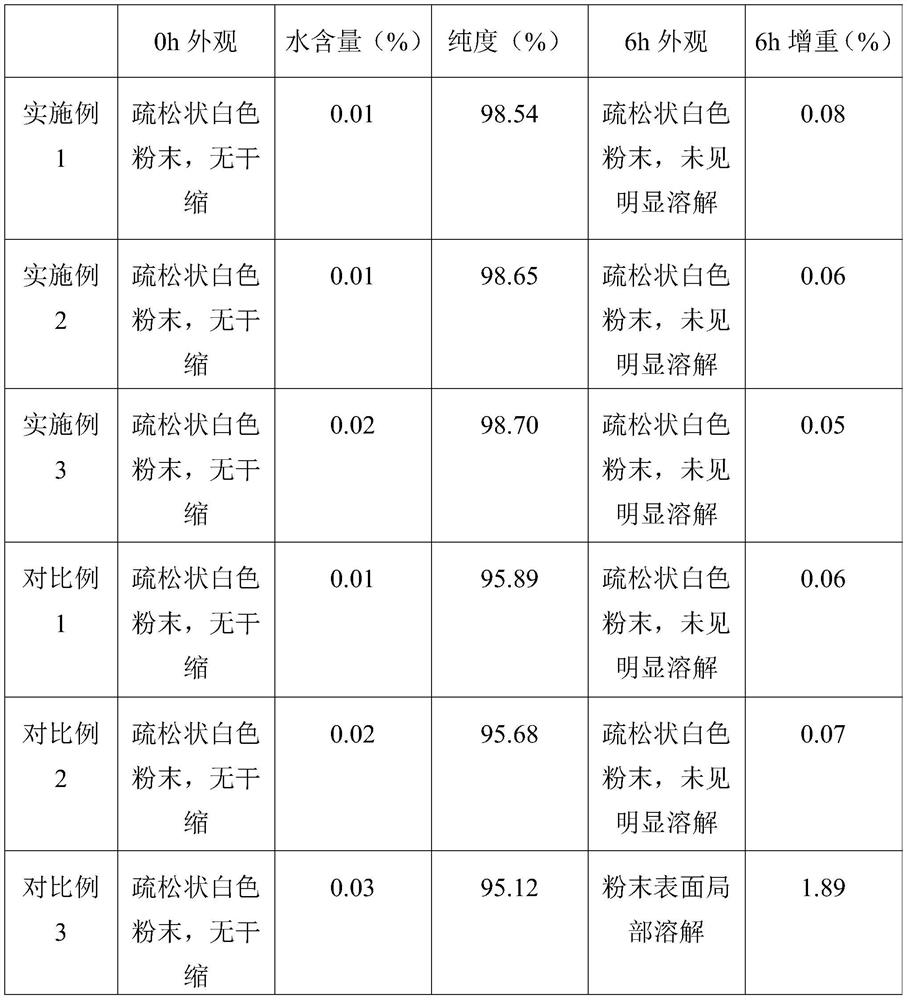
The technical field of the invention relates to pharmaceutical compositions of 9-(1,3-dihydroxy-2-propoxymethyl) guanine (ganciclovir) that are stable and contain more than 1% water content. One pharmaceutical composition includes ganciclovir having more than about 1% water content, and one or more pharmaceutically acceptable excipients. The ganciclovir retains at least about 97% of its initial purity after one month, at least about 96% of its initial purity after two months, and at least about 95% of its initial purity after three months when stored at 40° C. and 75% RH. In particular, the water content may be between about 2% and about 6%.
