Human cytomegalovirus containing foreign antigen
A technology of human cytomegalovirus and heterologous antigen, applied in the field of vaccine platform, can solve the problem that primary cells are difficult to achieve
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0081] Embodiment 1.HCMV-TR3 carrier platform
[0082] Clinical use of effector memory T cell-inducing CMV vectors requires vectors that are genetically stable and maintain persistent infection, but lack the ability to confer on immunocompromised subjects in which HCMV is pathogenic. Previous attenuation strategies for HCMV variants entering clinical trials relied on serial passage of the virus in fibroblasts (Plotkin SA et al., J Infect Dis 134, 470-475 (1976); incorporated herein by reference), attenuated versus non-attenuated Recombination of HCMV strains (Heineman J et al. 2006, supra) or generation of replication-deficient recombinant vectors (WO2013 / 036465; incorporated herein by reference). However, the resulting virus retains pathogenicity or loses beneficial characteristics, such as the ability to establish latent infection or secondary infection in a subject previously naturally infected with CMV.
[0083] An HCMV vector platform, HCMV-TR3, that overcomes these limi...
Embodiment 2
[0088] Example 2. HCMV-TR is superior to other HCMV strains in establishing latent infection
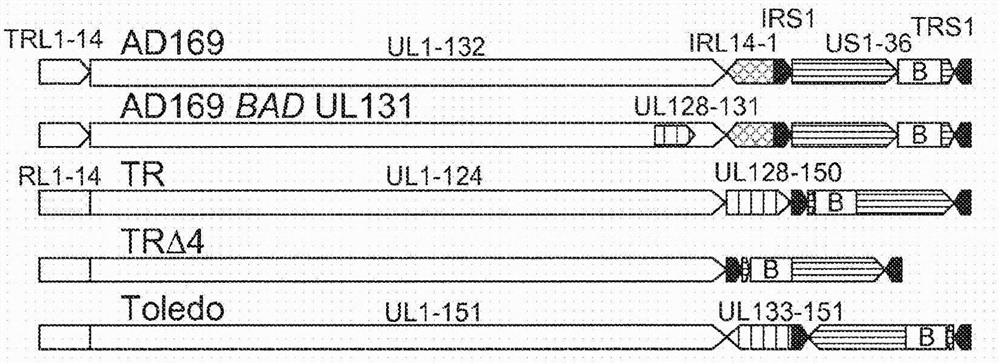
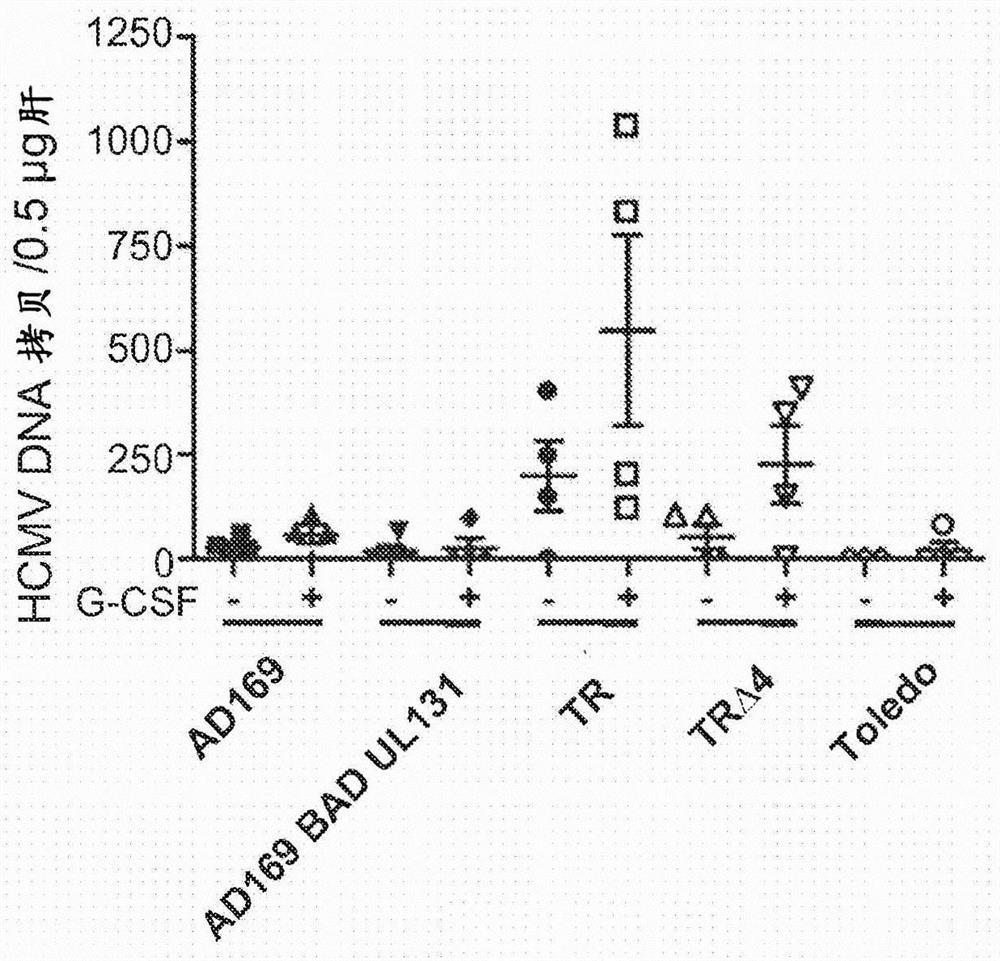
[0089] A humanized mouse model that allows for the study of HCMV latency and reactivation is described in Smith MS et al., Cell Host Microbe 8, 284-291 (2010) (incorporated herein by reference). This model was used to demonstrate that HCMV-TR is superior to other HCMV strains (AD169, Toledo) in establishing persistent infection. Persistent infection is important for the induction of effector memory T cells. The ability to generate persistent infection is independent of the UL128-150 region, which is mutated in many HCMV strains, including all strains previously used in clinical trials of HCMV vaccines (AD169, Towne and Toledo). Restoration of UL131A in the AD169 strain did not restore the ability to establish latency, but HCMV-TRΔ4 strains lacking UL128-150 maintained the ability to establish latency ( Figure 1B ). Note that these previous clinical trials did not involve HCMV con...
Embodiment 3
[0090] Example 3. HCMV-TR3 is sensitive to ganciclovir and contains the US2-7 region, whereas native HCMV-TR is not
[0091] HCMV TR was cloned by BAC recombination engineering from a virus isolate resistant to the antiviral drug ganciclovir (Smith IL et al., J Infect Dis 176, 69-77 (1997); incorporated herein by reference). Ganciclovir resistance is not a desirable trait in HCMV vectors since treatment with ganciclovir is important in the context of CMV-associated disease caused by HCMV-based vectors. Confirmation of ganciclovir resistance is shown in image 3 middle.
[0092] Insert the complete UL97 gene into HCMVTR ( figure 2 ) to produce a ganciclovir-sensitive carrier. Molecular cloning of further modified HCMV-TR. Insertion of the BAC cassette during the original cloning of the HCMV TR resulted in a deletion of the US2-7 region (Murphy et al., 2003, supra). US2-7 was subsequently identified as a region essential for reinfection of CMV-positive individuals (Hansen ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com