Method for detecting ganciclovir and related substances in ganciclovir ophthalmic gel
A detection method and technology for related substances, applied in the field of medicine, can solve problems such as incomplete separation and undetectable impurities, and achieve the effects of being conducive to detection and accurate quantification, good specificity, and improved separation.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
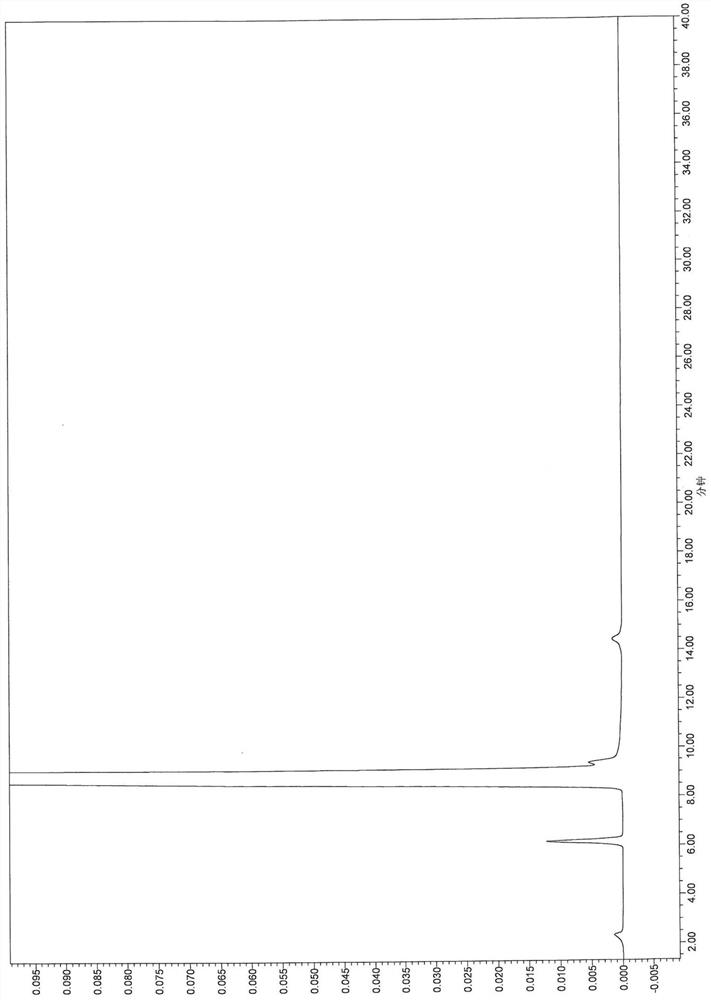
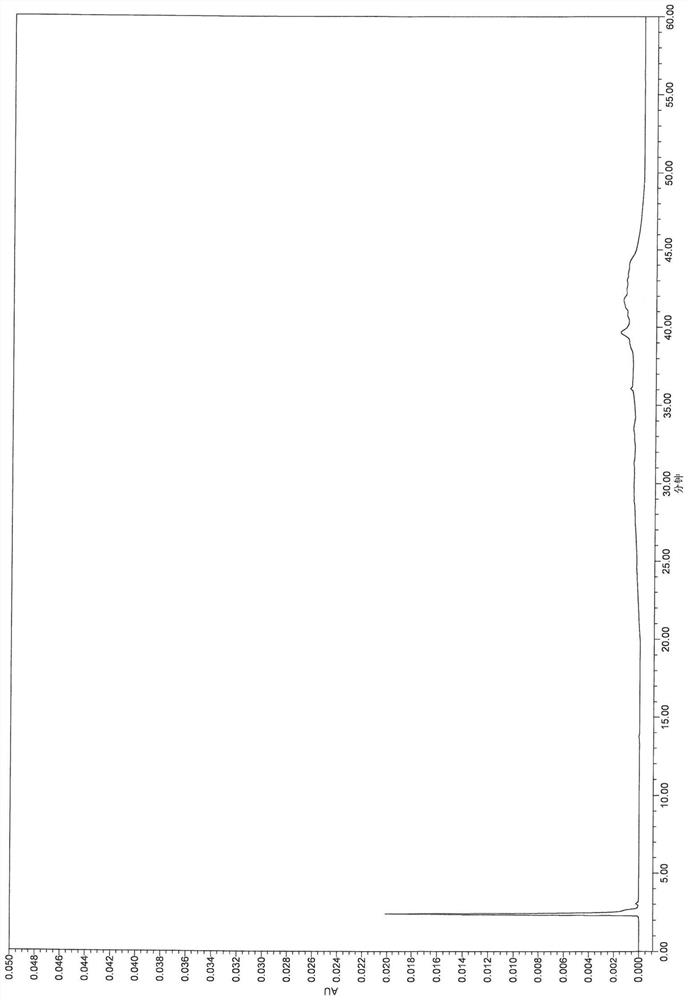
Examples
Embodiment 1
[0089] Example 1: Pre-processing research and verification of the test product preparation method
[0090] (1) Pre-processing research on the preparation of the test product
[0091] If the test product (i.e. the sample of ganciclovir ophthalmic gel to be tested) is directly diluted with mobile phase, the solution viscosity is high, it is difficult to filter, and it will lead to an increase in column pressure and a decrease in column efficiency. Carbomer must be effectively removed. followed by chromatographic analysis.
