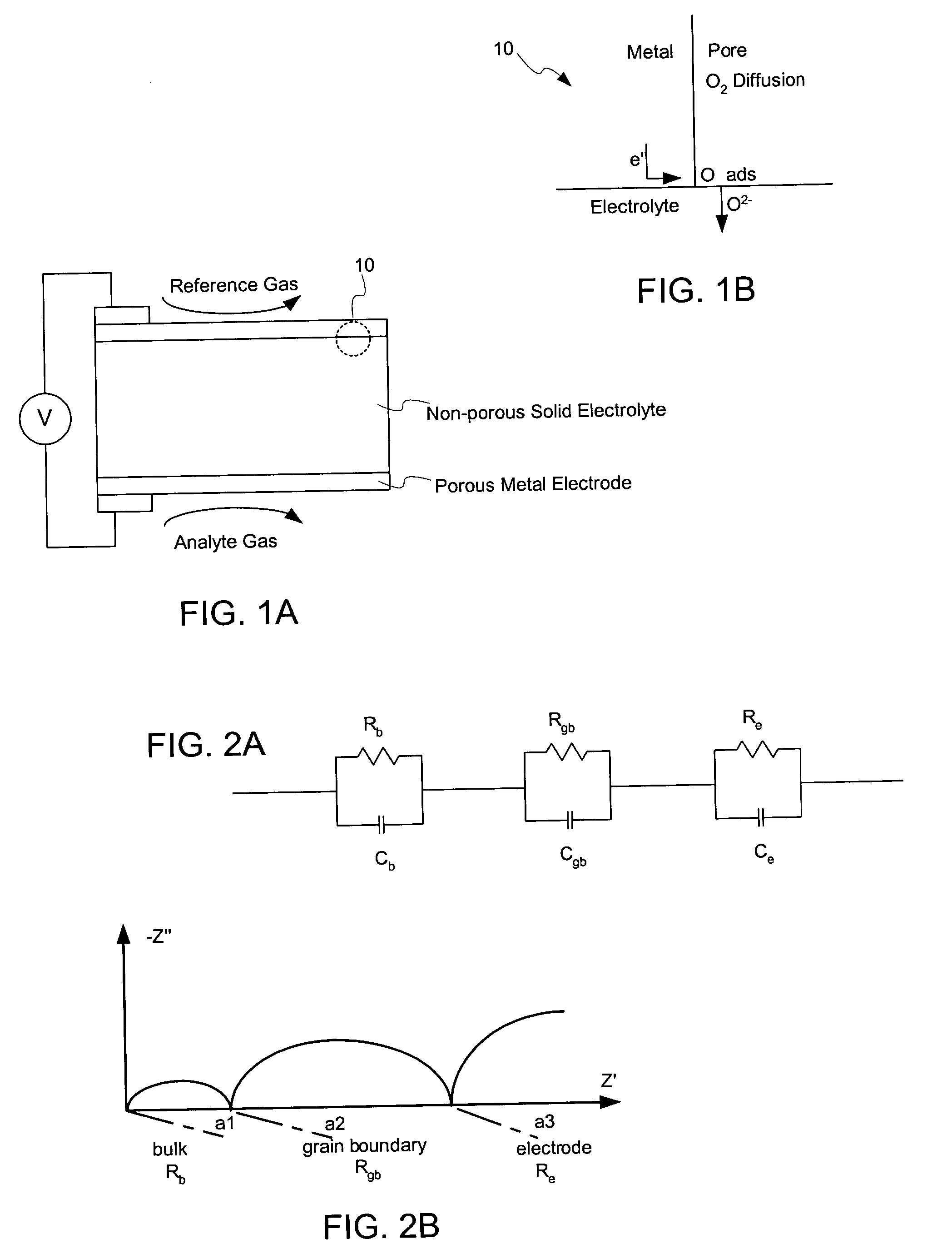
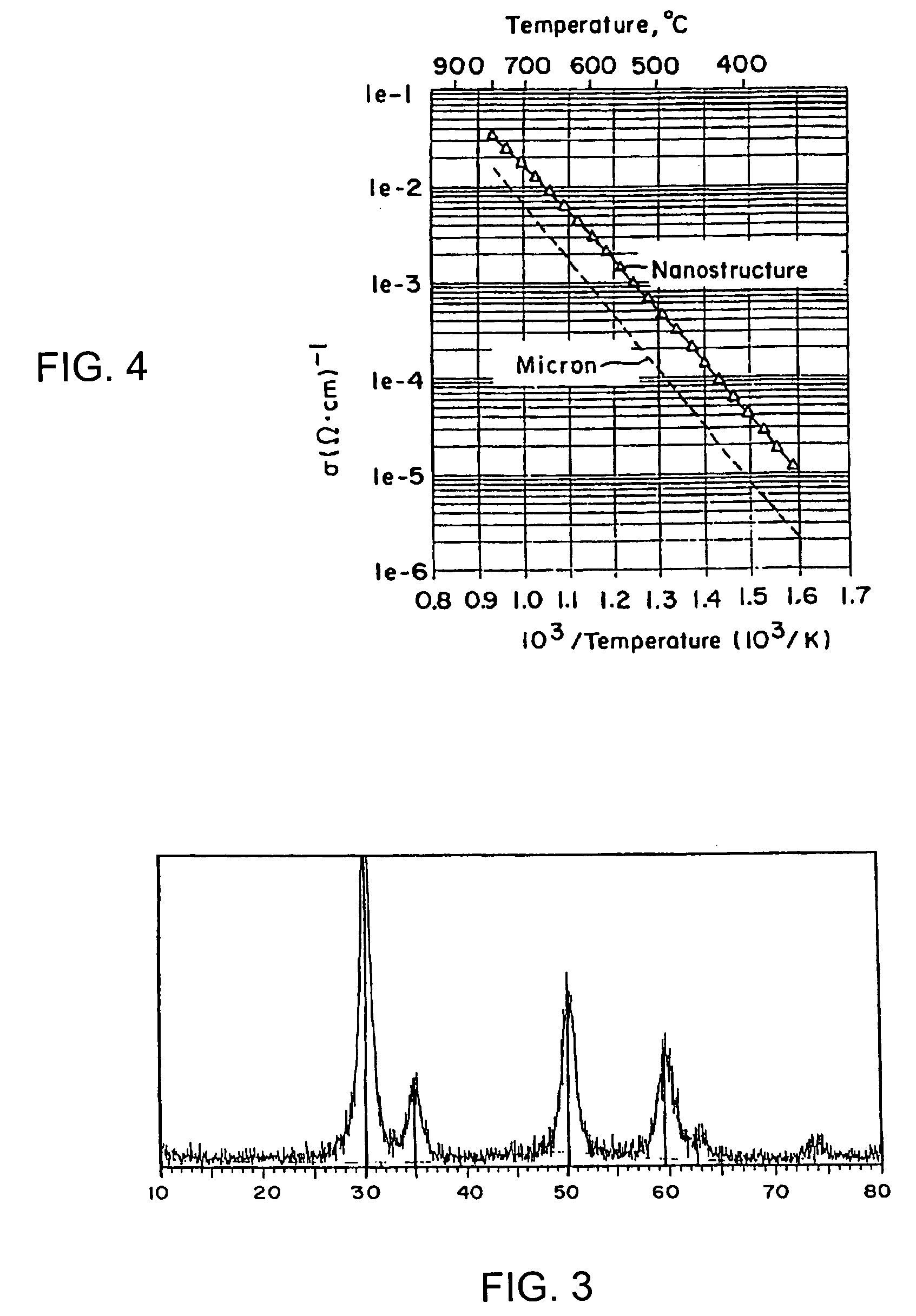
Patents
Literature
1305results about "Magnesia" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Niobium suboxide powder
ActiveUS20050013765A1High currentReduce residual currentOxide/hydroxide preparationLiquid electrolytic capacitorsCapacitorTungsten
A niobium suboxide powder comprising 100 to 600 ppm of magnesium is described. The niobium suboxide powder may (alternatively or in addition to 100 to 600 ppm of magnesium) further include 50 to 400 ppm of molybdenum and / or tungsten. The niobium suboxide powder is suitable for the production of: capacitors having an insulator layer of niobium pentoxide; capacitor anodes produced from the niobium suboxide powder; and corresponding capacitors.
Owner:TANIOBIS GMBH
Hydrogen production from carbonaceous material
