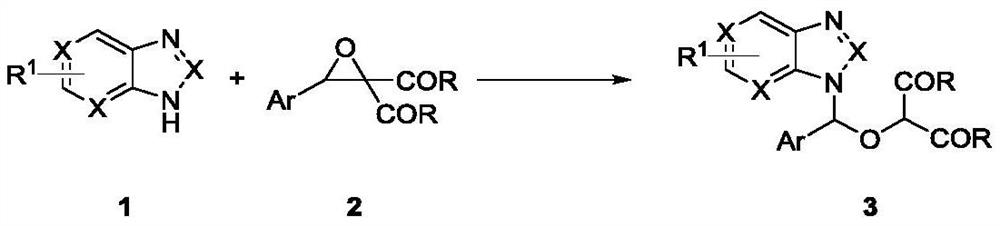
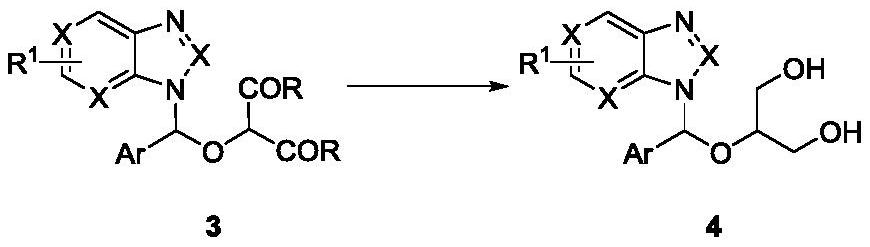
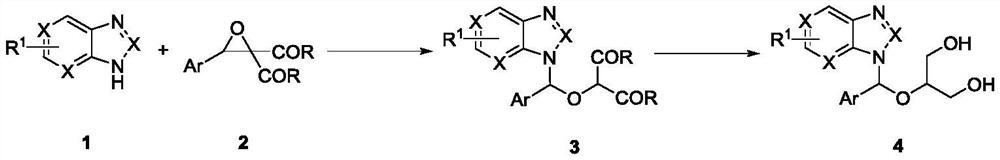
Method for synthesizing ganciclovir analogue
A technology for nucleoside analogs and acyclic nucleosides, applied in the field of ganciclovir analog synthesis, can solve the problems of low yield, insufficient research, inconvenient separation of allogeneic bodies by column chromatography, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Investigation of reaction conditions (taking entry 5 as an example):
[0021] In a reaction tube, benzotriazole 1a (0.1mmol, 12mg), phenyloxirane gem-diethylcarboxylate compound 2a (0.1mmol, 26.4mg), Y(OTf) 3 (5mol%, 2.7mg) and MS (30 mg) was added to the reaction tube, and 2 mL of 1,2-dichloroethane was added to the reaction system, and the reaction tube was placed in an 80° C. oil bath for 10 h. After TLC detection, the reaction was terminated, and the compound 3a was obtained by column chromatography after concentration, with a yield of 94%.
[0022]
[0023]
[0024] a Reaction conditions: 1a(0.1mmol), 2a(0.1mmol), catalyst(xmol%), MS(30mg) and solvent for 10h in the pressure tube. b The yield was determined by 1 HNMR using CH 2 Br 2 as an internal standard. c The ratio was determined by 1 H NMR Analysis of crude product. d Isolated yield of 3a in parentheses.
[0025] In the screening process of reaction conditions, the influence of different Lew...
Embodiment 2
[0032] In the reaction tube, benzotriazole 1a (0.1mmol, 12mg), o-tolyl oxide oxirane gem diethyl carboxyl compound 2b (0.1mmol, 27.8mg), Y (OTf) 3 (5mol%, 2.7mg) and MS (30 mg) was added to the reaction tube, and 2 mL of 1,2-dichloroethane was added to the reaction system, and the reaction tube was placed in an 80° C. oil bath for 10 h. After TLC detection, the reaction was terminated, and the compound 3b was obtained by column chromatography after concentration, with a yield of 84%. Colorless solid, 33.4mg, m.p.: 65.6-70.3℃. 1 H NMR (400MHz, CDCl 3 )δ8.21(d, J=8.0Hz, 1H), 8.03(d, J=8.0Hz, 1H), 7.44(s, 1H), 7.42-7.25(m, 4H), 7.12-7.09(m, 2H ),4.81(s,1H),4.35-4.27(m,2H),3.92(q,J=7.2Hz,2H),1.93(s,3H),1.30(t,J=7.2Hz,3H),1.02 (t,J=7.2Hz,3H). 13 C NMR (150MHz, CDCl 3 )δ165.8,165.1,147.0,136.2,132.4,131.5,131.3,129.8,128.0,126.7,126.3,124.5,120.1,111.7,87.2,76.4,62.6,62.2,19.0,14.2,13.8)m / ESI :[M+Na] + Calcd for C 21 h 23 N 3 NaO 5 420.1530; Found 420.1524.
Embodiment 3
[0034]In the reaction tube, benzotriazole 1a (0.1mmol, 12mg), p-tolyl oxirane gem diethyl ester compound 2c (0.1mmol, 27.8mg), Y (OTf) 3 (5mol%, 2.7mg) and MS (30 mg) was added to the reaction tube, and 2 mL of 1,2-dichloroethane was added to the reaction system, and the reaction tube was placed in an 80° C. oil bath for 10 h. After TLC detection, the reaction was terminated, and after concentration, column chromatography obtained compound 3c with a yield of 87%. Colorless oil, 34.6mg, 1 H NMR (400MHz, CDCl 3 )δ8.07-8.05(m,1H),7.37-7.29(m,5H),7.25-7.22(m,1H),7.18(d,J=8Hz,2H),4.68(s,1H),4.36- 4.27(m,2H),3.92(q,J=7.2Hz,2H),2.35(s,3H),1.31(t,J=7.2Hz,3H),1.00(t,J=7.2Hz,3H). 13 C NMR (100MHz, CDCl 3 )δ165.9, 165.0, 147.2, 139.5, 131.8, 131.4, 129.5, 127.9, 126.2, 124.6, 120.0, 112.2, 89.5, 76.5, 62.6, 62.2, 21.4, 14.2, 13.7. HRMS (ESI) m / z: [M+ Na] + Calcd for C 21 h 23 N 3 NaO 5 420.1530; Found 420.1530.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com