Method for preparing bird-nest type monodisperse magnesium oxide
A magnesium oxide, monodisperse technology, applied in the direction of magnesium oxide, can solve the problems that affect the production and application of magnesium oxide, the shape of magnesium oxide is difficult to control, and the particle size distribution is uneven, so as to achieve easy operation, controllable shape, The effect of short reaction times
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
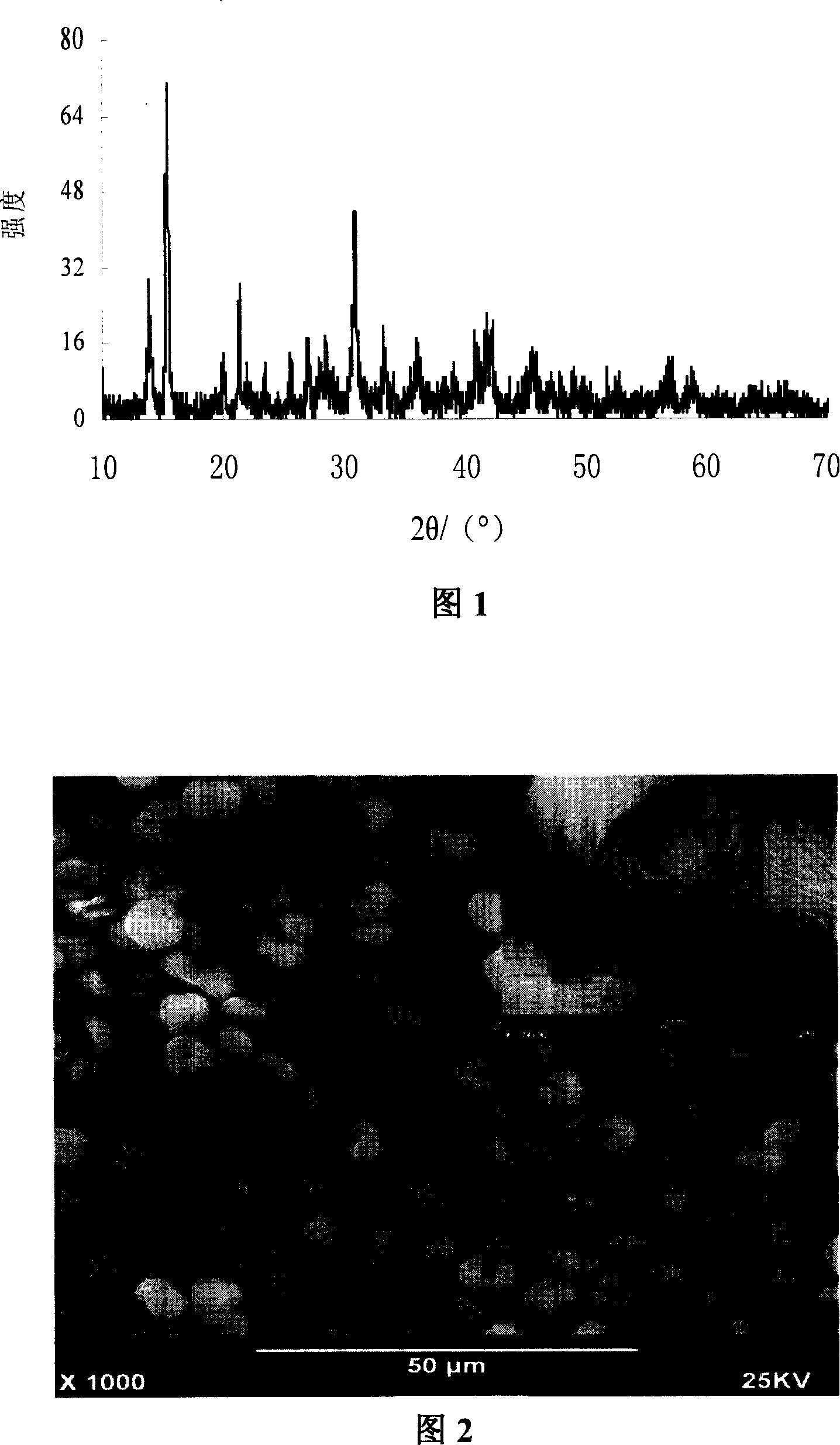
[0022] Weigh 16g of magnesium nitrate in a 250ml beaker, add 60-150ml of secondary deionized water, specifically 90ml, transfer it to a 500ml three-necked round bottom flask; then weigh 9g of potassium carbonate in a 250ml beaker , add 120-300ml of secondary deionized water, specifically 180ml; heat the magnesium nitrate and potassium carbonate solutions to 100°C respectively, then quickly add potassium carbonate to the magnesium nitrate solution heated in an oil bath, and stir quickly to Aging for 0.5 hour, filtering, and drying; the X-ray diffraction patterns and scanning electron microscope images of the resulting product are shown in Figures 1 and 2. It can be seen from the figure that the product is bird’s nest-shaped basic magnesium carbonate with uniform particle size distribution and controllable shape, Mg 5 (CO 3 ) 4 (OH) 2 (H 2 O) 4 .