InactiveUS6790430B1Avoid the needCalcium/strontium/barium carbonatesGas turbine plantsCalcinationExothermic reaction
Owner:BOARD OF SUPERVISORS OF LOUISIANA STATE UNIV & AGRI & MECHANICAL COLLEGE +1
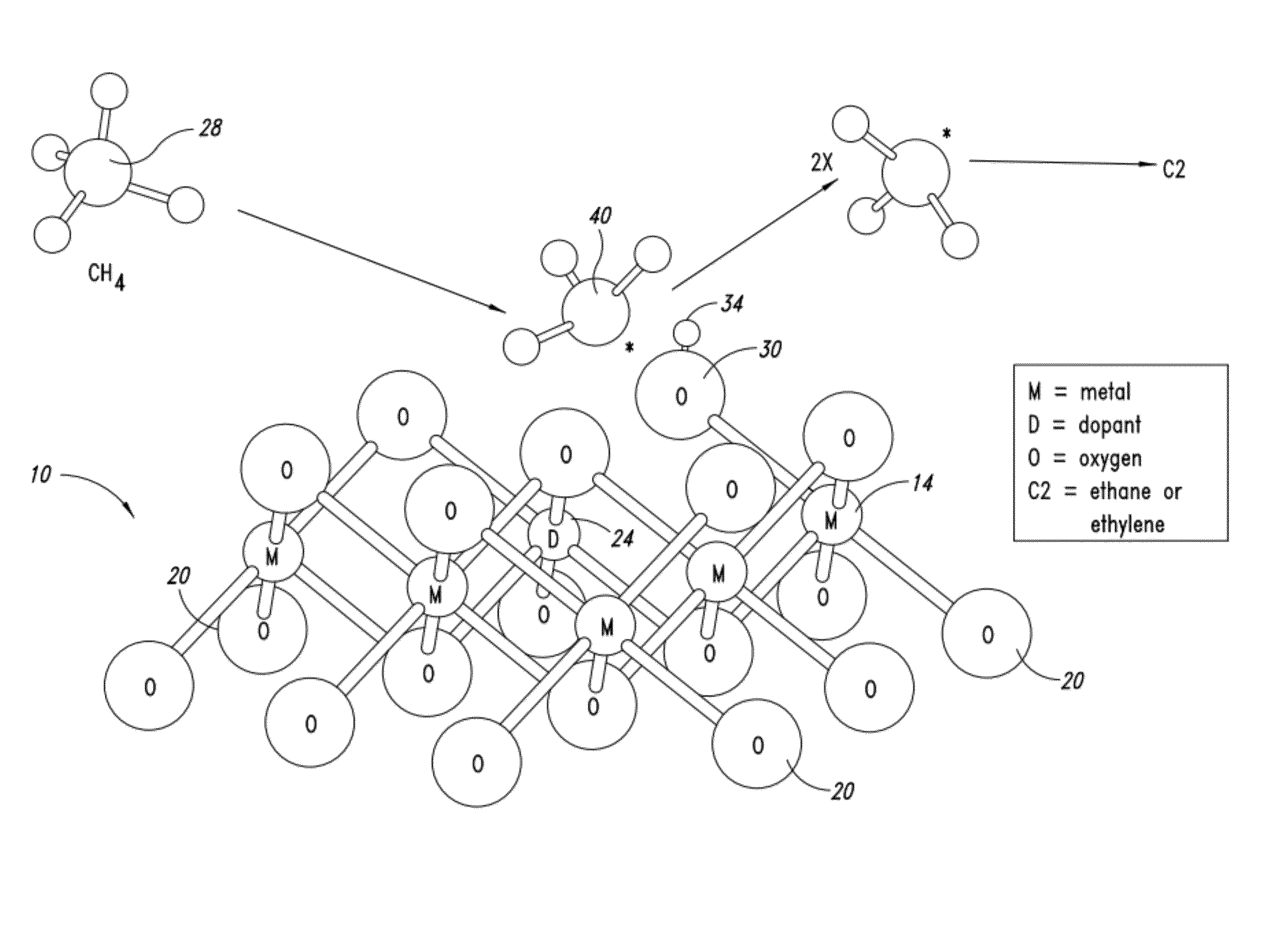
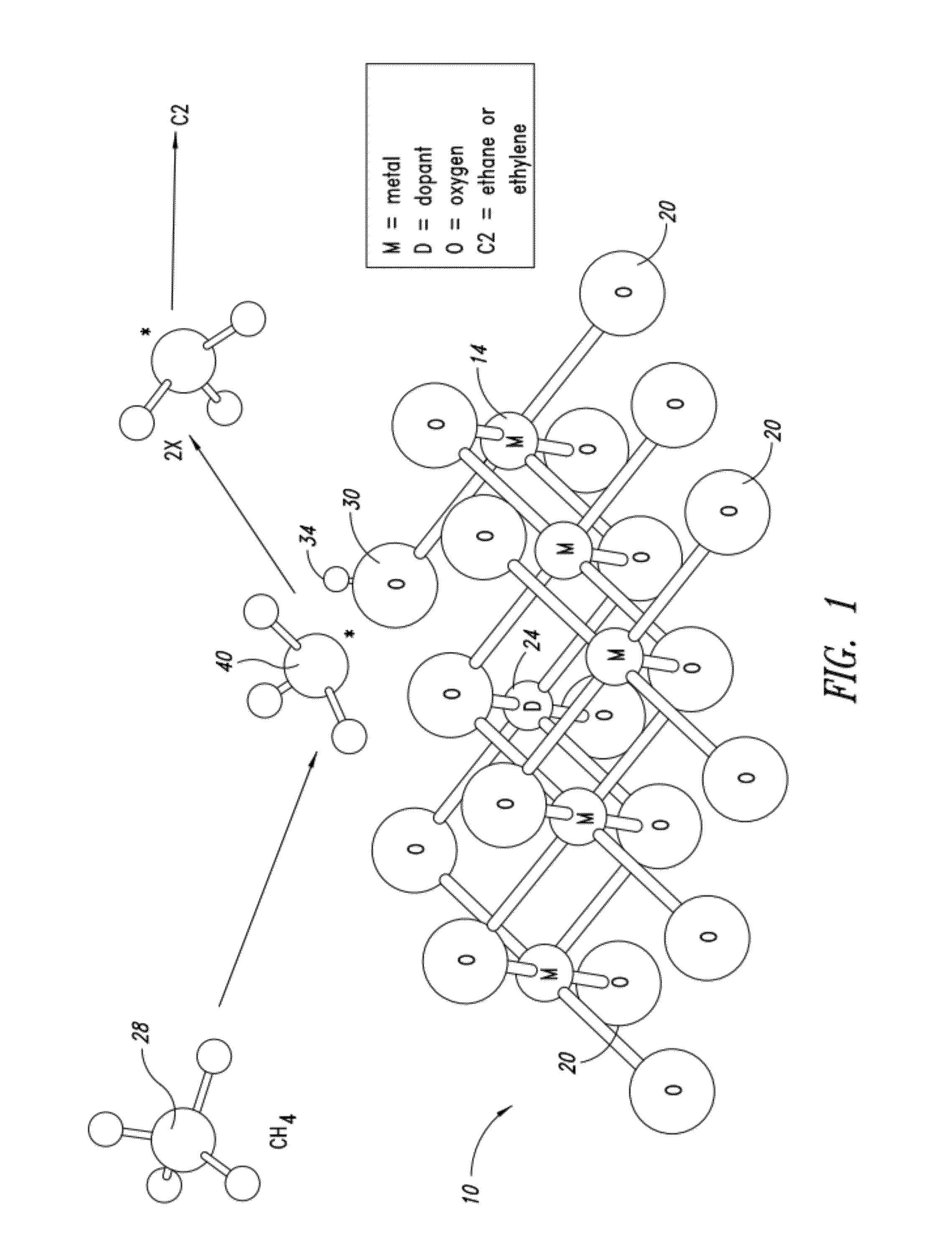
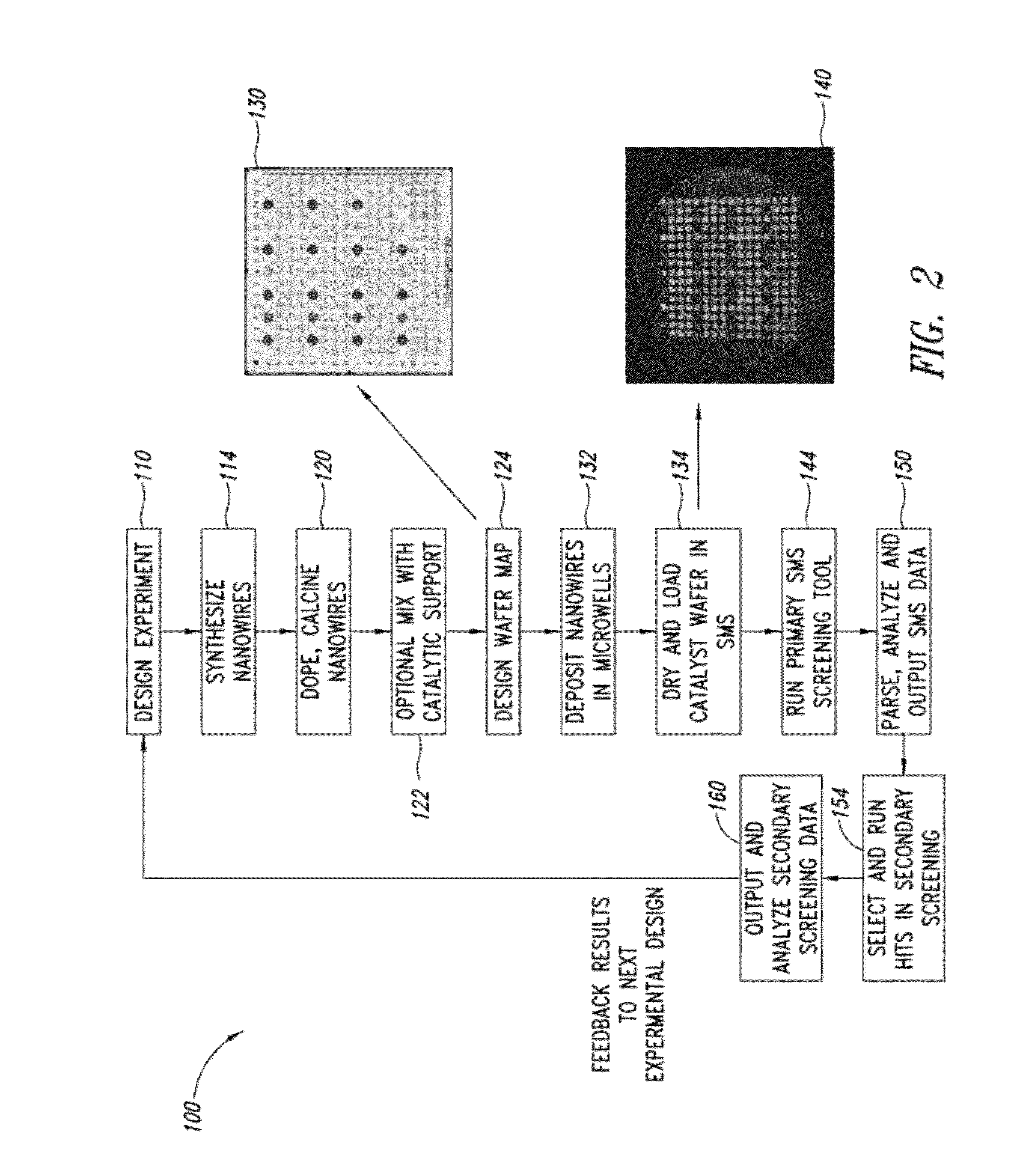
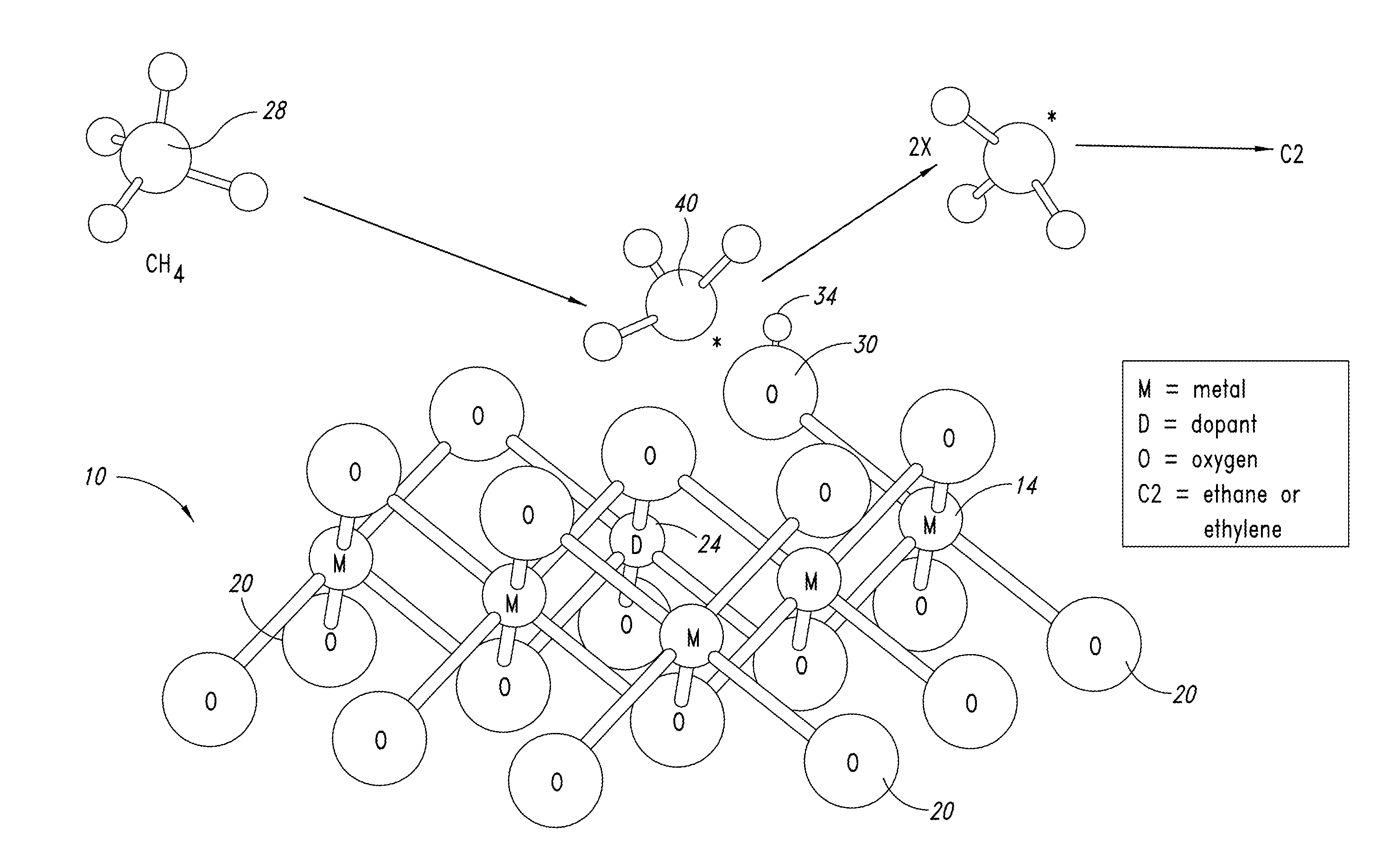
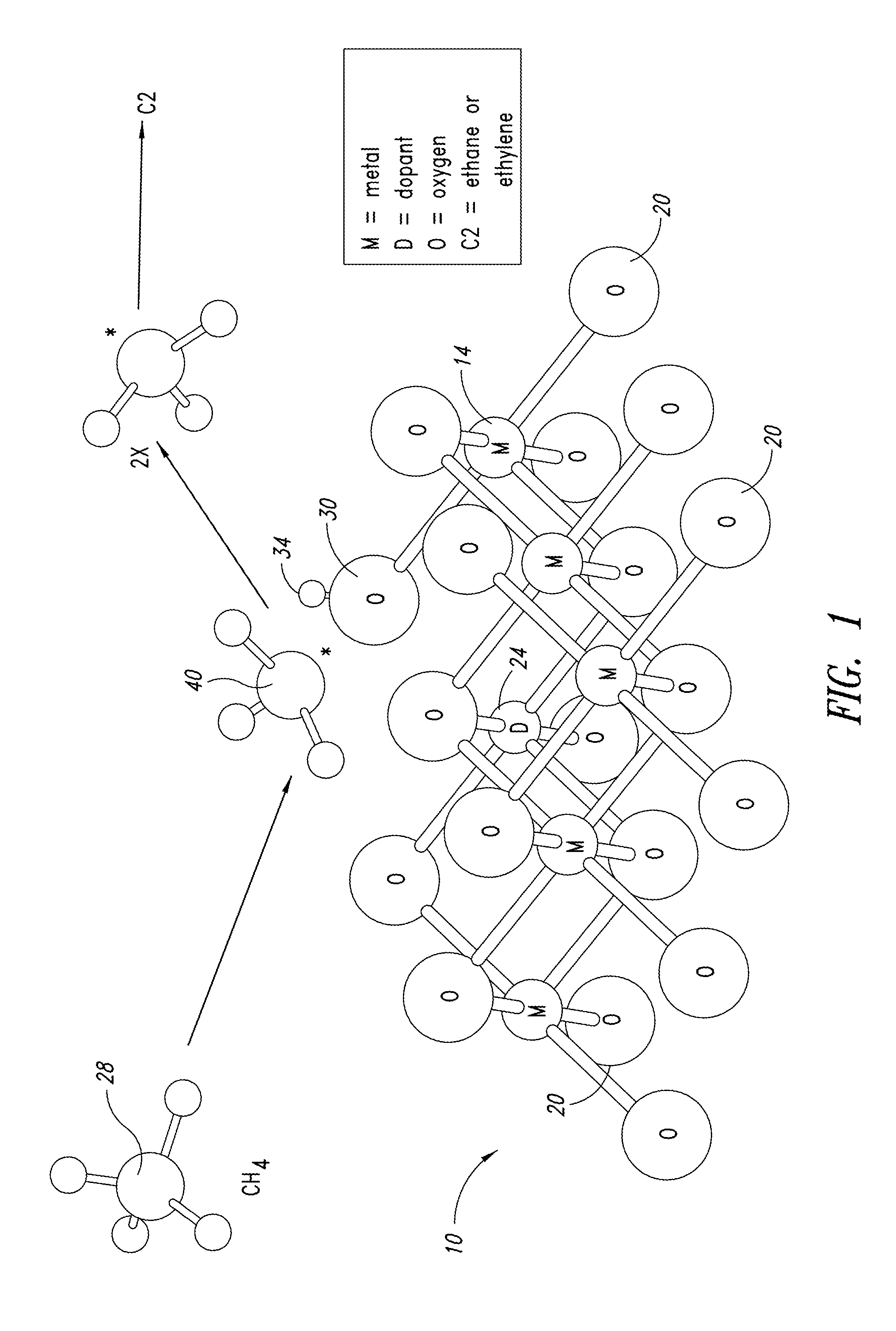
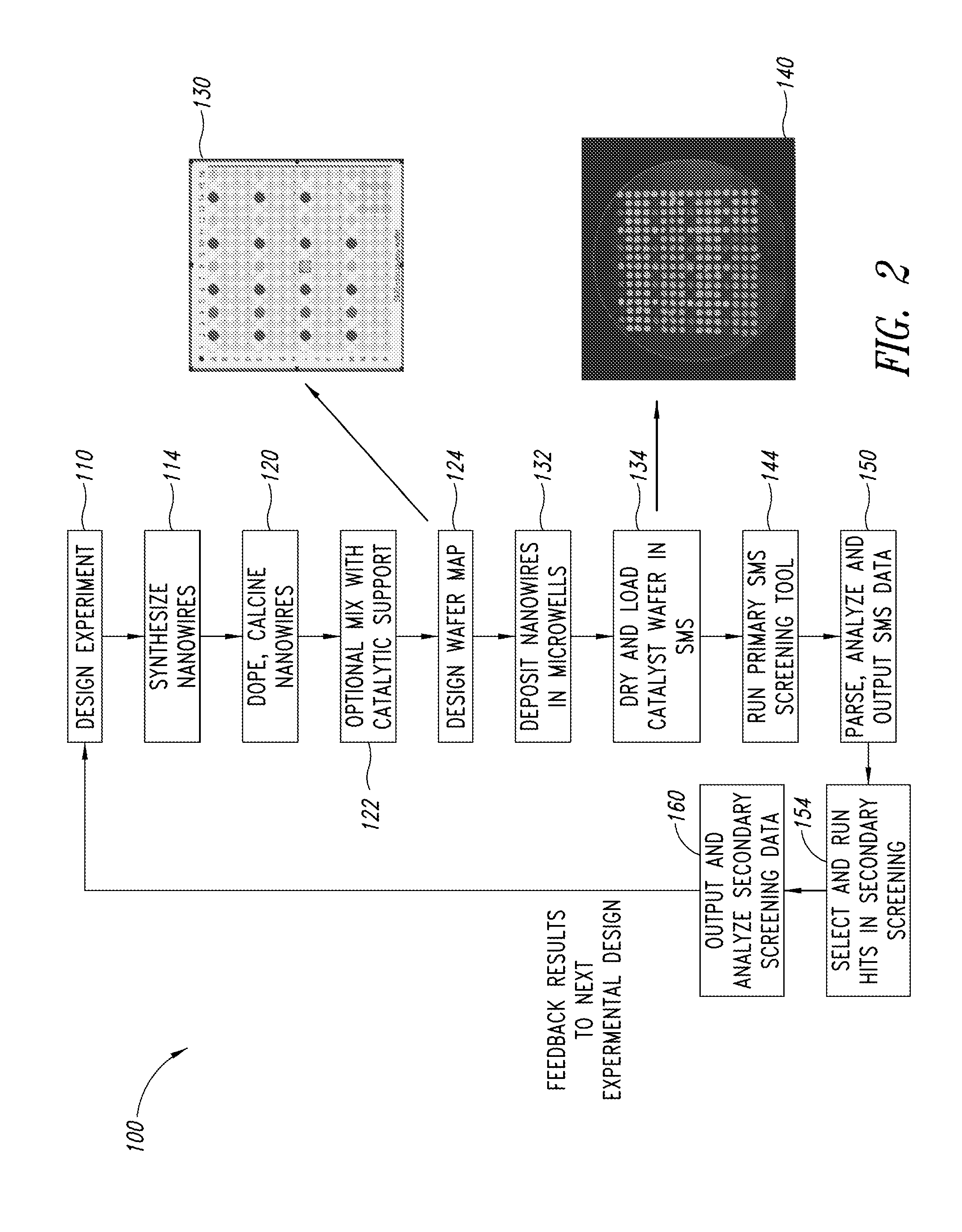
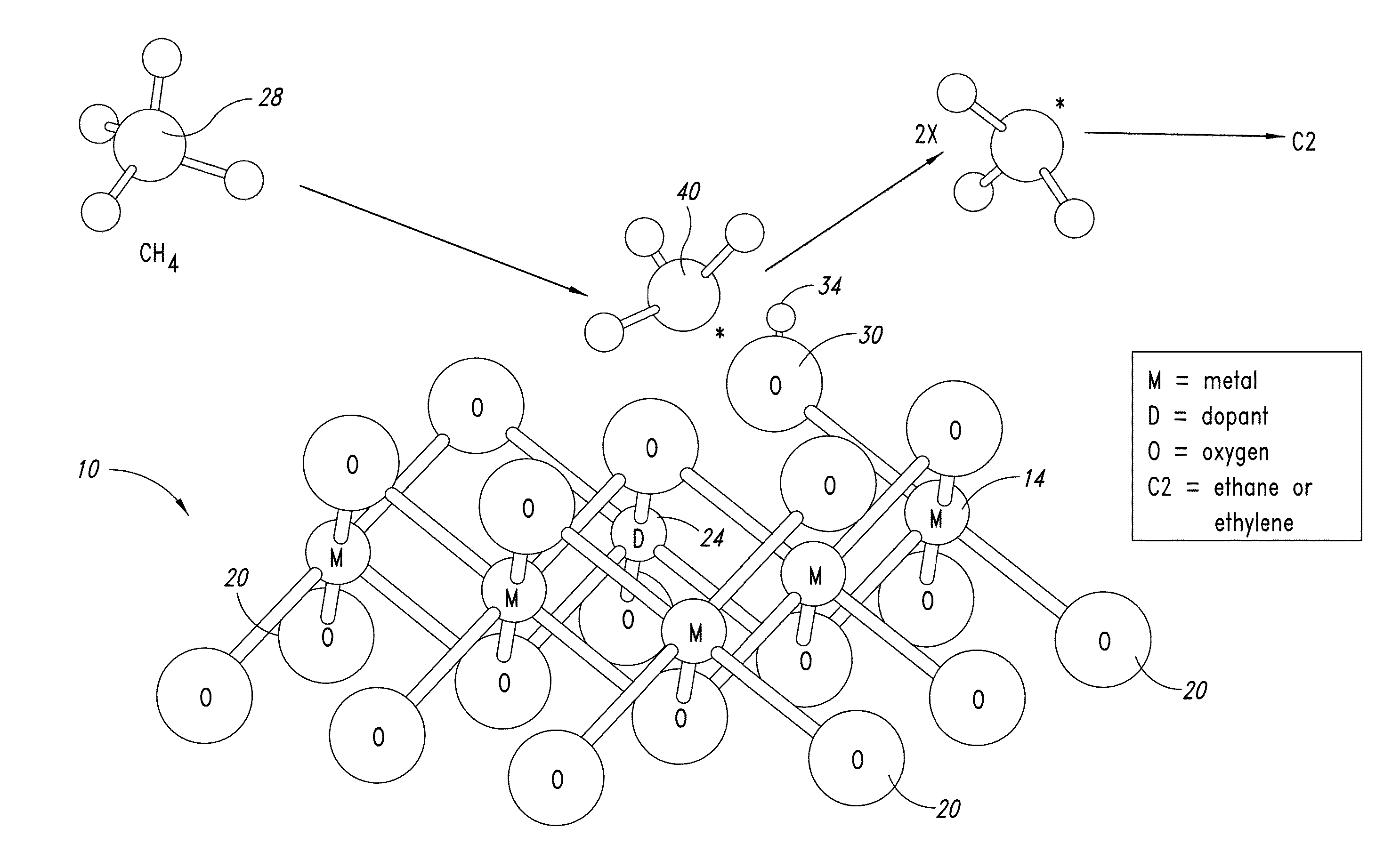
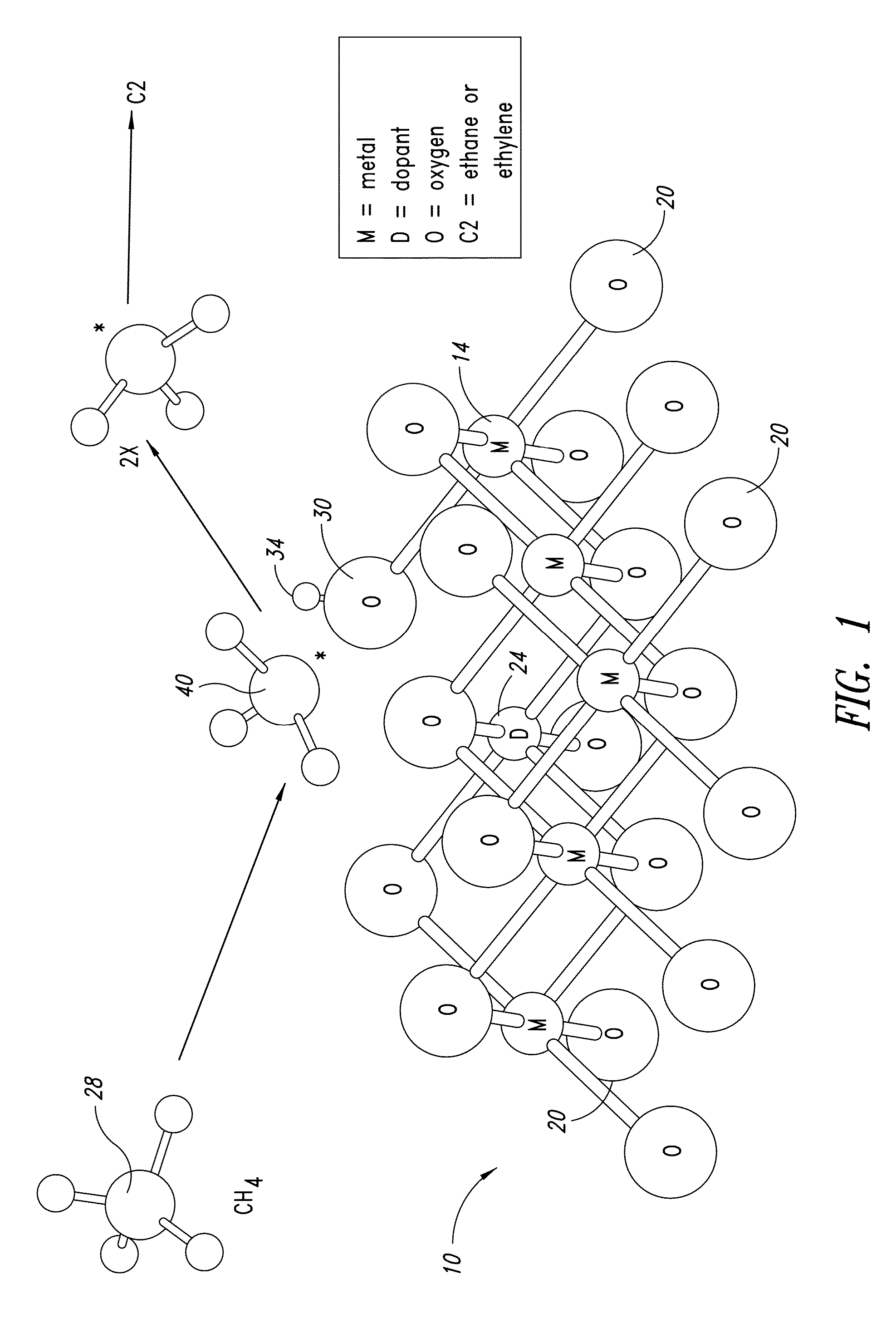
Nanowire catalysts
Nanowires useful as heterogeneous catalysts are provided. The nanowire catalysts are useful in a variety of catalytic reactions, for example, the oxidative coupling of methane to ethylene. Related methods for use and manufacture of the same are also disclosed.
