A kind of method utilizing carbon-hydrogen bond activation to synthesize acyclovir and ganciclovir
A technology of carbon-hydrogen bond activity and ganciclovir, which is applied in the field of synthesis of raw materials, can solve the problems of low total yield and long side chain synthesis route, and achieve short reaction route, cheap raw materials and high atom economy. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
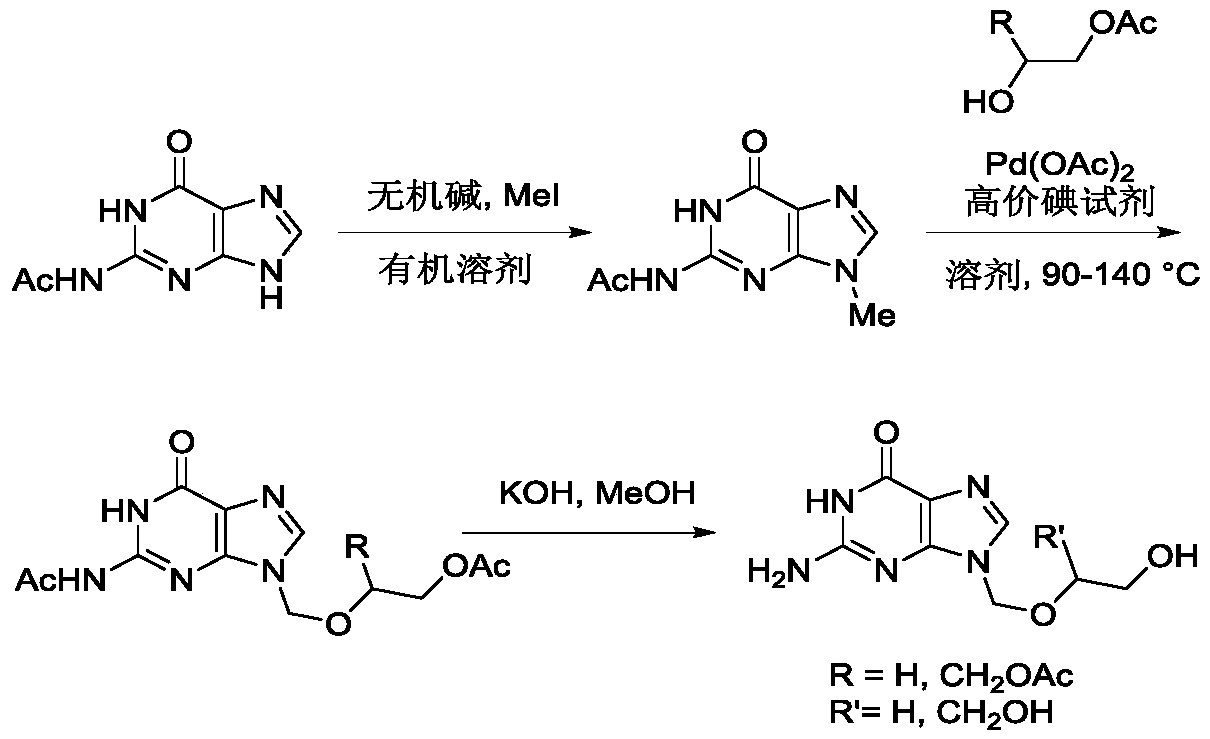
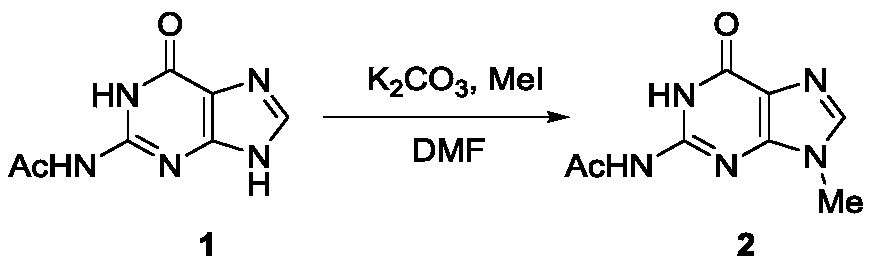
[0021] In the first step, the reaction formula is as follows:
[0022]
[0023] 5 mmol of N2-acetylguanine (1) was dissolved in 15 mL of anhydrous DMF, 10 mmol of potassium carbonate and 6 mmol of methyl iodide were added, stirred and reacted at room temperature for 8 hours, then 30 mL of ethyl acetate was added, fully Stir, transfer to a separatory funnel, wash twice with water, once with saturated brine, collect the organic phase, dry over anhydrous sodium sulfate, remove the solvent under reduced pressure, and purify the residue by silica gel column chromatography to obtain light yellow syrupy N9 -Methyl-N2-acetyl protected guanine (2), the product yield was 78%.
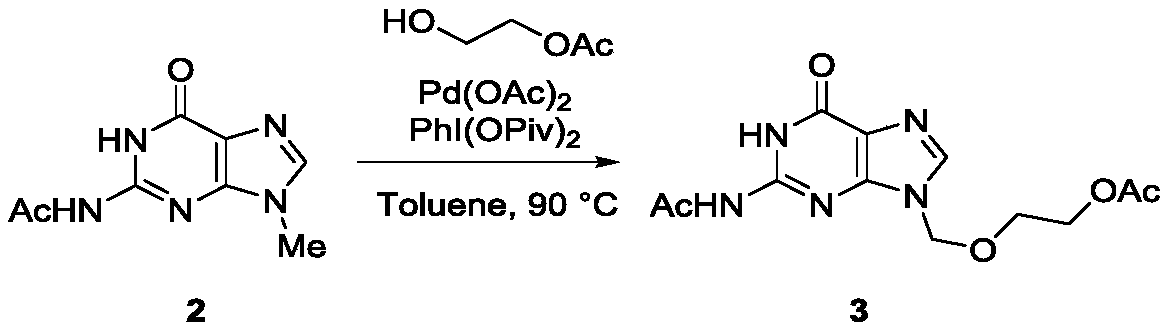
[0024] In the second step, the reaction formula is as follows:
[0025]
[0026] 5 mmol of N9-methyl-N2-acetylguanine (2) was dissolved in 20 mL of anhydrous toluene, 0.5 mmol of palladium acetate was added, 6 mmol of PhI(OPiv)2 and 6 mmol of monoacetyl-protected Ethylene glycol (HOCH2CH2OAc), the tempera...
Embodiment 2
[0033] In the first step, the reaction formula is as follows:
[0034]
[0035] 5 mmol of N2-acetylguanine (1) was dissolved in 15 mL of anhydrous DMF, 10 mmol of potassium carbonate and 6 mmol of methyl iodide were added, stirred and reacted at room temperature for 8 hours, then 30 mL of ethyl acetate was added, fully Stir, transfer to a separatory funnel, wash twice with water, once with saturated brine, collect the organic phase, dry over anhydrous sodium sulfate, remove the solvent under reduced pressure, and purify the residue by silica gel column chromatography to obtain light yellow syrupy N9 -Methyl-N2-acetyl protected guanine (2), the product yield was 78%.
[0036] In the second step, the reaction formula is as follows:
[0037]
[0038] 5 mmol of N9-methyl-N2-acetylguanine (2) was dissolved in 20 mL of anhydrous toluene, 0.5 mmol of palladium acetate was added, 6 mmol of PhI(OPiv)2 and 6 mmol of 1,3-acetyl Glycerol, the temperature rose to 120 degrees Celsiu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com