Embodiment 2
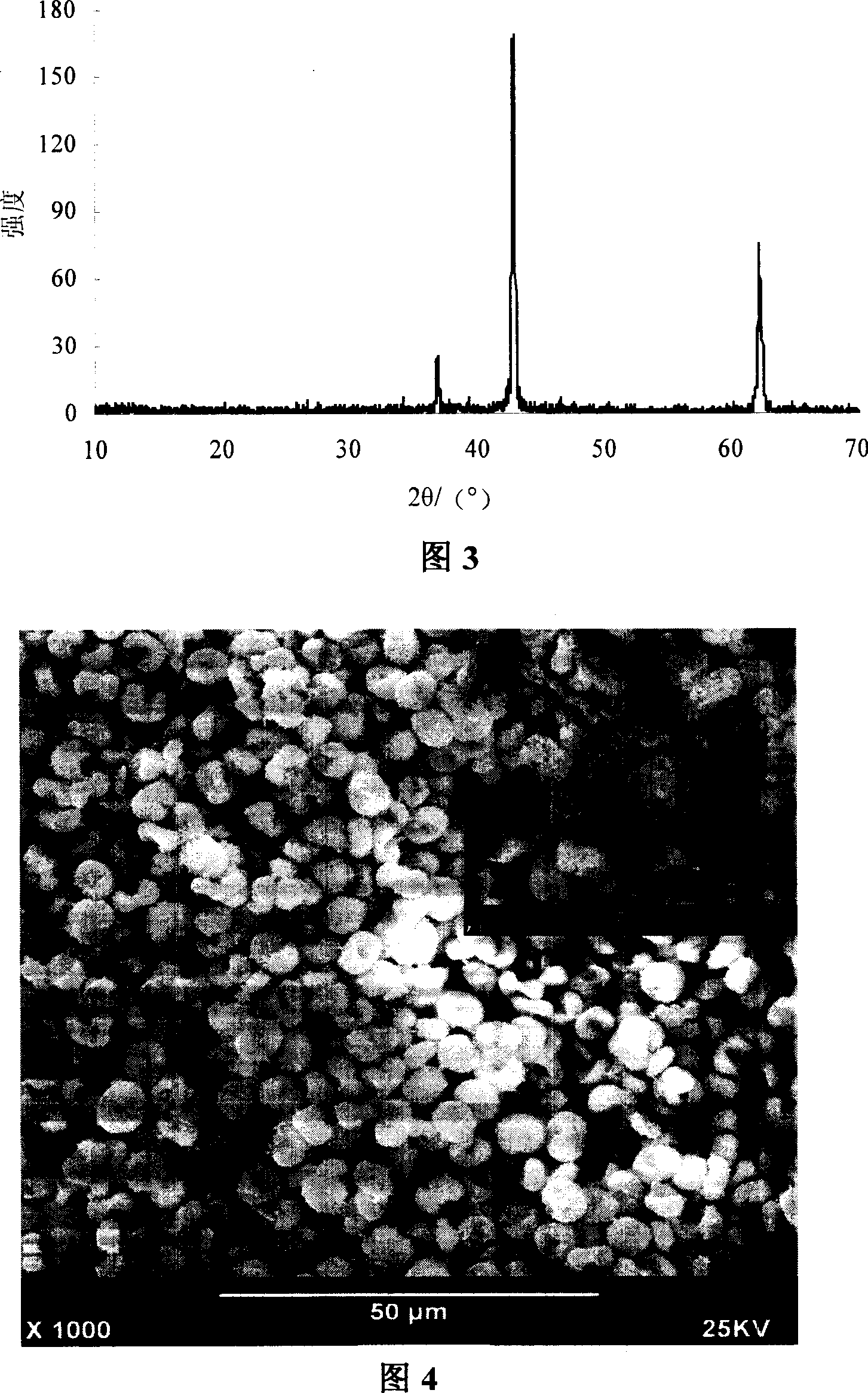
[0024] Weigh 16g of magnesium nitrate in a 250ml beaker, add 60-150ml of secondary deionized water, specifically 90ml, transfer it to a 500ml three-necked round bottom flask; then weigh 9g of potassium carbonate in a 250ml beaker , add 120-300ml of secondary deionized water, specifically 180ml; heat the magnesium nitrate and potassium carbonate solutions to 80°C respectively, then quickly add potassium carbonate to the magnesium nitrate solution heated in the oil bath, and stir quickly evenly. Aging for 1 hour, filtering, drying, and then roasting at a high temperature of 550°C; the X-ray diffraction patterns and scanning electron microscope images of the obtained product are shown in Figure 3 and Figure 4 . It can be seen from the figure that the product is bird's nest-like MgO with uniform particle size distribution and controllable shape.