Owner:SILURIA TECH INC
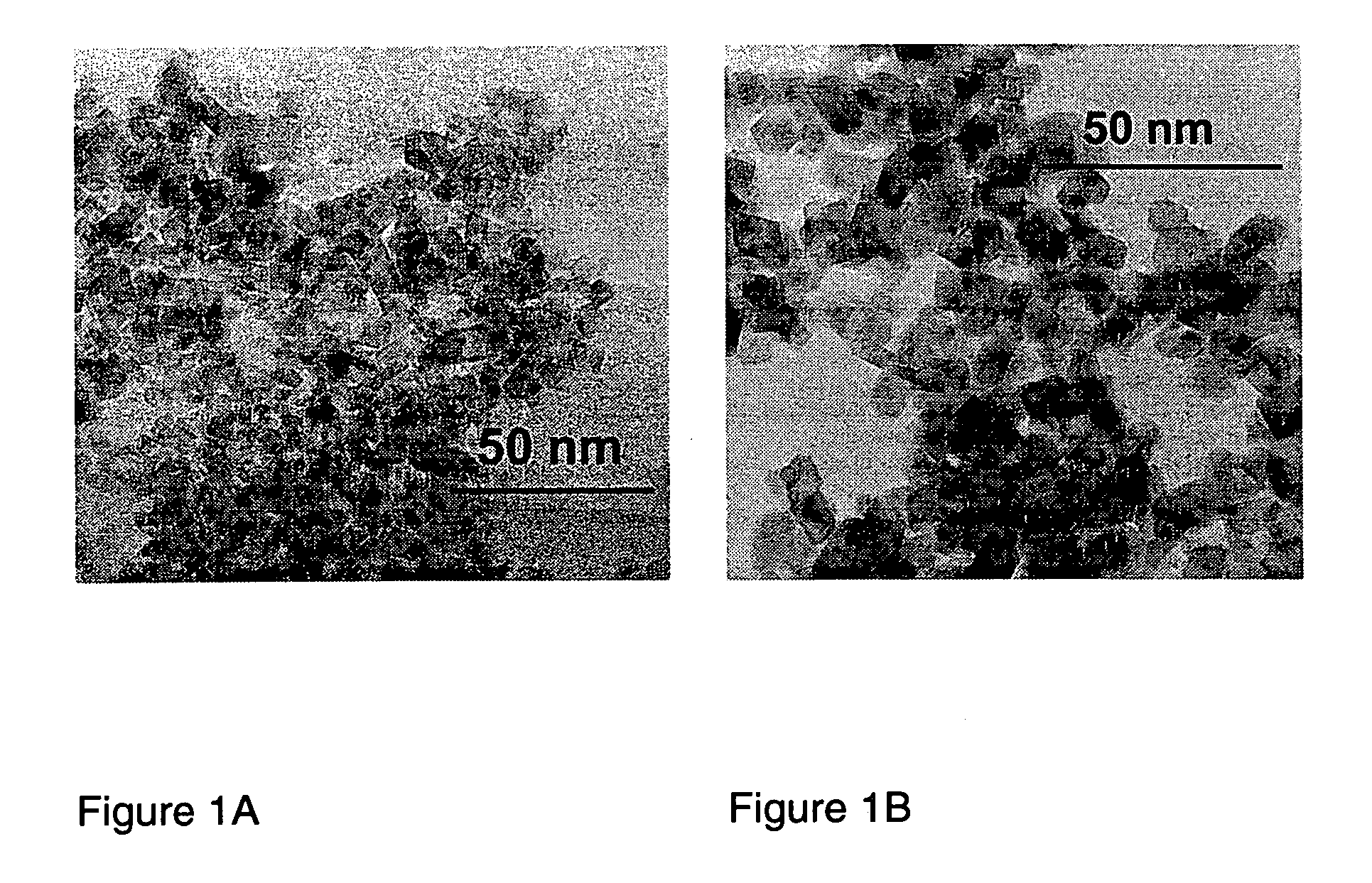
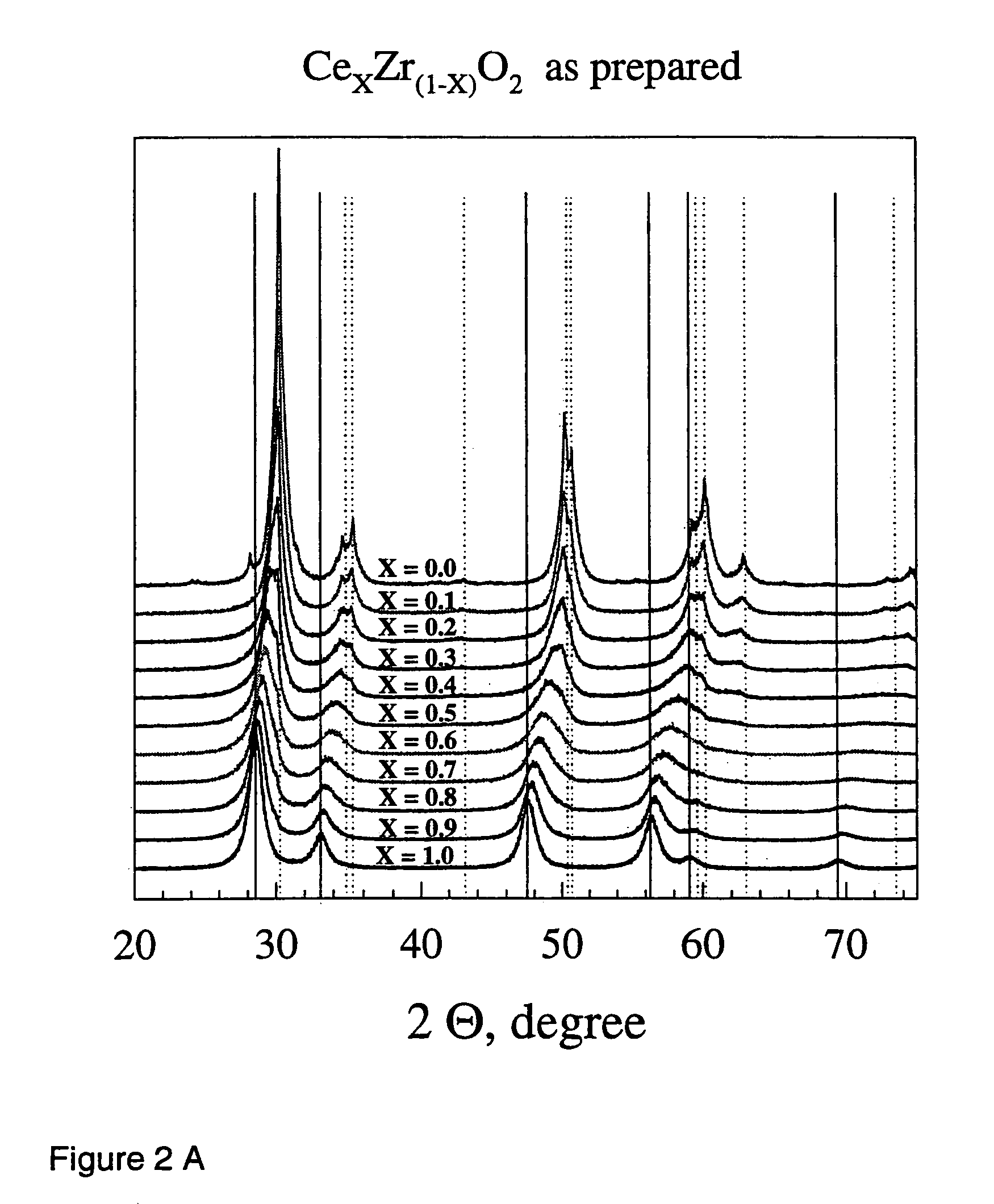
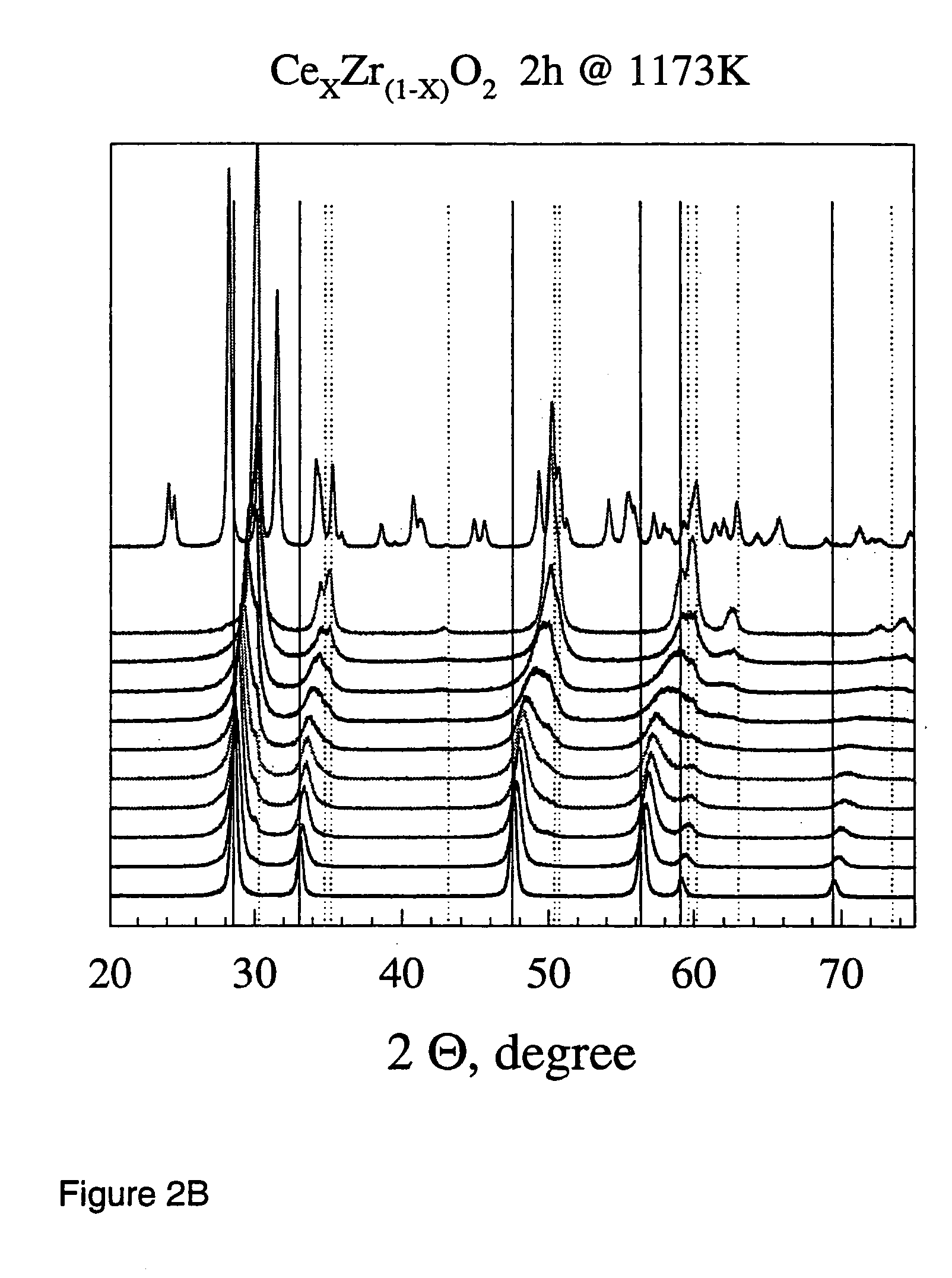
Flame made metal oxides
ActiveUS7211236B2Add featureWell mixedMaterial nanotechnologyZirconium oxidesSpray pyrolysisCarboxylic acid
Described is a method for the production of metal oxides by flame spray pyrolysis, in particular mixed metal oxides such as ceria / zirconia, and metal oxides obtainable by said method. Due to high enthalpy solvents with a high carboxylic acid content said metal oxides have improved properties. For example ceria / zirconia has excellent oxygen storage capacity at high zirconium levels up to more than 80% of whole metal content.
Owner:EIDGENOSSISCHE TECHN HOCHSCULE ZURICH
Process for the production of ultrafine powders of metal oxides
InactiveUS6503475B1Low costHigh yield rateAlkaline earth titanatesMaterial nanotechnologyDiluentBiological activation
A process for the production of ultrafine powders that includes subjecting a mixture of precursor metal compound and a non-reactant diluent phase to mechanical milling whereby the process of mechanical activation reduces the microstructure of the mixture to the form of nano-sized grains of the metal compound uniformly dispersed in the diluent phase. The process also includes heat treating the mixture of nano-sized grains of the metal compound uniformly dispersed in the diluent phase to convert the nano-sized grains of the metal compound into a metal oxide phase. The process further includes removing the diluent phase such that the nano-sized grains of the metal oxide phase are left behind in the form of an ultrafine powder.
Owner:SAMSUNG CORNING PRECISION MATERIALS CO LTD +1
Polymer nanocomposite implants with enhanced transparency and mechanical properties for administration within humans or animals
Polymer nanocomposite implants with nanofillers and additives are described. The nanofillers described can be any composition with the preferred composition being those composing barium, bismuth, cerium, dysprosium, europium, gadolinium, hafnium, indium, lanthanum, neodymium, niobium, praseodymium, strontium, tantalum, tin, tungsten, ytterbium, yttrium, zinc, and zirconium. The additives can be of any composition with the preferred form being inorganic nanopowders comprising aluminum, calcium, gallium, iron, lithium, magnesium, silicon, sodium, strontium, titanium. Such nanocomposites are particularly useful as materials for biological use in applications such as drug delivery, biomed devices, bone or dental implants.
Owner:PPG IND OHIO INC
Nanotechnology for drug delivery, contrast agents and biomedical implants
A nanocomposite structure comprising a nanostructured filler or carrier intimately mixed with a matrix, and methods of making such a structure. The nanostructured filler has a domain size sufficiently small to alter an electrical, magnetic, optical, electrochemical, chemical, thermal, biomedical, or tribological property of either filler or composite by at least 20%.