Owner:RANBAXY LAB LTD
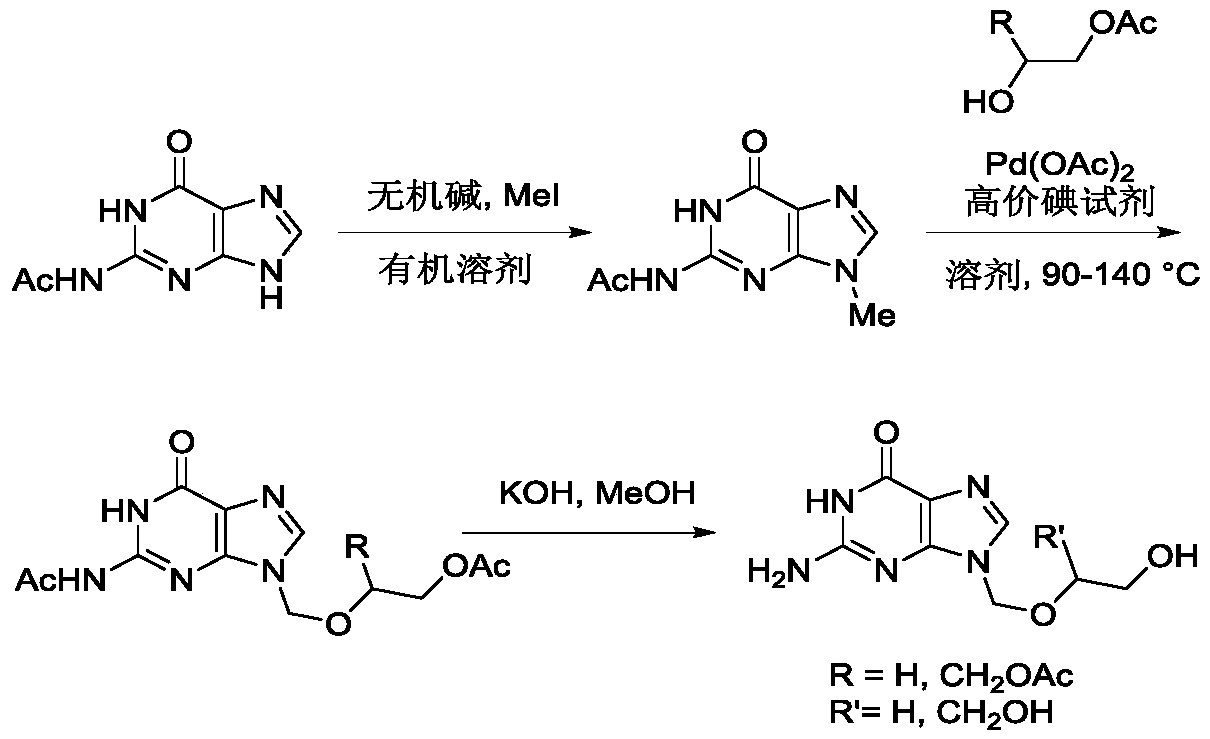
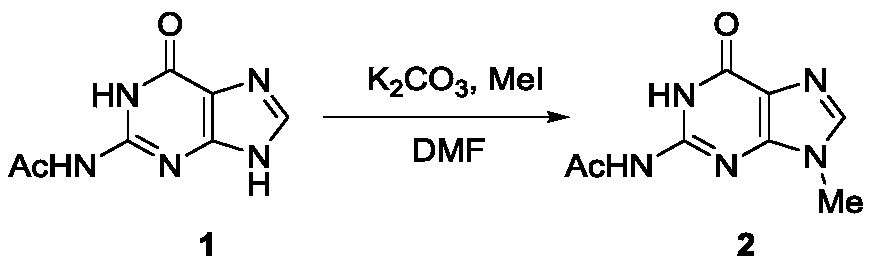
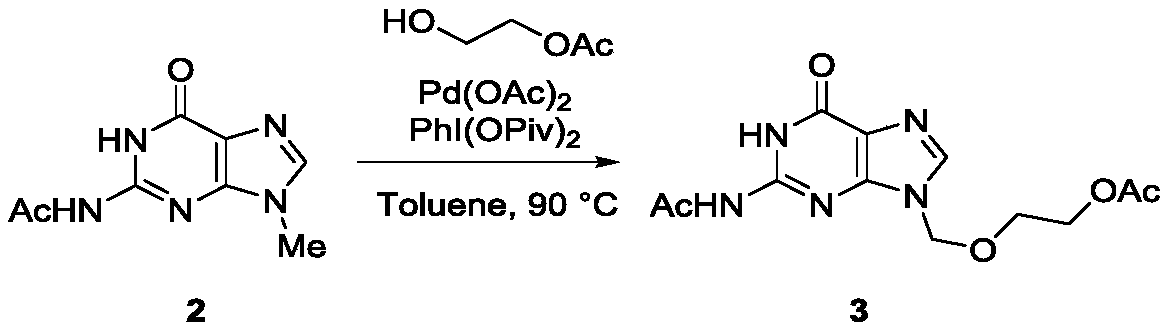
A kind of method utilizing carbon-hydrogen bond activation to synthesize acyclovir and ganciclovir
The invention discloses a method for synthesizing acyclovir and ganciclovir by carbon-hydrogen bond activation and belongs to the field of organic synthesis. The method comprises that inexpensive guanine as a raw material undergoes methyl protection on 9th NH, a high-valent iodine reagent and monoacetyl-protected ethylene glycol or 1, 2-isopropylidene-protected glycerol are added into the raw material under catalysis of palladium acetate, the mixture undergo a heating reaction to produce acetyl-protected acyclovir or acetyl-protected ganciclovir, and the acetyl group is removed by an inorganicalkali alcohol solution so that acyclovir and ganciclovir are obtained. The method utilizes cheap and easily available raw materials, prevents use risk and corrosive reagents, has the advantages of short reaction route, simple operation, high atomic economy and high total product yield, provides a novel synthesis route of acyclovir and ganciclovir and has a potential application prospect.