[0092]Adopt the method precipitation carbomer of 5% magnesium sulfate solution 4ml to add the sodium hydroxide solution 1ml of 0.1mol / L in the prior art: this method salt concentration is bigger, easily damages chromatographic column, is not suitable for HPLC method to measure, and because Due to the difference in preparation prescription and process, when this method is used to treat this product, the precipitate cannot be condensed, and the transfer an...
Embodiment 2
[0103] Example 2: Durability Study (1)
[0104] Instruments and conditions: Waterse 2695 liquid chromatography system, UV detector; Chromatographic column: Waters AtlantisT3 (5μm, 4.6mm×250mm); Detection wavelength: 252nm; Column temperature: 35°C; Flow rate: 1.0ml / min; Injection volume: 40 μl; mobile phase A is purified water, mobile phase B is methanol, and gradient elution is performed according to Table 2.
[0105] Table 2: Gradient elution parameters
[0106] time (minutes) Mobile phase A(%) Mobile phase B(%) 0 100 0 15 100 0 35 47 53 37 100 0 52 100 0
[0107] Experimental procedure: take an appropriate amount of ganciclovir reference substance, quantitatively dilute it with mobile phase A to make a solution containing about 0.15 mg per 1 ml, shake well, and use it as the ganciclovir reference substance solution; take another ganciclovir EP and mix One impurity reference substance was dissolved in 1ml of ganciclovir ref...
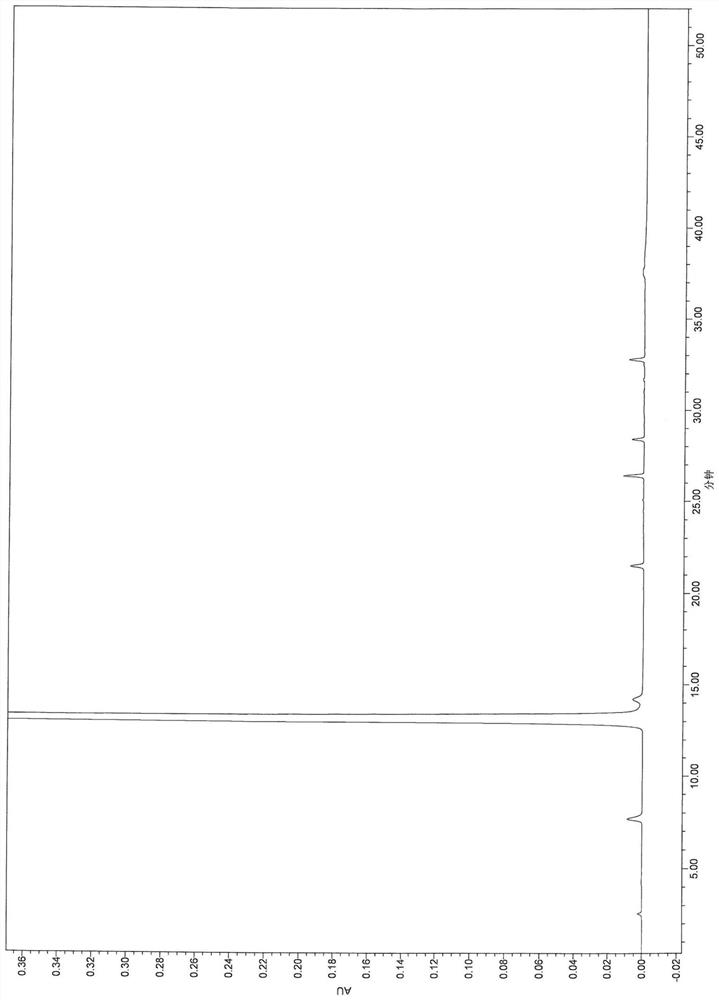
Embodiment 3
[0109] Example 3: Durability Study (2)
[0110] The instruments, chromatographic conditions and experimental procedures were the same as in Example 2, except that methanol-water (3:97) was used as mobile phase A.
[0111] The results show that the main component can be completely separated from adjacent impurities and each impurity, and the specificity is good.
PUM
| Property | Measurement | Unit |
|---|---|---|
| separation | aaaaa | aaaaa |
| separation | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com