Owner:PPG IND OHIO INC
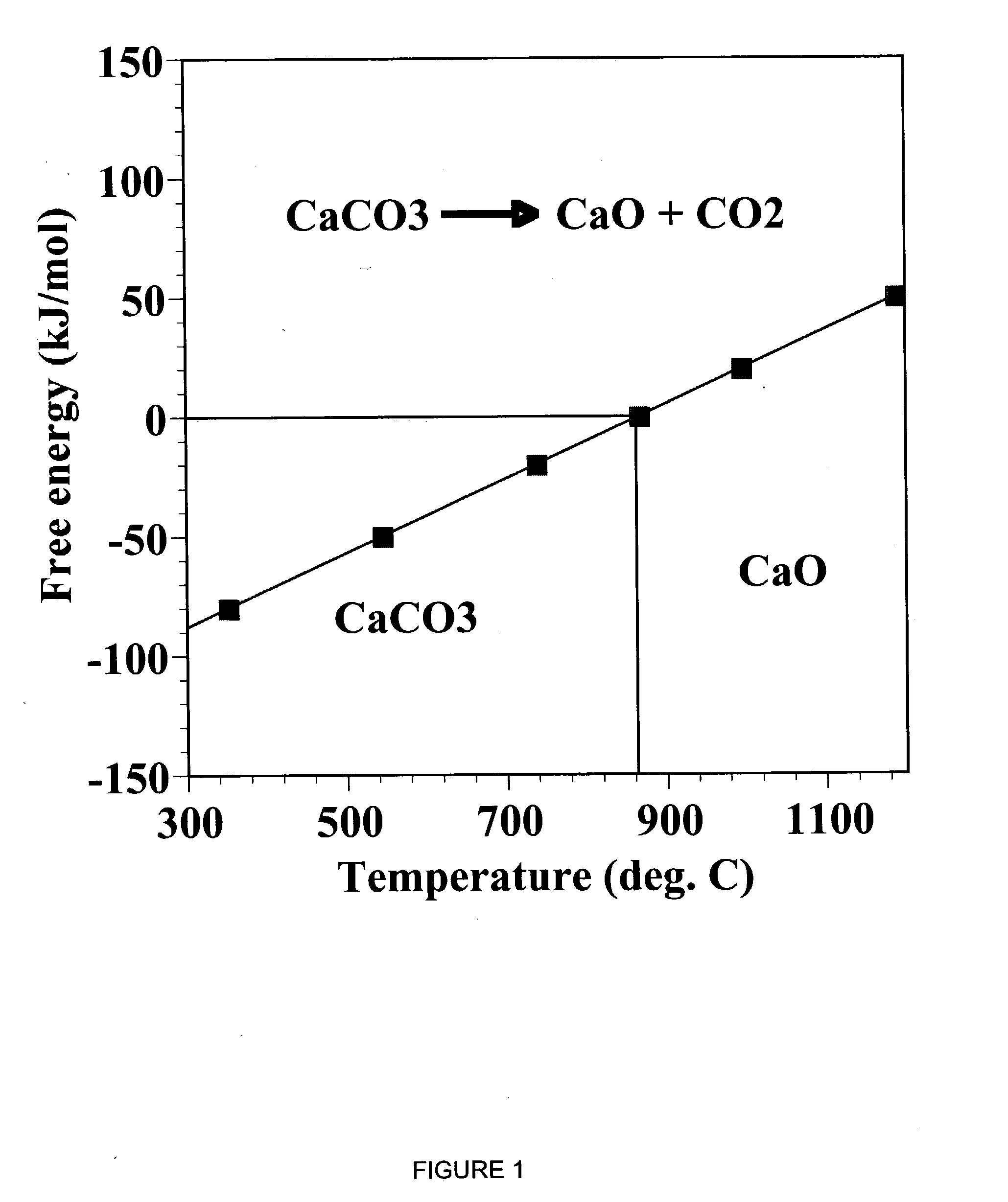
Sorbent for separation of carbon dioxide (CO2) from gas mixtures
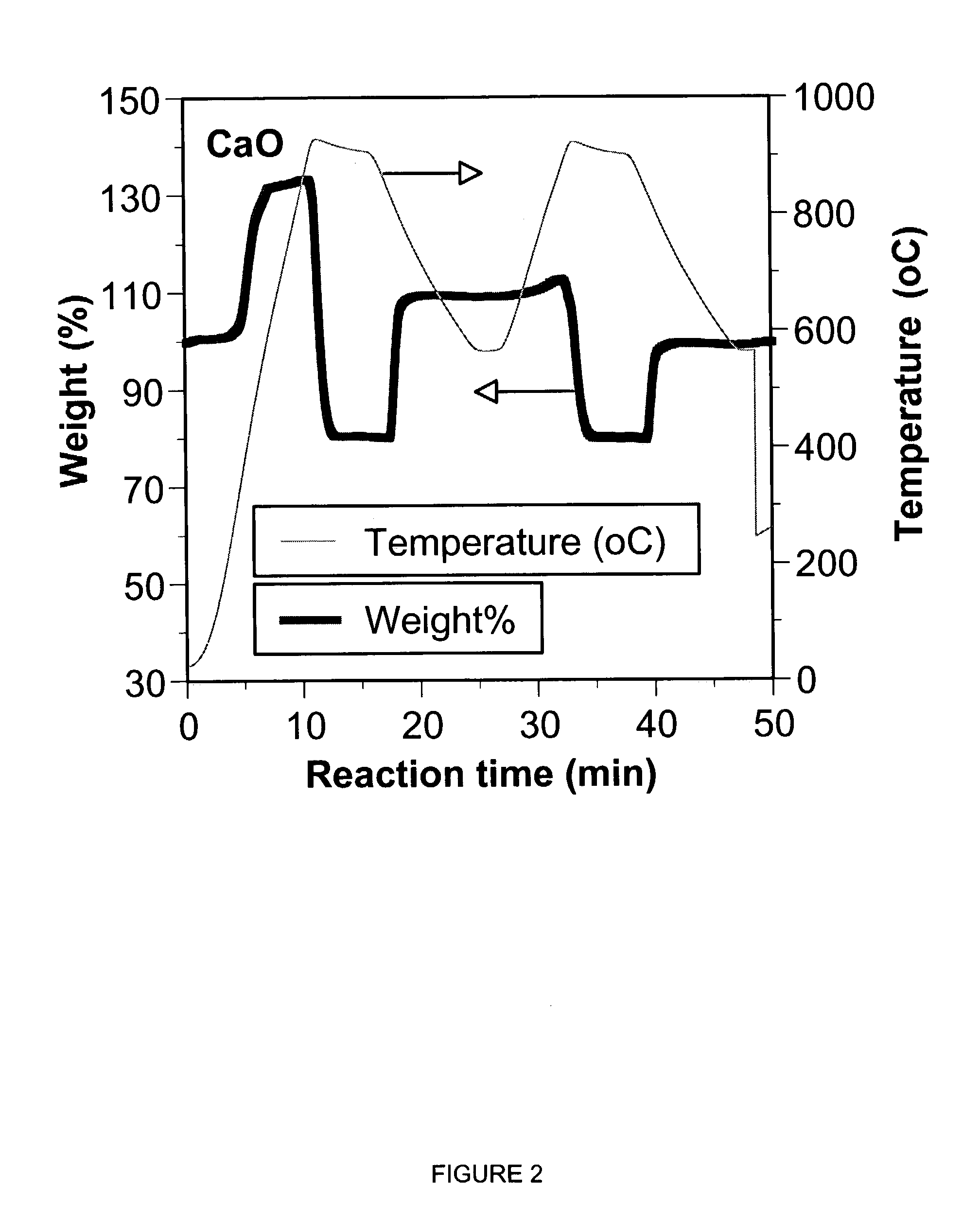
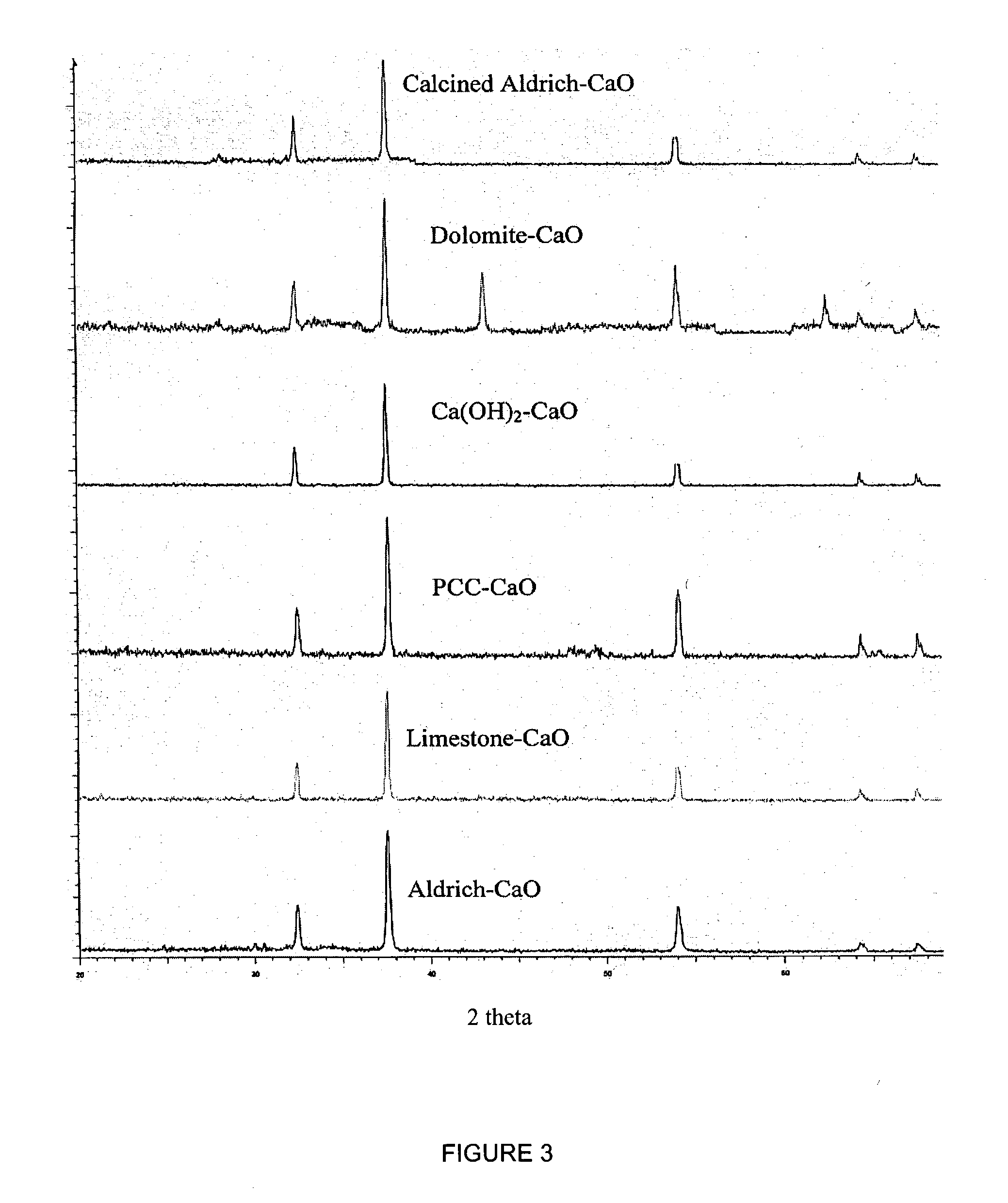
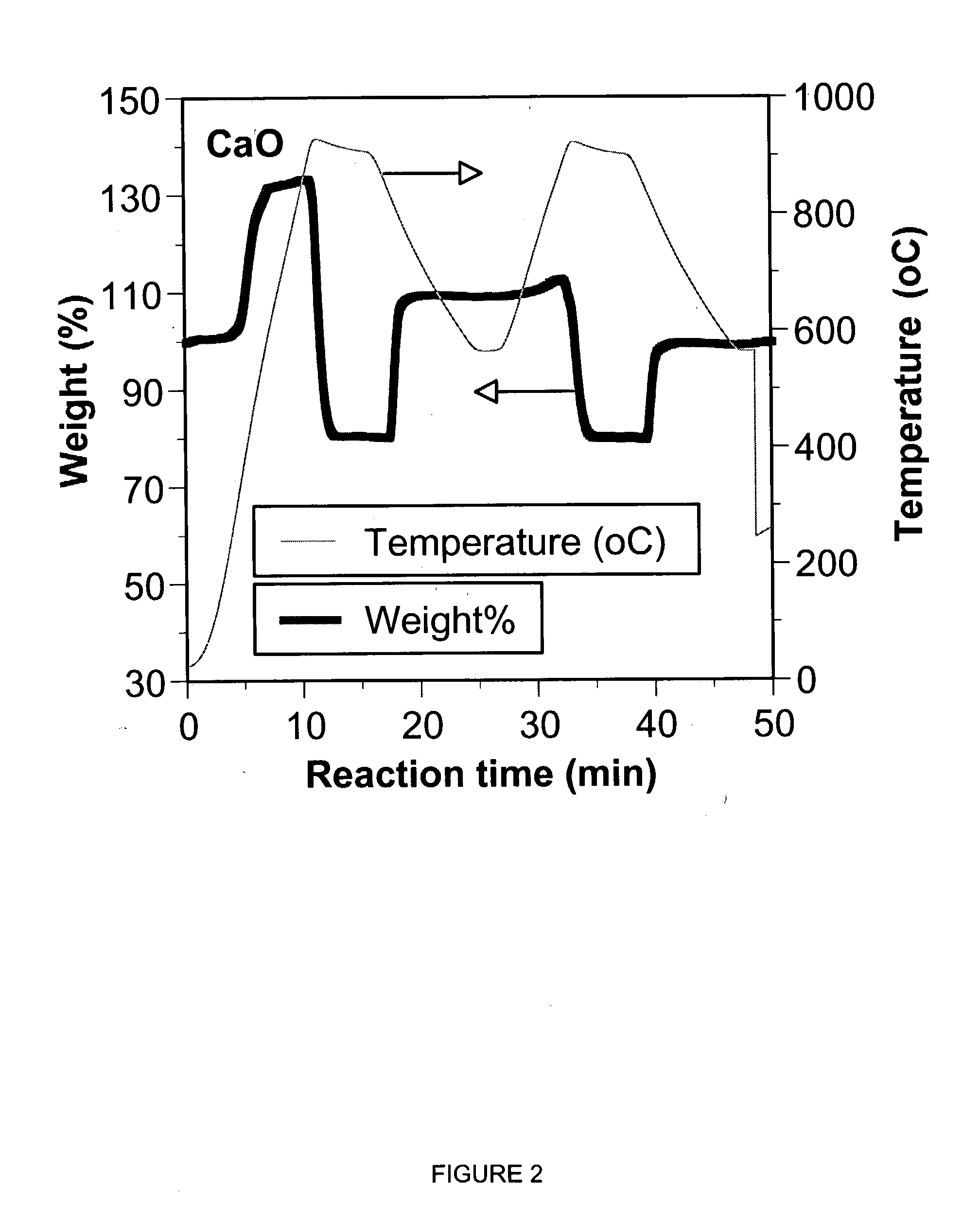
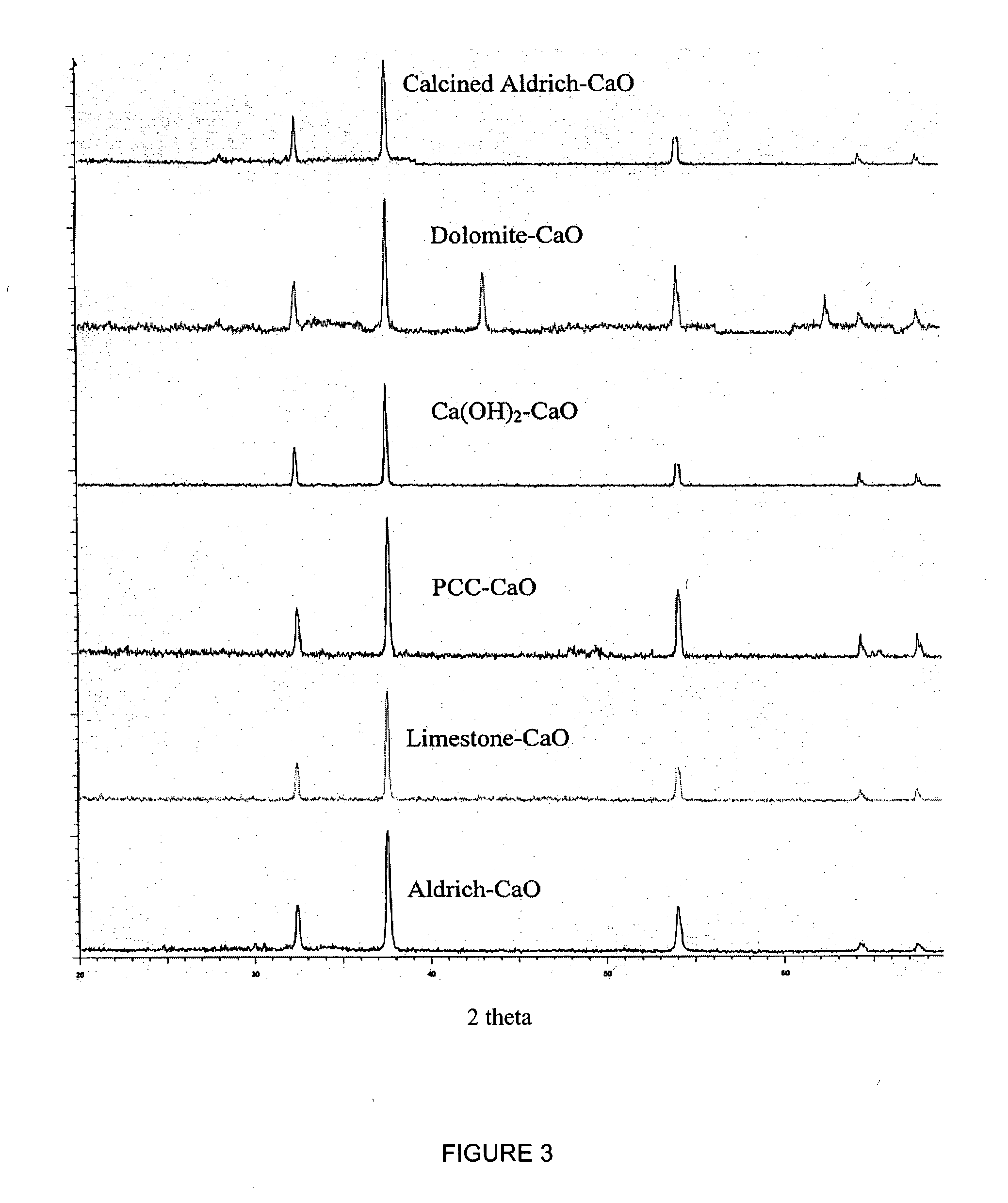
A reaction-based process has been developed for the selective removal of carbon dioxide (CO2) from a multicomponent gas mixture to provide a gaseous stream depleted in CO2 compared to the inlet CO2 concentration in the stream. The proposed process effects the separation of CO2 from a mixture of gases (such as flue gas / fuel gas) by its reaction with metal oxides (such as calcium oxide). The Calcium based Reaction Separation for CO2 (CaRS-CO2) process consists of contacting a CO2 laden gas with calcium oxide (CaO) in a reactor such that CaO captures the CO2 by the formation of calcium carbonate (CaCO3). Once “spent”, CaCO3 is regenerated by its calcination leading to the formation of fresh CaO sorbent and the evolution of a concentrated stream of CO2. The “regenerated” CaO is then recycled for the further capture of more CO2. This carbonation-calcination cycle forms the basis of the CaRS-CO2 process. This process also identifies the application of a mesoporous CaCO3 structure, developed by a process detailed elsewhere, that attains >90% conversion over multiple carbonation and calcination cycles. Lastly, thermal regeneration (calcination) under vacuum provided a better sorbent structure that maintained reproducible reactivity levels over multiple cycles.
Owner:THE OHIO STATES UNIV
Inorganic dopants, inks and related nanotechnology
InactiveUS6849109B2Facilitated DiffusionLower transition temperatureSelenium/tellurium compundsCell electrodesIndiumCerium
Ink compositions with modified properties result from using a powder size below 100 nanometers. Colored inks are illustrated. Nanoscale coated, uncoated, whisker inorganic fillers are included. The pigment nanopowders taught comprise one or more elements from the group actinium, aluminum, antimony, arsenic, barium, beryllium, bismuth, cadmuim, calcium, cerium, cesium, chalcogenide, cobalt, copper, dysprosium, erbium, europium, gadolinium, gallium, gold, hafnium, hydrogen, indium, iridium, iron, lanthanum, lithium, magnesium, manganese, mendelevium, mercury, molybdenum, neodymium, neptunium, nickel, niobium, nitrogen, oxygen, osmium, palladium, platinum, potassium, praseodymium, promethium, protactinium, rhenium, rubidium, scandium, silver, sodium, strontium, tantalum, terbium, thallium, thorium, tin, titanium, tungsten, vanadium, ytterbium, yttrium, zinc, and zirconium.