Owner:XINYANG NORMAL UNIVERSITY
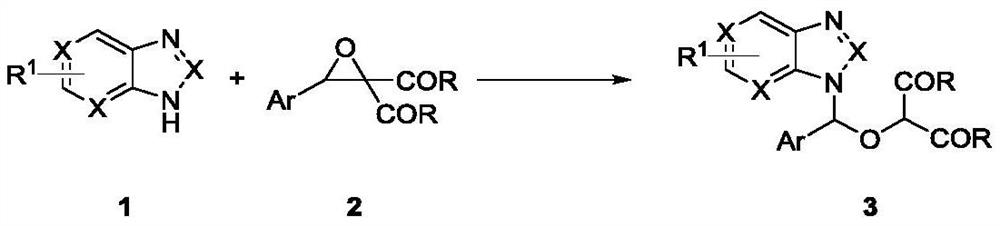
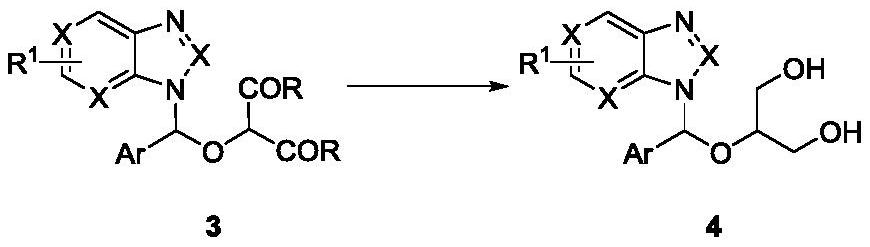
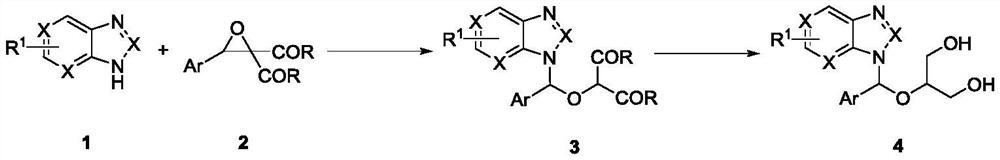
Method for synthesizing ganciclovir analogue
ActiveCN112778224AReaction raw materials are readily availableSingle reaction chemistryOrganic chemistryBulk chemical productionMolecular sieveAryl
The invention discloses a method for synthesizing a ganciclovir analogue, and belongs to the technical field of synthesis of medical intermediates. N-aryl heterocycle 1 and D-A ethylene oxide 2 are used as raw materials, Lewis acid is used as a catalyst, a molecular sieve is added, and a non-cyclic nucleoside analogue 3 is obtained through reaction. The reaction is relatively single in chemical selectivity, high in regioselectivity and good to excellent in yield. The non-cyclic purine nucleoside 3 is further reduced to obtain a ganciclovir analogue 4. The raw materials are easy to obtain, the operation is simple, and a new way is provided for synthesis of the ganciclovir analogue.