Embodiment 3
[0026] Weigh 16g of magnesium nitrate in a 250ml beaker, add 60-150ml of secondary deionized water, specifically 90ml, transfer it to a 500ml three-necked round bottom flask; then weigh 9g of potassium carbonate in a 250ml beaker , add 120-300ml of secondary deionized water, specifically 180ml;
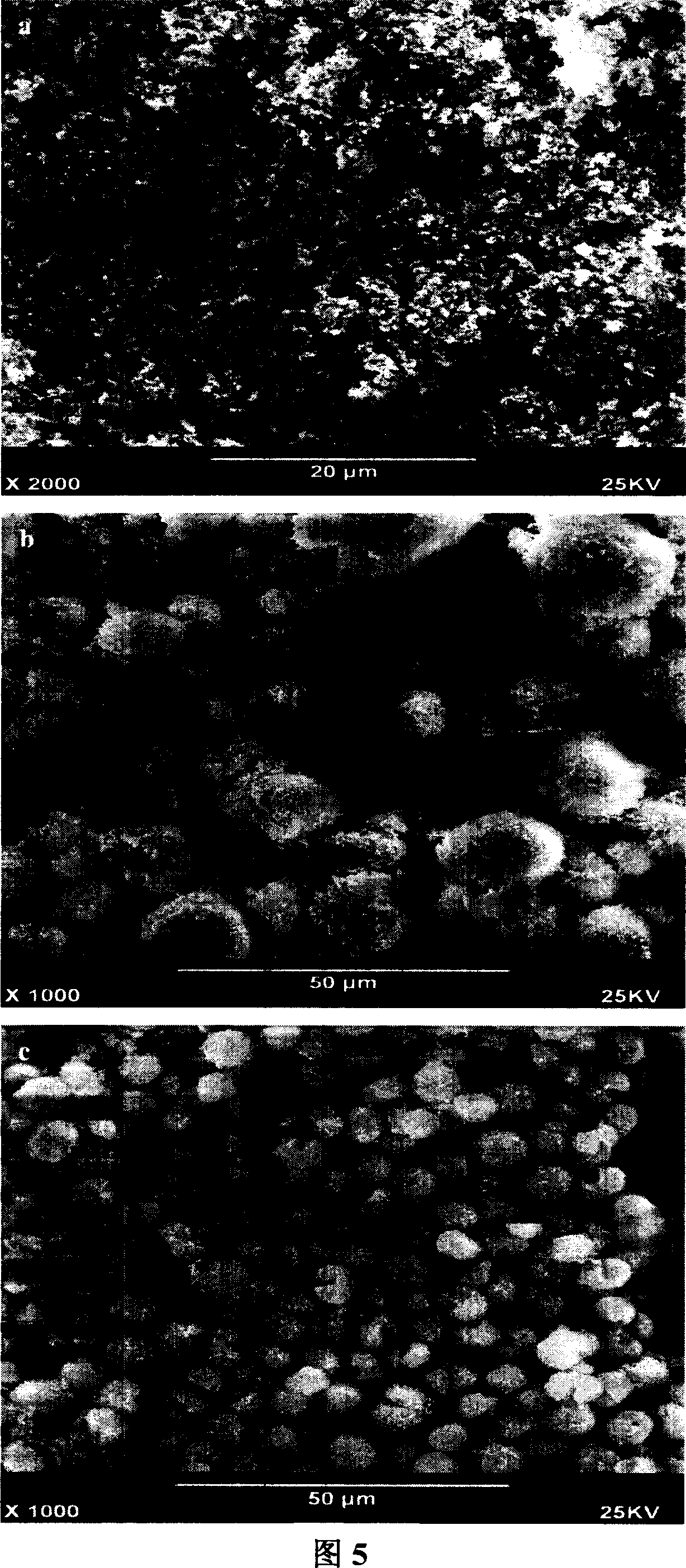
[0027] (a) Potassium carbonate was quickly added to the magnesium nitrate solution heated in an oil bath, stirred vigorously, aged for 2 hours, filtered, dried, and then roasted at a high temperature of 600°C; the scanning electron microscope image of the obtained product is shown in Figure 5a. As can be seen from the figure, the particle size of the product is relatively small.
[0028] (b) Heating the magnesium nitrate solution to 90°C, quickly adding potassium carbonate to the magnesium nitrate solution heated in an oil bath, stirring rapidly, aging for 3 hours, filtering, drying, and then roasting at a high temperature of 700°C; the obtained product The SEM image is shown in Figu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com