Owner:PPG IND OHIO INC
Nanowire catalysts and methods for their use and preparation
ActiveUS20130165728A1Material nanotechnologyManganese oxides/hydroxidesNanowireOxidative coupling of methane
Nanowires useful as heterogeneous catalysts are provided. The nanowire catalysts are useful in a variety of catalytic reactions, for example, the oxidative coupling of methane to C2 hydrocarbons. Related methods for use and manufacture of the same are also disclosed.
Owner:LUMMUS TECH LLC
Polymer templated nanowire catalysts
InactiveUS20130158322A1Improve drawing legibilityMaterial nanotechnologyManganese oxides/hydroxidesNanowirePolymer science
Nanowires useful as heterogeneous catalysts are provided. The nanowire catalysts are prepared by polymer templated methods and are useful in a variety of catalytic reactions, for example, the oxidative coupling of methane to ethane and / or ethylene. Related methods for use and manufacture of the same are also disclosed.
Owner:SILURIA TECH INC
Nanostructured powders and related nanotechnology
InactiveUS7081267B2Low costReduce operating costsNitrogen compoundsSelenium/tellurium compundsThermal energyOxygen
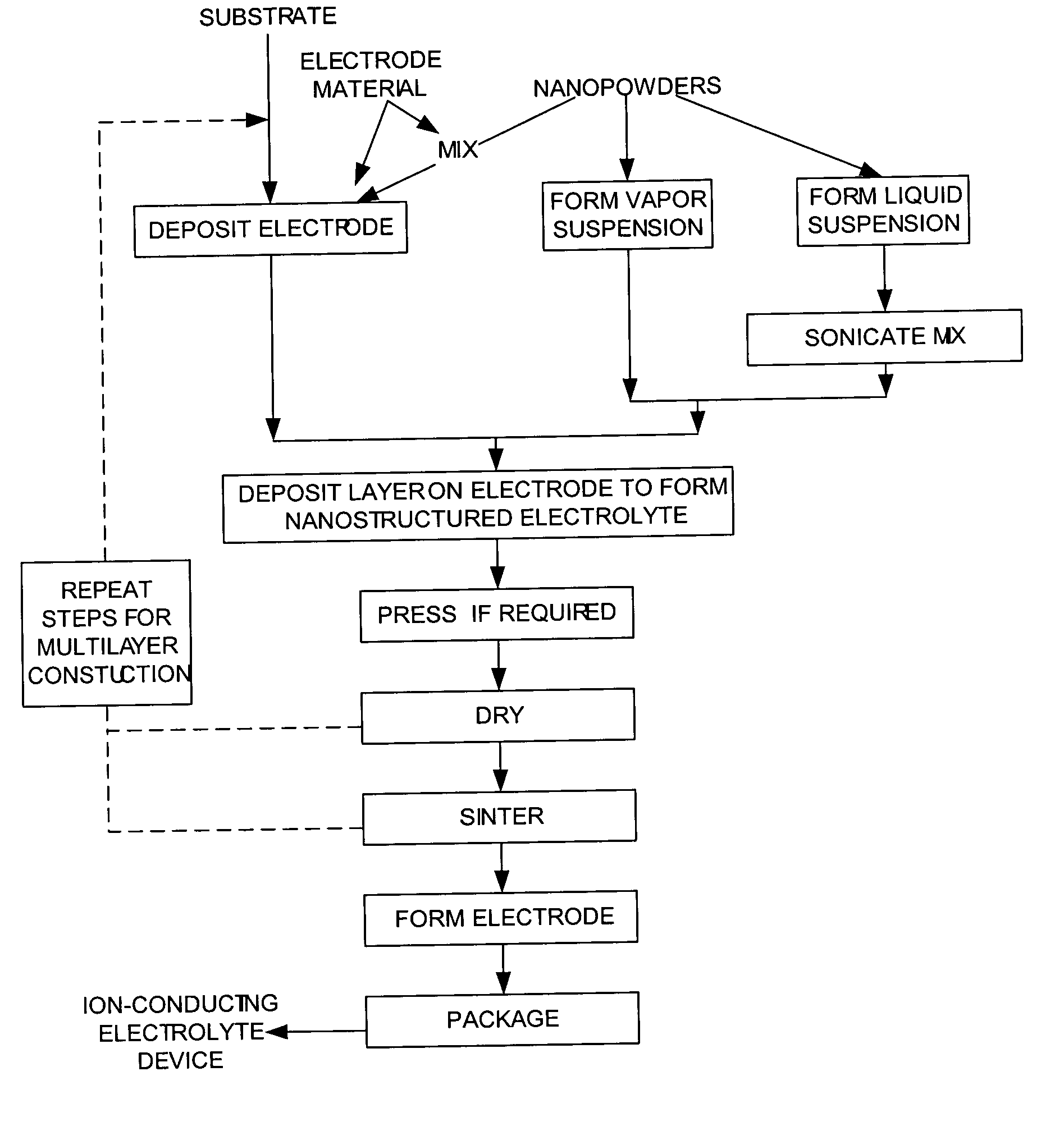
Methods to manufacture nanoscale particles comprising metals, alloys, intermetallics, ceramics are disclosed. The thermal energy is provided by plasma, internal energy, heat of reaction, microwave, electromagnetic, direct electric arc, pulsed electric arc and / or nuclear. The process is operated at some stage above 3000K and at high velocities. The invention can be utilized to prepare nanopowders for nanostructured products and devices such as ion conducting solid electrolytes for a wide range of applications, including sensors, oxygen pumps, fuel cells, batteries, electrosynthesis reactors and catalytic membranes.
Owner:PPG IND OHIO INC
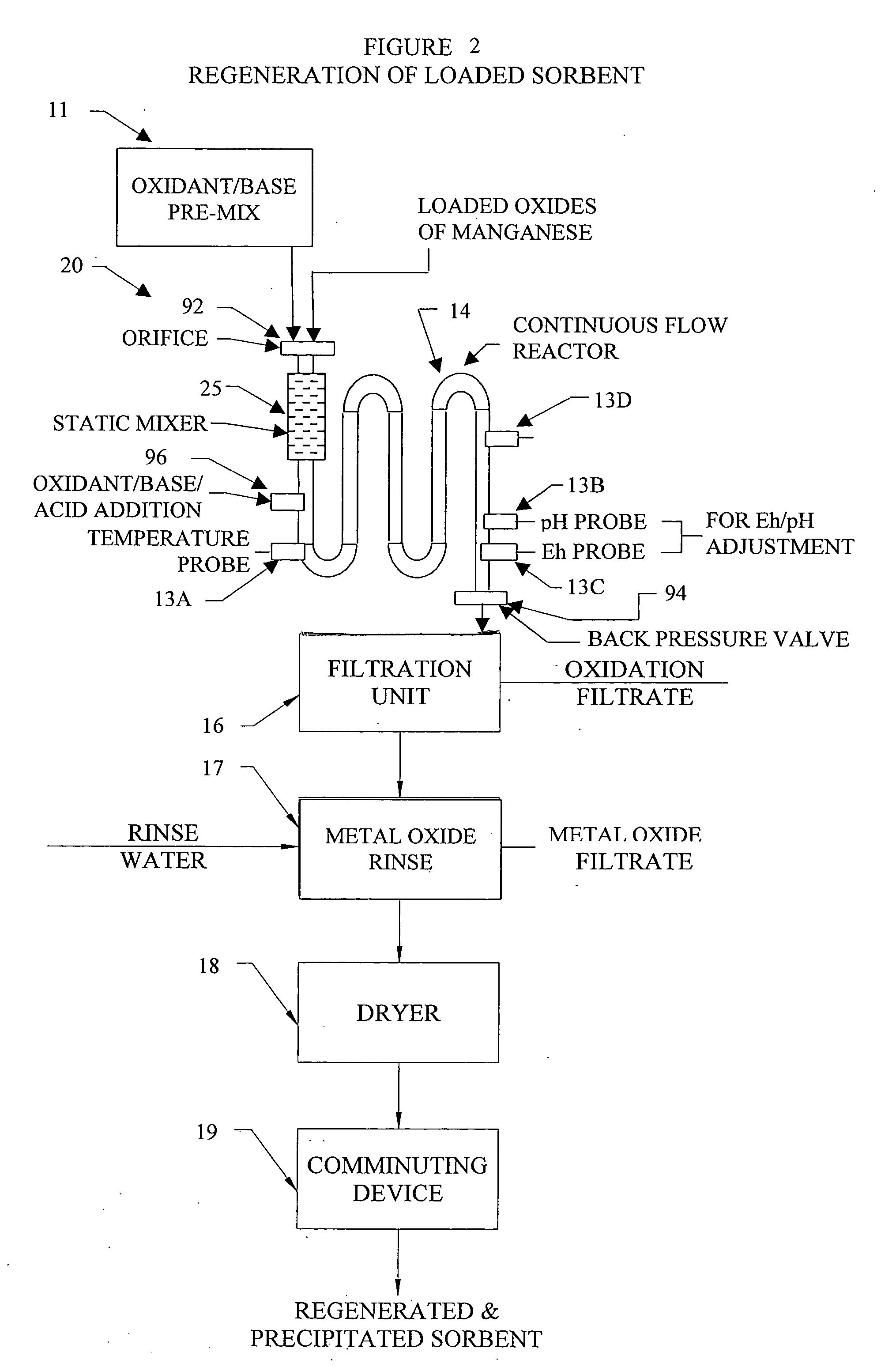
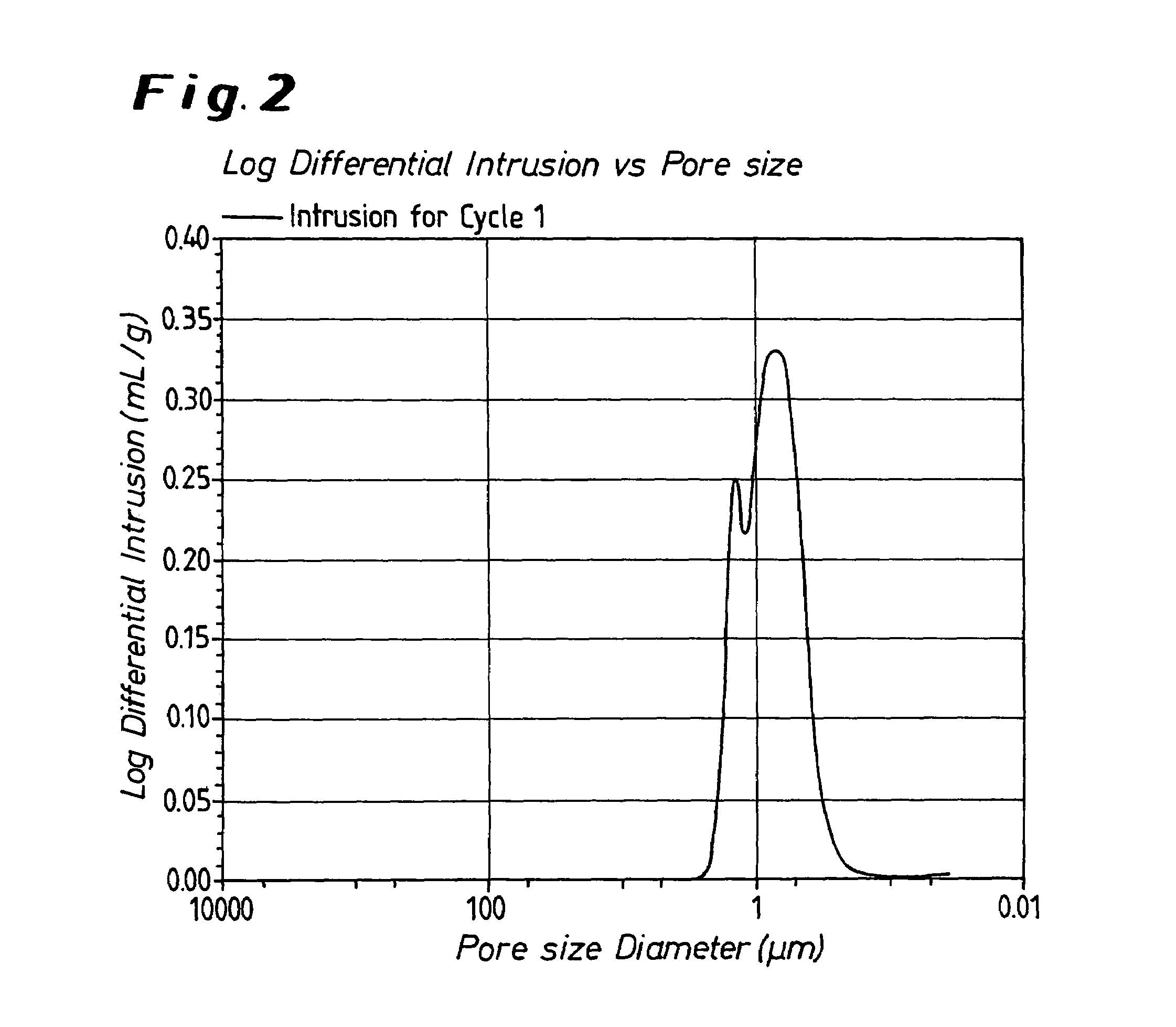
Metal oxide processing methods and systems
InactiveUS20050074380A1Move quicklyIncrease load capacityCombination devicesTemperatue controlIndustrial gasBatch processing
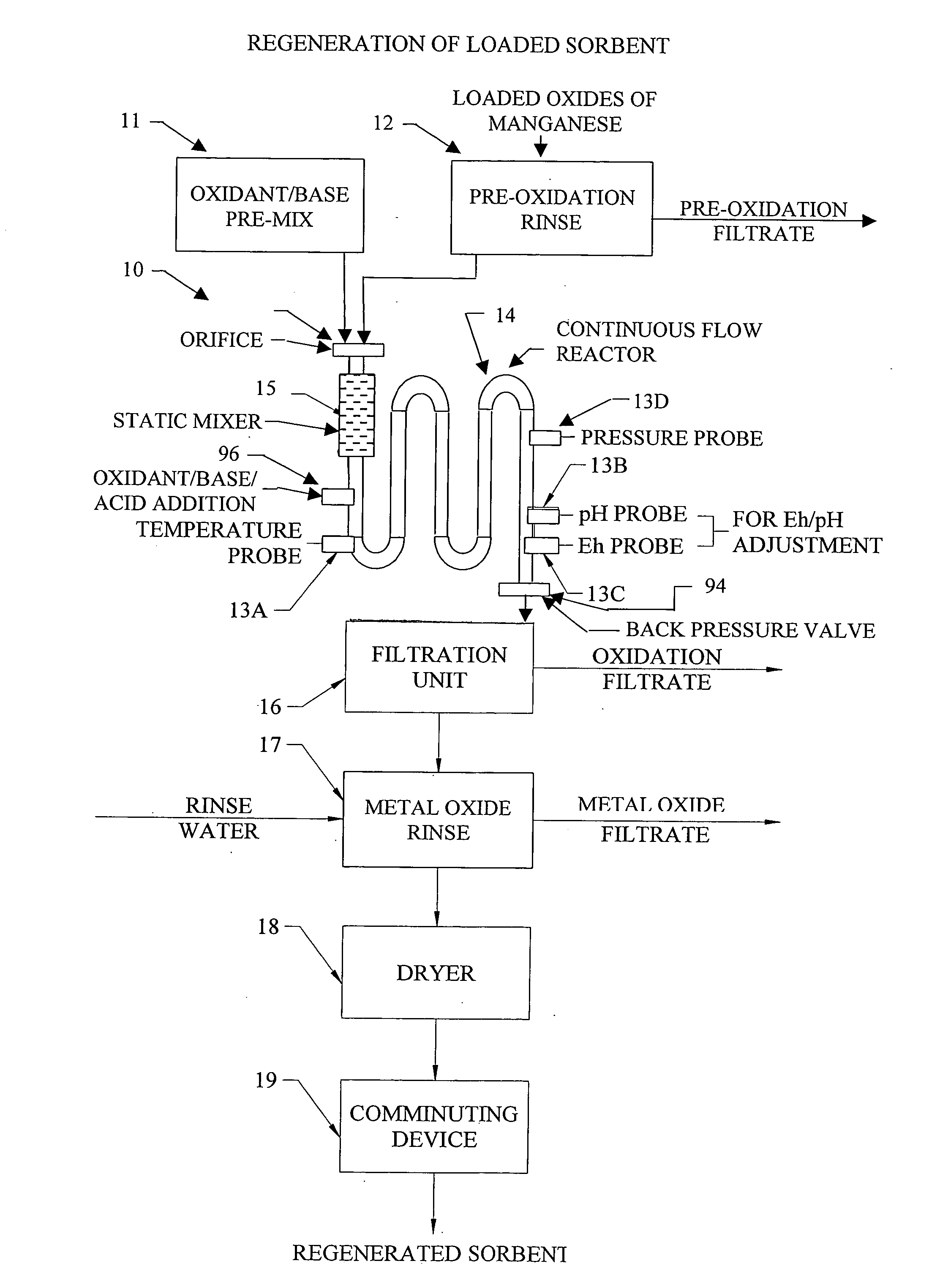
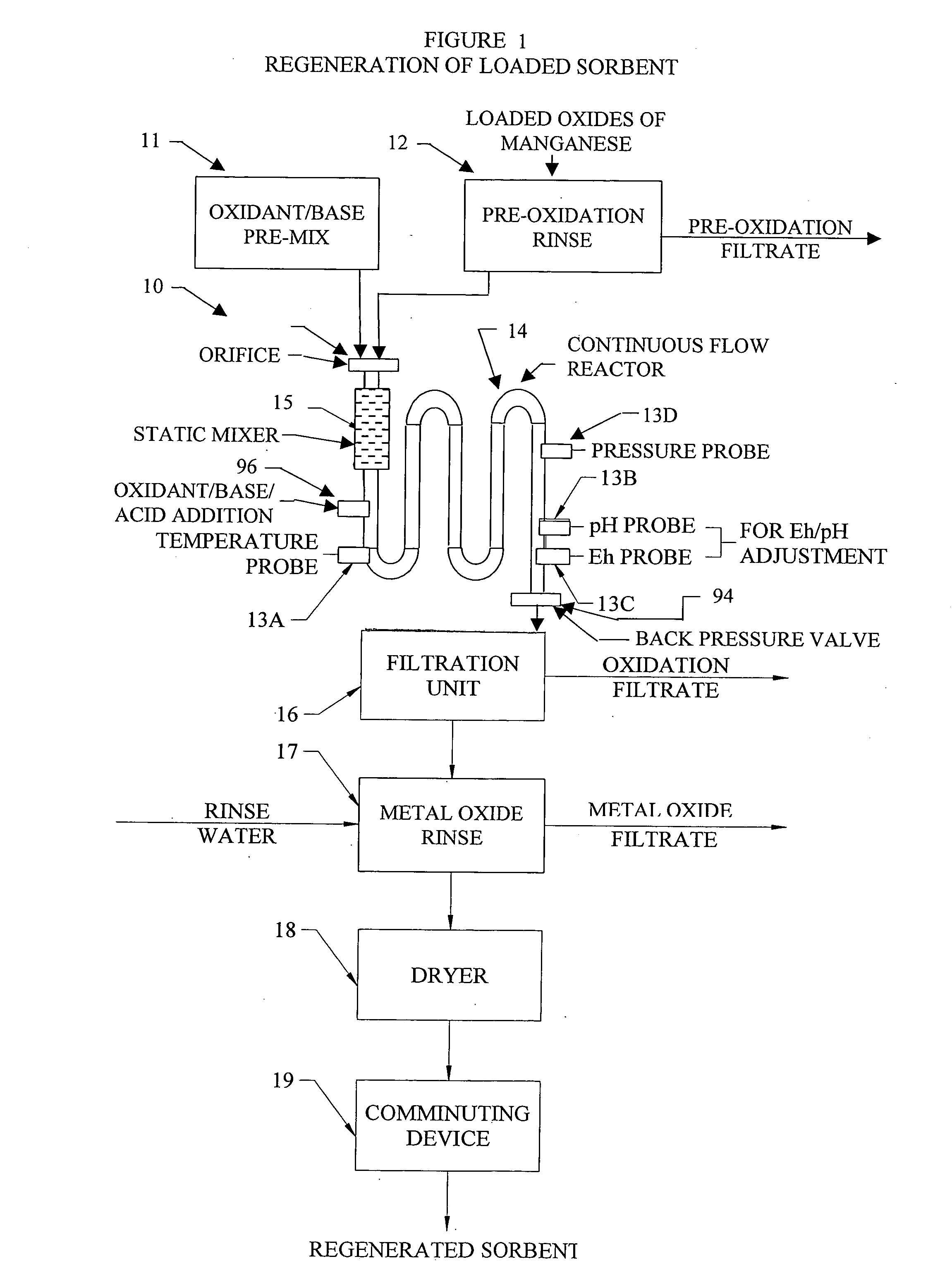
Methods and systems for processing metal oxides from metal containing solutions. Metal containing solutions are mixed with heated aqueous oxidizing solutions and processed in a continuous process reactor or batch processing system. Combinations of temperature, pressure, molarity, Eh value, and pH value of the mixed solution are monitored and adjusted so as to maintain solution conditions within a desired stability area during processing. This results in metal oxides having high or increased pollutant loading capacities and / or oxidation states. These metal oxides may be processed according to the invention to produce co-precipitated oxides of two or more metals, metal oxides incorporating foreign cations, metal oxides precipitated on active and inactive substrates, or combinations of any or all of these forms. Metal oxides thus produced are, amongst other uses; suitable for use as a sorbent for capturing or removing target pollutants from industrial gas streams or drinking water or aqueous streams or for personal protective respirators.