Owner:HENAN NORMAL UNIV
Method for recycling ganciclovir condensation compound synthesis mother liquor
The invention discloses a method for recycling ganciclovir condensation compound synthesis mother liquor, which comprises the following steps: after the condensation reaction is finished, treating and separating out a ganciclovir condensation compound isomer, then evaporating the centrifugal mother liquor to dryness a solvent, and adding methanol and toluene to separate out a ganciclovir condensation compound; transferring the mother liquor after centrifuging the ganciclovir condensation compound into a reaction kettle to concentrate methanol and toluene; adding water into the base solution to separate out a ganciclovir condensation compound isomer, and centrifuging the ganciclovir condensation compound isomer; the recovered methanol toluene is directly used as toluene after being washed twice; the ganciclovir condensation compound isomer recovered from the mother liquor can also be put into the condensation reaction for continuous use; according to the method, resource recycling and limited resource waste are well achieved, and the method has large economic benefits and good social benefits.
Owner:HUBEI HONGYUAN PHARMA
Method for recovering side chain in ganciclovir condensation compound mother liquor
PendingCN113929580AIncrease valueLow raw material costOrganic compound preparationCarboxylic acid esters separation/purificationSide chainDistillation
The invention discloses a method for recovering a side chain in ganciclovir condensation compound mother liquor, which comprises the following steps of: after the condensation reaction is finished, transferring mother liquor obtained after ganciclovir condensation compound centrifuging into a reaction kettle to concentrate methanol and toluene; adding water into the base solution to separate out a ganciclovir condensation compound isomer; adding water and toluene into mother liquor obtained after centrifuging of the ganciclovir condensation compound isomer, stirring and filtering, layering filtrate, taking a toluene layer, extracting a water layer twice with toluene, and merging the toluene layer; and then carrying out atmospheric distillation and vacuum rectification on toluene to obtain a ganciclovir side chain. By the implementation of the method, resource recycling is better realized, waste of limited resources is reduced, the raw material cost of ganciclovir is reduced, and great economic benefits and good social benefits are achieved.
Owner:HUBEI HONGYUAN PHARMA
Preparation method of ganciclovir sodium freeze-dried powder for injection
ActiveCN113476413AGuaranteed purityGood lookingPowder deliveryPharmaceutical non-active ingredientsCelluloseActivated carbon
The invention discloses a preparation method of ganciclovir sodium freeze-dried powder for injection, which sequentially comprises the following steps: adding mannitol into water for injection, then adding activated carbon, stirring and standing, and then performing coarse filtering and decarburization; enabling the mannitol solution subjected to rough filtration and decarburization to pass through a sterile mixed cellulose film to prepare a purified mannitol solution; adding ganciclovir sodium into the purified mannitol solution, and then preparing a ganciclovir sodium solution through the sterile mixed cellulose film; carrying out temperature control on the ganciclovir sodium solution, and regulating a pH value with CO2; filling a bottle with the ganciclovir sodium solution, and carrying out freeze-drying after semi-plug pressing; and after the freeze-drying is finished, performing plug pressing and re-pressing to prepare the ganciclovir sodium freeze-dried powder for injection. As a protective agent of the ganciclovir sodium freeze-dried powder, the mannitol is more suitable for storage of the ganciclovir sodium freeze-dried powder, and the purity of the ganciclovir sodium freeze-dried powder can be better guaranteed.
Owner:HAINAN HAILING CHEMIPHARMA CORP
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com