Owner:ENVIROSCRUB TECH CORP
Niobium suboxide powder
ActiveUS7381396B2Reduce residual currentReduce volatilityOxide/hydroxide preparationLiquid electrolytic capacitorsTungstenSuboxide
A niobium suboxide powder comprising 100 to 600 ppm of magnesium is described. The niobium suboxide powder may (alternatively or in addition to 100 to 600 ppm of magnesium) further include 50 to 400 ppm of molybdenum and / or tungsten. The niobium suboxide powder is suitable for the production of: capacitors having an insulator layer of niobium pentoxide; capacitor anodes produced from the niobium suboxide powder; and corresponding capacitors.
Owner:TANIOBIS GMBH
Recovery of common salt and marine chemicals from brine
InactiveUS6776972B2High purityLow costGeneral water supply conservationSeawater treatmentSaline waterEvaporation
A new process for recovery of common salt, potassium chloride, concentrated magnesium chloride with enriched bromide, and high purity magnesia from brine in an integrated manner, said process comprises preparation of calcium chloride by reaction of hydrochloric acid generated in the process with limestone, desulfatation of brine with calcium chloride, production of sodium chloride of superior quality in solar pans, solar evaporation of bittern thereby producing carnallite and end bittern, processing carnallite through established processes to produce potassium chloride, recovering end bittern containing highly concentrated magnesium chloride and enriched bromide and calcination of a part of the end bittern after solidification to produce high purity magnesia and hydrochloric acid utilizable in the process.
Owner:COUNCIL OF SCI & IND RES
Separation of carbon dioxide (CO2) from gas mixtures by calcium based reaction separation (CaRS-CO2) process
A reaction-based process has been developed for the selective removal of carbon dioxide (CO2) from a multicomponent gas mixture to provide a gaseous stream depleted in CO2 compared to the inlet CO2 concentration in the stream. The proposed process effects the separation of CO2 from a mixture of gases (such as flue gas / fuel gas) by its reaction with metal oxides (such as calcium oxide). The Calcium based Reaction Separation for CO2 (CaRS-CO2) process consists of contacting a CO2 laden gas with calcium oxide (CaO) in a reactor such that CaO captures the CO2 by the formation of calcium carbonate (CaCO3). Once “spent”, CaCO3 is regenerated by its calcination leading to the formation of fresh CaO sorbent and the evolution of a concentrated stream of CO2. The “regenerated” CaO is then recycled for the further capture of more CO2. This carbonation-calcination cycle forms the basis of the CaRS-CO2 process. This process also identifies the application of a mesoporous CaCO3 structure, developed by a process detailed elsewhere, that attains >90% conversion over multiple carbonation and calcination cycles. Lastly, thermal regeneration (calcination) under vacuum provided a better sorbent structure that maintained reproducible reactivity levels over multiple cycles.
Owner:THE OHIO STATES UNIV
Process of making hydrophobic metal oxide nanoparticles
InactiveUS7081234B1Uniform coatingSulfur compoundsCopper oxides/halidesMetal oxide nanoparticlesTransport layer
A process of treating metal oxide nanoparticles that includes mixing metal oxide nanoparticles, a solvent, and a surface treatment agent that is preferably a silane or siloxane is described. The treated metal oxide nanoparticles are rendered hydrophobic by the surface treatment agent being surface attached thereto, and are preferably dispersed in a hydrophobic aromatic polymer binder of a charge transport layer of a photoreceptor, whereby π—π interactions can be formed between the organic moieties on the surface of the nanoparticles and the aromatic components of the binder polymer to achieve a stable dispersion of the nanoparticles in the polymer that is substantially free of large sized agglomerations.
Owner:XEROX CORP
Flame-Retardant Magnesium Hydroxide Compositions and Associated Methods of Manufacture and Use
InactiveUS20070176155A1Improve resistance performanceSuitable for usePigmenting treatmentGlass/slag layered productsParticulatesPolymer resin
The invention provides a submicron magnesium hydroxide particulate composition comprising a first distribution of magnesium hydroxide particles having a D50 of no more than about 0.30 μm, a D90 of no more than about 1.5 μm, and a BET surface area of at least about 35 m2 / g, which can be used as a flame-retardant additive for synthetic polymers, optionally in combination with other flame-retardant additives such as nanoclays and larger-sized magnesium hydroxide particulate compositions. Polymeric resins comprising the submicron magnesium hydroxide particles and methods of manufacturing submicron magnesium hydroxide particles are also provided.
Owner:MARTIN MARIETTA MATERIALS
Methods of Making Binary Metal Oxide Nanostructures and Methods of Controlling Morphology of Same
ActiveUS20100278720A1Reduce crystallinityControl dimensionalityCopper oxides/halidesManganese oxides/hydroxidesPorous membraneNanostructure
The present invention includes a method of producing a crystalline metal oxide nanostructure. The method comprises providing a metal salt solution and providing a basic solution; placing a porous membrane between the metal salt solution and the basic solution, wherein metal cations of the metal salt solution and hydroxide ions of the basic solution react, thereby producing a crystalline metal oxide nanostructure.
Owner:WONG STANISLAUS S +1
Recovery of common salt and marine chemicals from brine
InactiveUS20030080066A1High purityLow costGeneral water supply conservationSeawater treatmentSaline waterEvaporation
A new process for recovery of common salt, potassium chloride, concentrated magnesium chloride with enriched bromide, and high purity magnesia from brine in an integrated manner, said process comprises preparation of calcium chloride by reaction of hydrochloric acid generated in the process with limestone, desulfatation of brine with calcium chloride, production of sodium chloride of superior quality in solar pans, solar evaporation of bittern thereby producing carnallite and end bittern, processing carnallite through established processes to produce potassium chloride, recovering end bittern containing highly concentrated magnesium chloride and enriched bromide and calcination of a part of the end bittern after solidification to produce high purity magnesia and hydrochloric acid utilizable in the process.
Owner:COUNCIL OF SCI & IND RES
Flame-retardant magnesium hydroxide compositions and associated methods of manufacture and use
InactiveUS7514489B2Improve resistance performanceSuitable for usePigmenting treatmentGlass/slag layered productsParticulatesPolymer resin
Owner:MARTIN MARIETTA MATERIALS
Magnetic Metal Oxide Biochar Composite Particles, and Their Use in Recovering Pollutants From Aqueous Solution
InactiveUS20180016162A1Cheap manufacturingEconomical to useBio-organic fraction processingWaste water treatment from animal husbandryPhosphateAgricultural runoff
Composite particles are disclosed comprising magnesium oxide, iron oxide, and biochar; and methods of making and using the composite particles. The composite particles may be used to recover solutes including phosphate, nitrate, ammonium, and organic compounds from aqueous solution, and the resulting solute-loaded particles may be used as a fertilizer to enhance plant growth. The composites be used to remove pollutants from agricultural runoff, wastewater, and surface water. The particles possess magnetic properties that enhance their recovery following solute adsorption.
Owner:BOARD OF SUPERVISORS OF LOUISIANA STATE UNIV & AGRI & MECHANICAL COLLEGE
Process for the Production of Carbon Graphenes and other Nanomaterials
InactiveUS20120068124A1Low costAvoid insufficient purityMaterial nanotechnologyNon-metal conductorsMultiple layerOxidation reduction
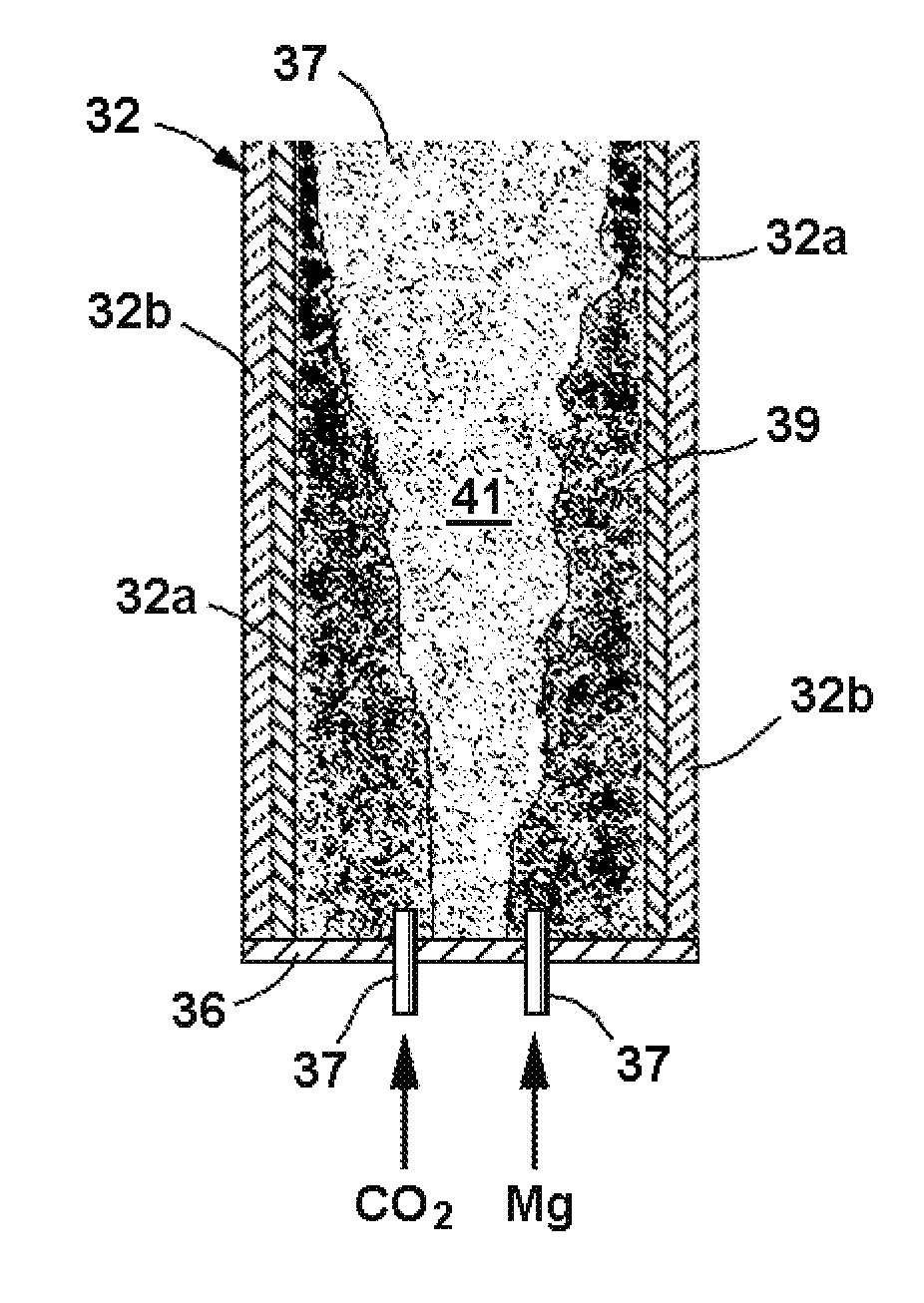
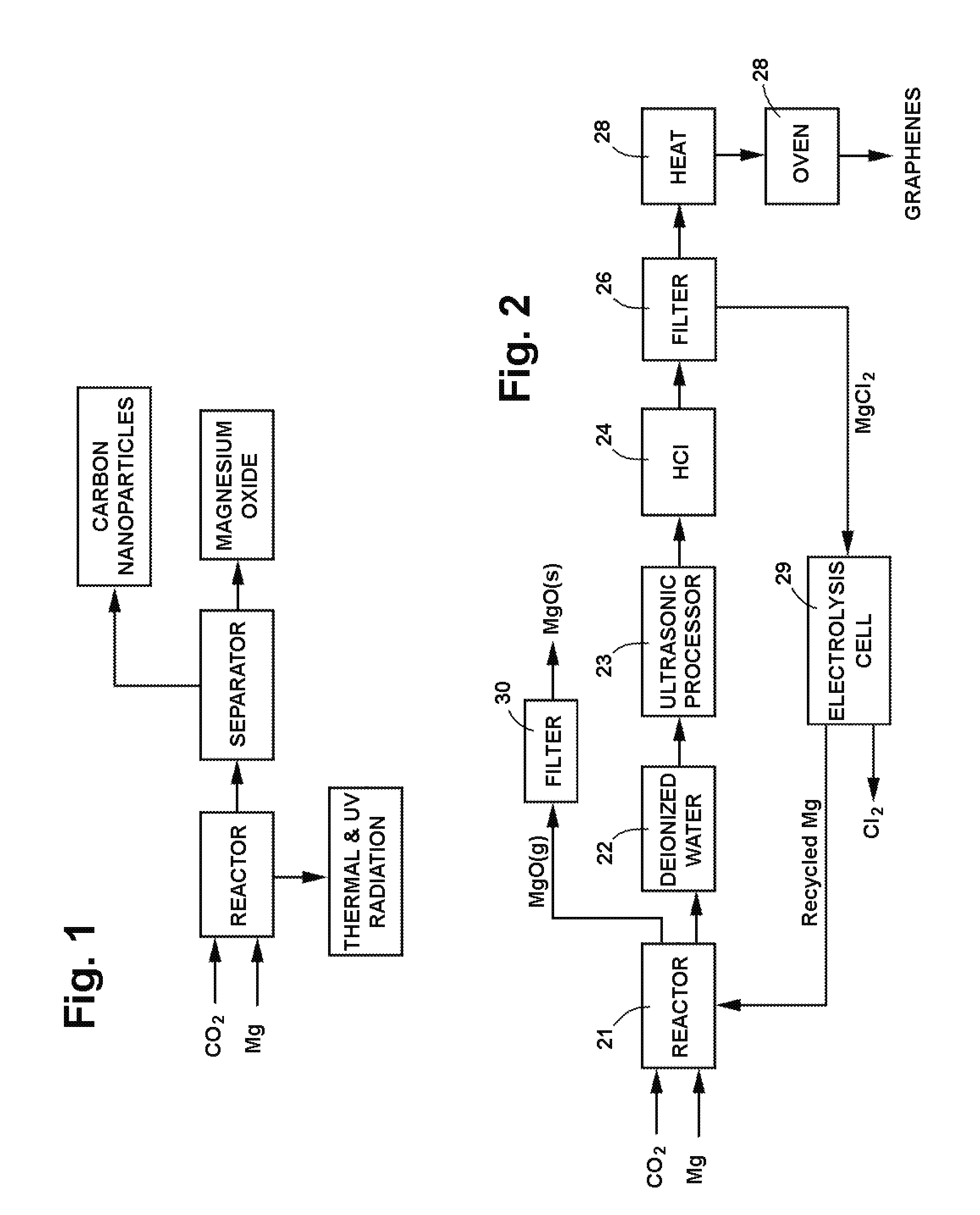
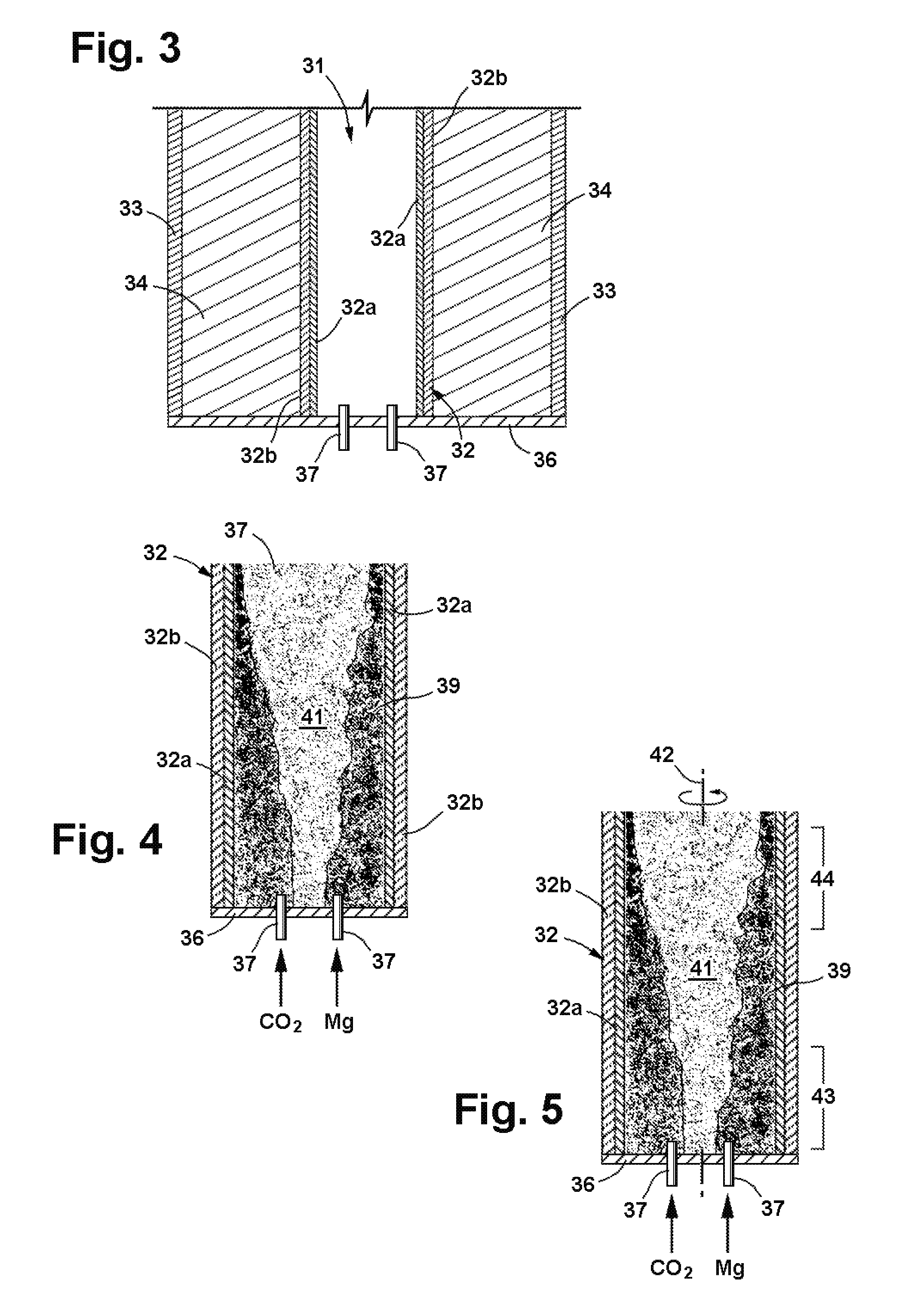
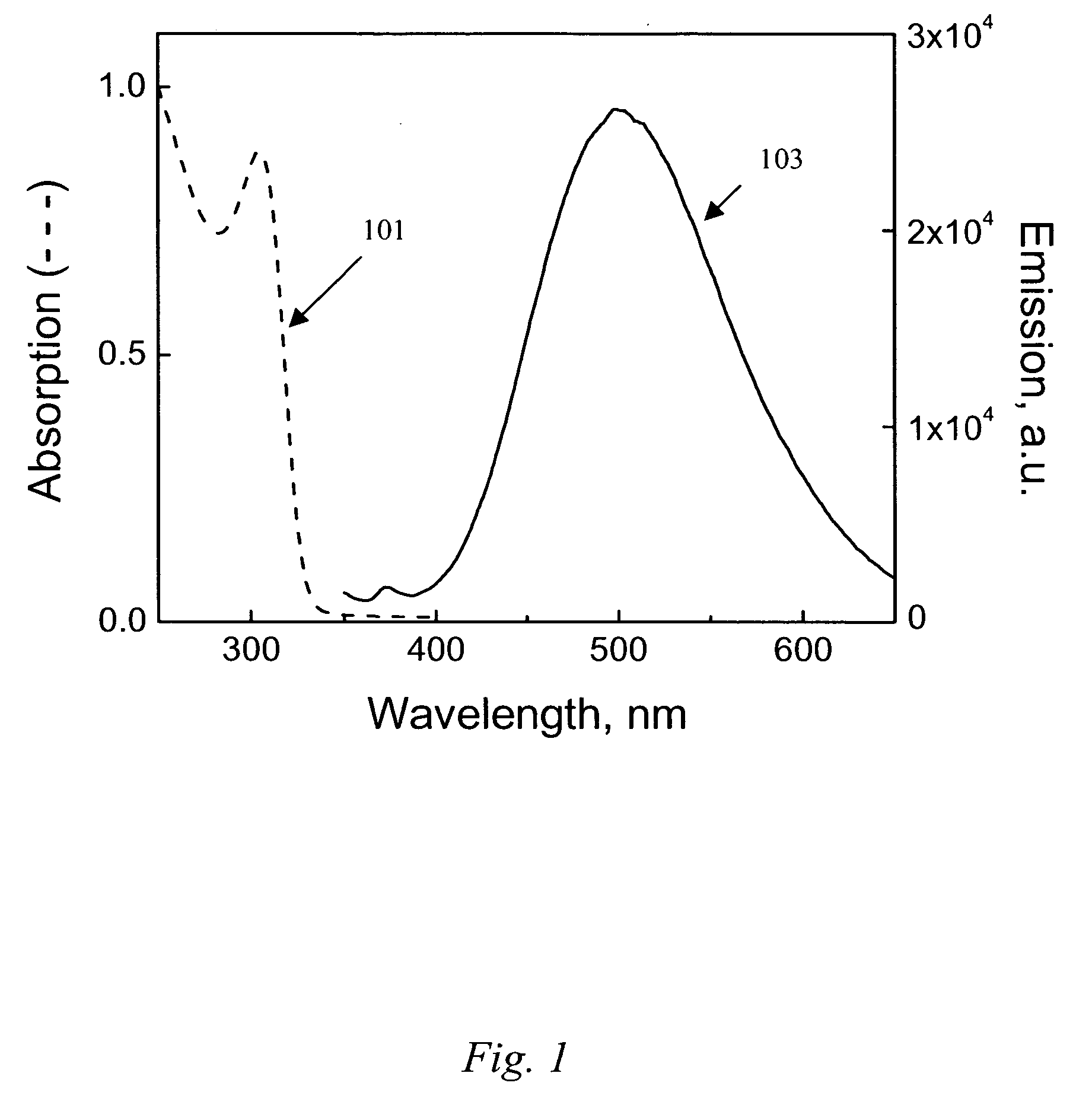
Process for producing nanomaterials such as graphenes, graphene composites, magnesium oxide, magnesium hydroxides and other nanomaterials by high heat vaporization and rapid cooling. In some of the preferred embodiments, the high heat is produced by an oxidation-reduction reaction of carbon dioxide and magnesium as the primary reactants, although additional materials such as reaction catalysts, control agents, or composite materials can be included in the reaction, if desired. The reaction also produces nanomaterials from a variety of other input materials, and by varying the process parameters, the type and morphology of the carbon nanoproducts and other nanoproducts can be controlled. The reaction products include novel nanocrystals of MgO (percilase) and MgAl2O4 (spinels) as well as composites of these nanocrystals with multiple layers of graphene deposited on or intercalated with them.
Owner:GRAPHENE TECH
Process for preparing nano-sized metal oxide particles
InactiveUS20050260122A1Efficiently provideNanosized metal oxide particles more efficientlyNanostructure manufactureGold compoundsHigh concentrationAlcohol
The present invention is directed to novel sol-gel methods in which metal oxide precursor and an alcohol-based solution are mixed to form a reaction mixture that is then allowed to react to produce nanosized metal oxide particles. The methods of the present invention are more suitable for preparing nanosized metal oxide than are previously-described sol-gel methods. The present invention can provide for nanosized metal oxide particles more efficiently than the previously-described sol-gel methods by permitting higher concentrations of metal oxide precursor to be employed in the reaction mixture. The foregoing is provided by careful control of the pH conditions during synthesis and by ensuring that the pH is maintained at a value of about 7 or higher.
Owner:KANEKA CORP +1
Powdered lime composition, method of preparing same and use thereof
ActiveUS7744678B2Improve performanceImprove efficiencyGas treatmentOxide/hydroxide preparationDesorptionFlue gas
Powdered lime composition having a BET specific surface area that is equal to or greater than 25 m2 / g and a BJH total pore volume obtained from nitrogen desorption that is equal to or greater than 0.1 cm3 / g, and furthermore comprising an alkali metal. The alkali metal content is equal to or greater than 0.2% and equal to or less than 3.5% based on the total weight of the composition. There is also described a method of preparing the composition and use thereof to reduce flue gases.
Owner:LHOIST RECH & DEV SA
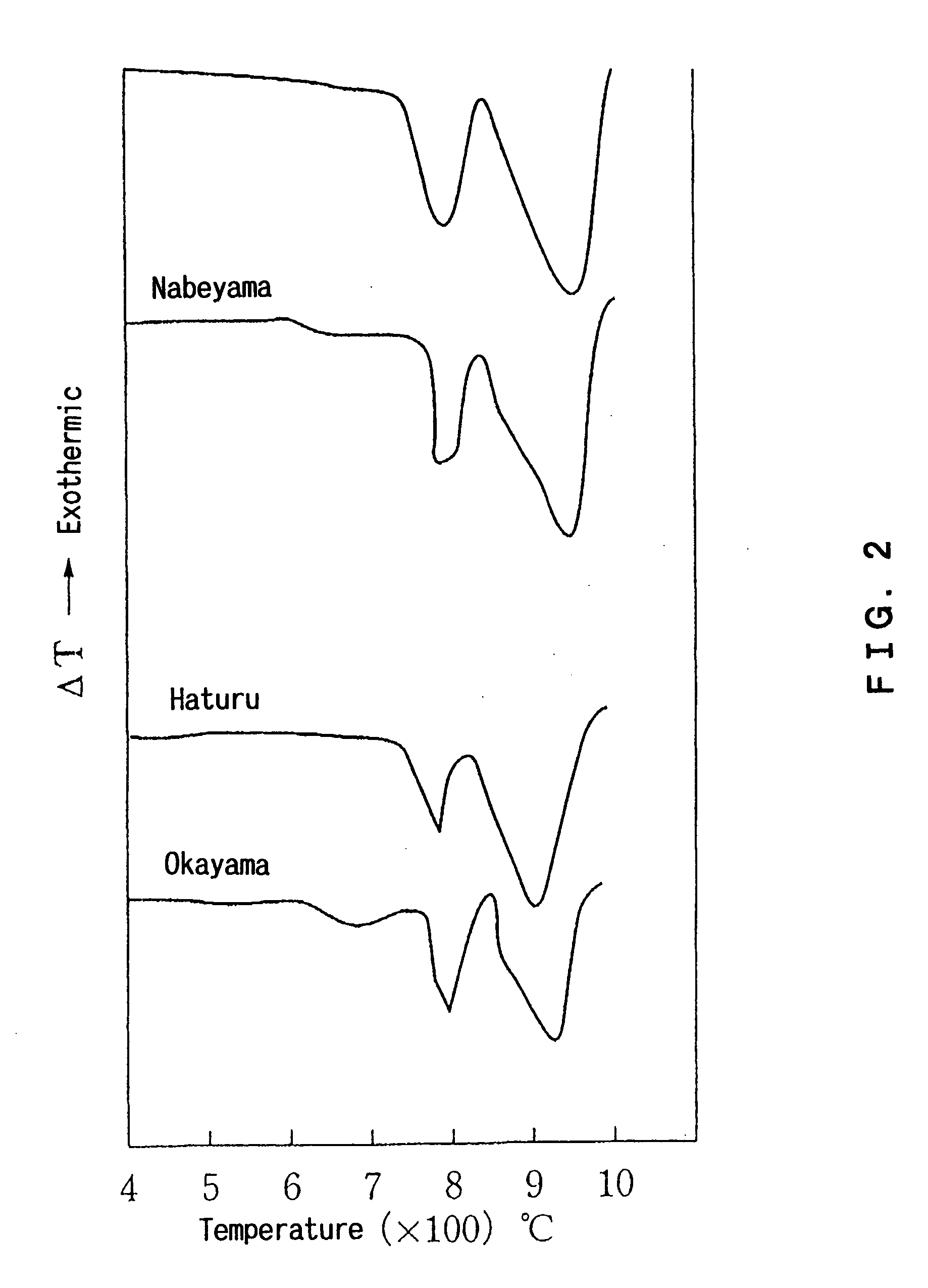
Additive for plastic and plastic
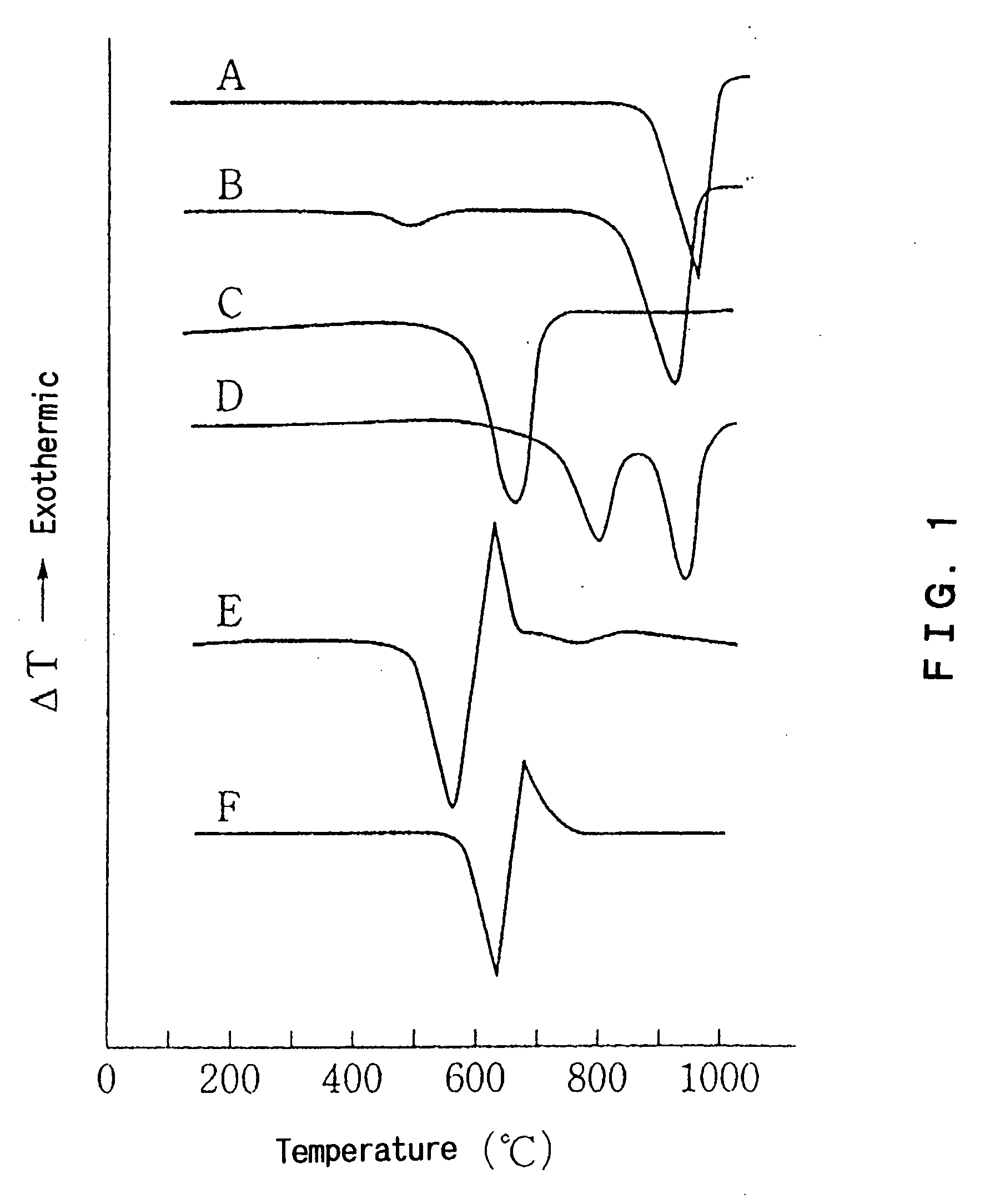
InactiveUS20060188428A1Small amountCalcium/strontium/barium carbonatesMagnesium carbonatesCALCIUM CARBONATE/MAGNESIUM CARBONATEMagnesium carbonate / Magnesium Oxide
Disclosed is an additive for a plastic, comprising fine particles obtained by calcination and slaking of a dolomite which exhibits two endothermic peaks in the differential thermal analysis, said fine particles containing calcium carbonate, magnesium carbonate, magnesium oxide, calcium hydroxide and magnesium hydroxide as main chemical components and also containing an ignition loss component in an amount of 10 to 40% by weight based on the weight of said fine particles. A plastic hating hydrogen chloride scavenging properties and antimicrobial properties imparted by incorporating the additive for a plastic is also disclosed.
Owner:OSAKA MUNICIPAL TECHN RES INST +2
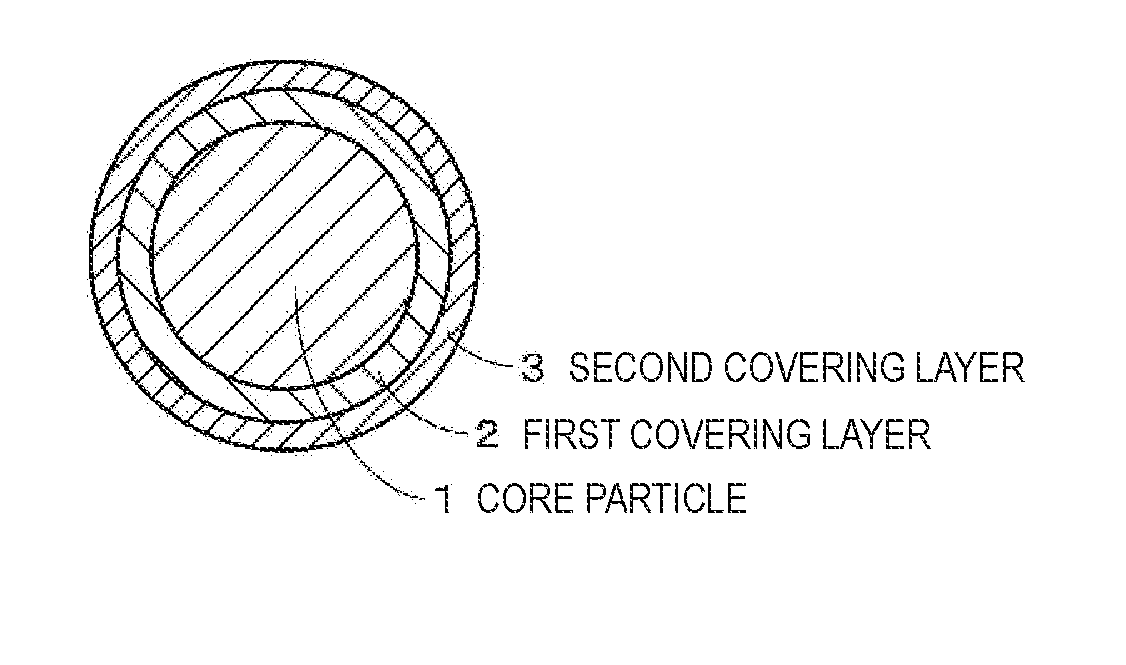
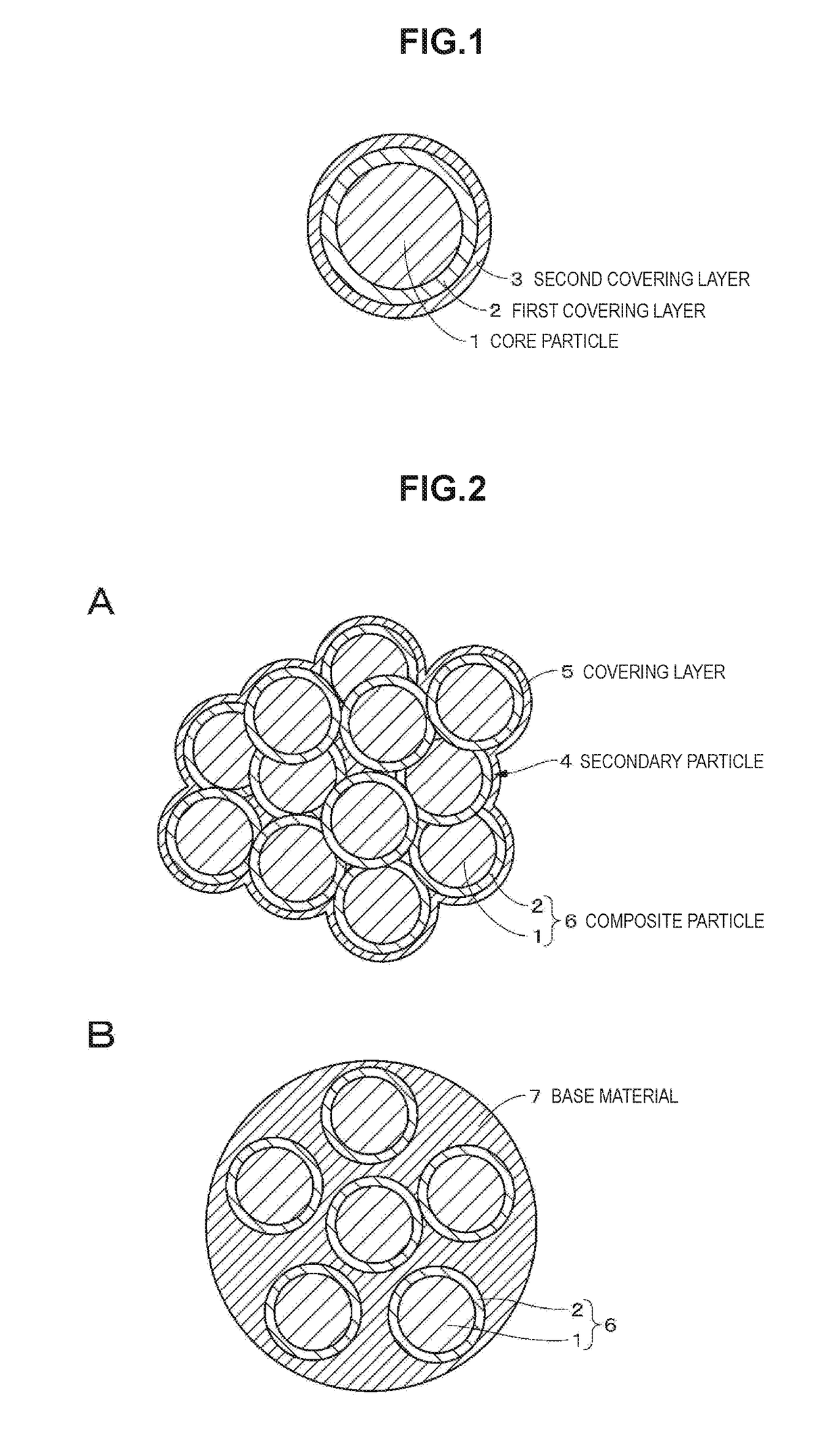
Positive electrode active material, positive electrode, battery, battery pack, electronic device, electric vehicle, power storage device, and power system
ActiveUS20170207444A1Large capacityExcellent cycle characteristicsMagnesium halidesCell electrodesFluorideCrystallinity
A positive electrode active material includes: a particle including a lithium composite oxide; a first layer that is provided on a surface of the particle and includes a lithium composite oxide; and a second layer that is provided on a surface of the first layer. The lithium composite oxide included in the particle and the lithium composite oxide included in the first layer have the same composition or almost the same composition, the second layer includes an oxide or a fluoride, and the lithium composite oxide included in the first layer has lower crystallinity than the lithium composite oxide included in the particle.
Owner:MURATA MFG CO LTD
Magnesium hydroxide particles, process for producing the same, and resin composition containing the particles
InactiveUS6676920B1Maintain good propertiesOxide/hydroxide preparationSilicaShell moldingSingle crystal
Owner:KYOWA CHEM IND
Method for mass preparing hollow nano cages in high quality
InactiveCN101284663AHigh purityHigh mesoporosityIndividual molecule manipulationMagnesiaMagnesium saltReaction temperature
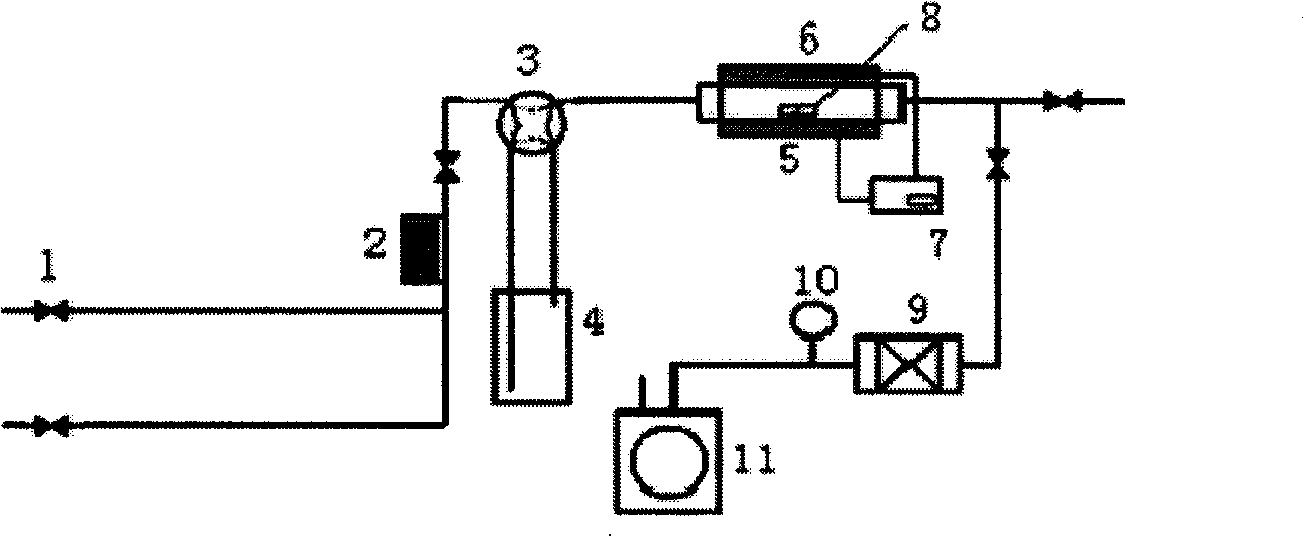
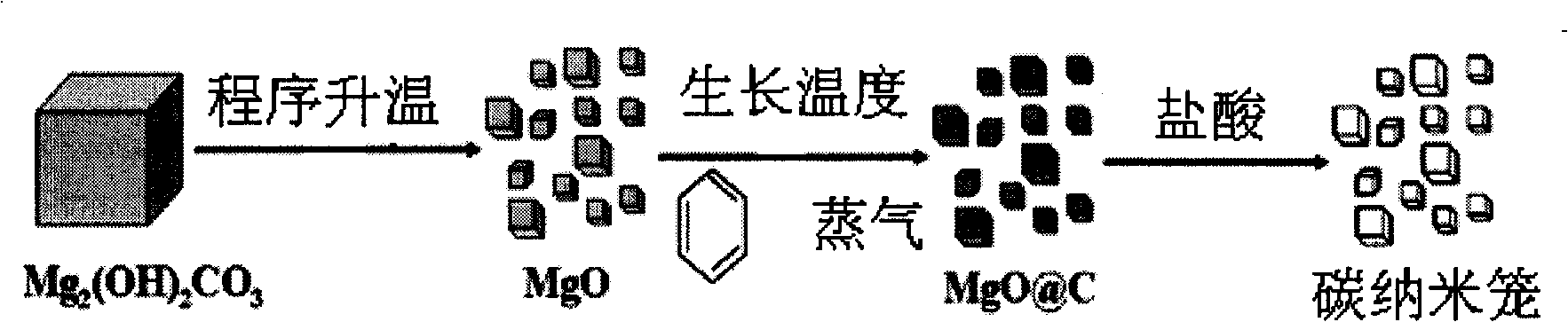
A method for mass production of high-quality hollow carbon nanocages comprises the following steps: 1) light magnesium carbonate or magnesium carbonate is added into a reaction tube and spread evenly, the tube is put into a tubular furnace, air is pumped out of the tube, inert gas such as N2 and Ar is injected; the reaction temperature is increased to 670 DEG C-900 DEG C, (C) source vapor is introduced, and reaction is carried out for 5 to 240 minutes under the protection of 10-500sccm inert gas atmosphere; the (C) source gas is led to the reaction area of the tubular furnace by the inert gas flow, carbonized on the surfaces of nano particles generated in situ, and covered to form a MgO@C structure; after the reaction, the temperature inside the reaction tube drops to the room temperature under the protection of the inert gas; 2) powder is collected from the reaction tube, dipped in sufficient hydrochloric acid or sulphuric acid for 5 to 720 minutes to remove the kernel of MgO, filtered, washed to neutrality by using deionized water, and dried to obtain the hollow carbon nanocages; and 3) the magnesium salt filtrate is recycled. The nanocages prepared by the method have high purity, and the price for the precursor is low, thereby facilitating recovery and reuse.
Owner:NANJING UNIV
Plasma display panel and method for manufacturing the same
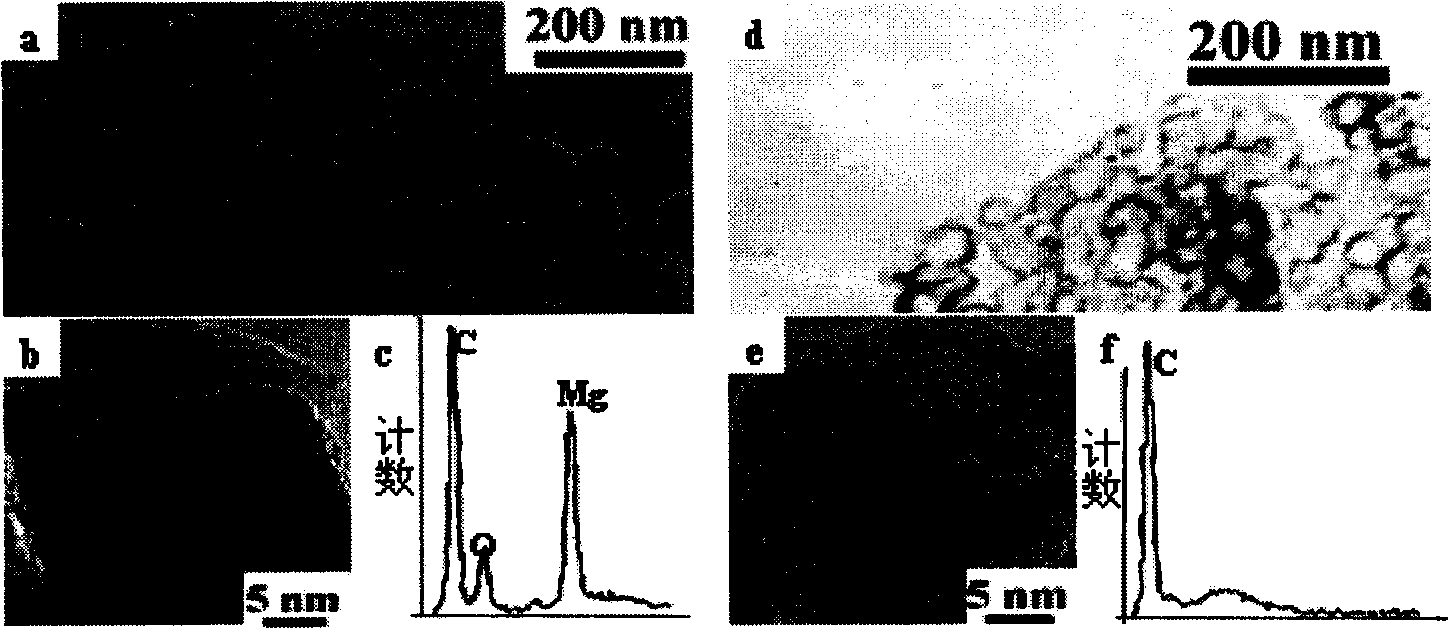
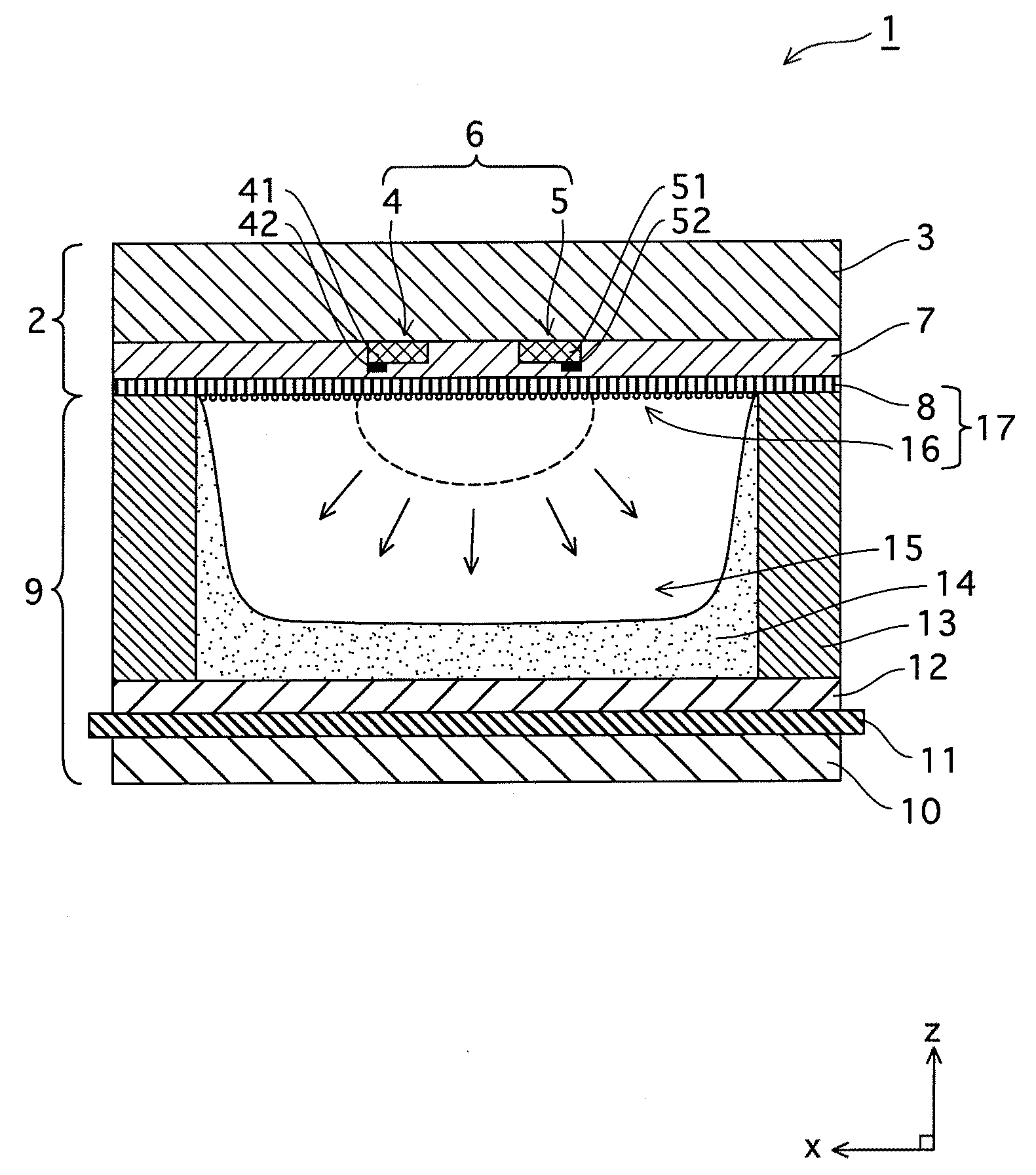
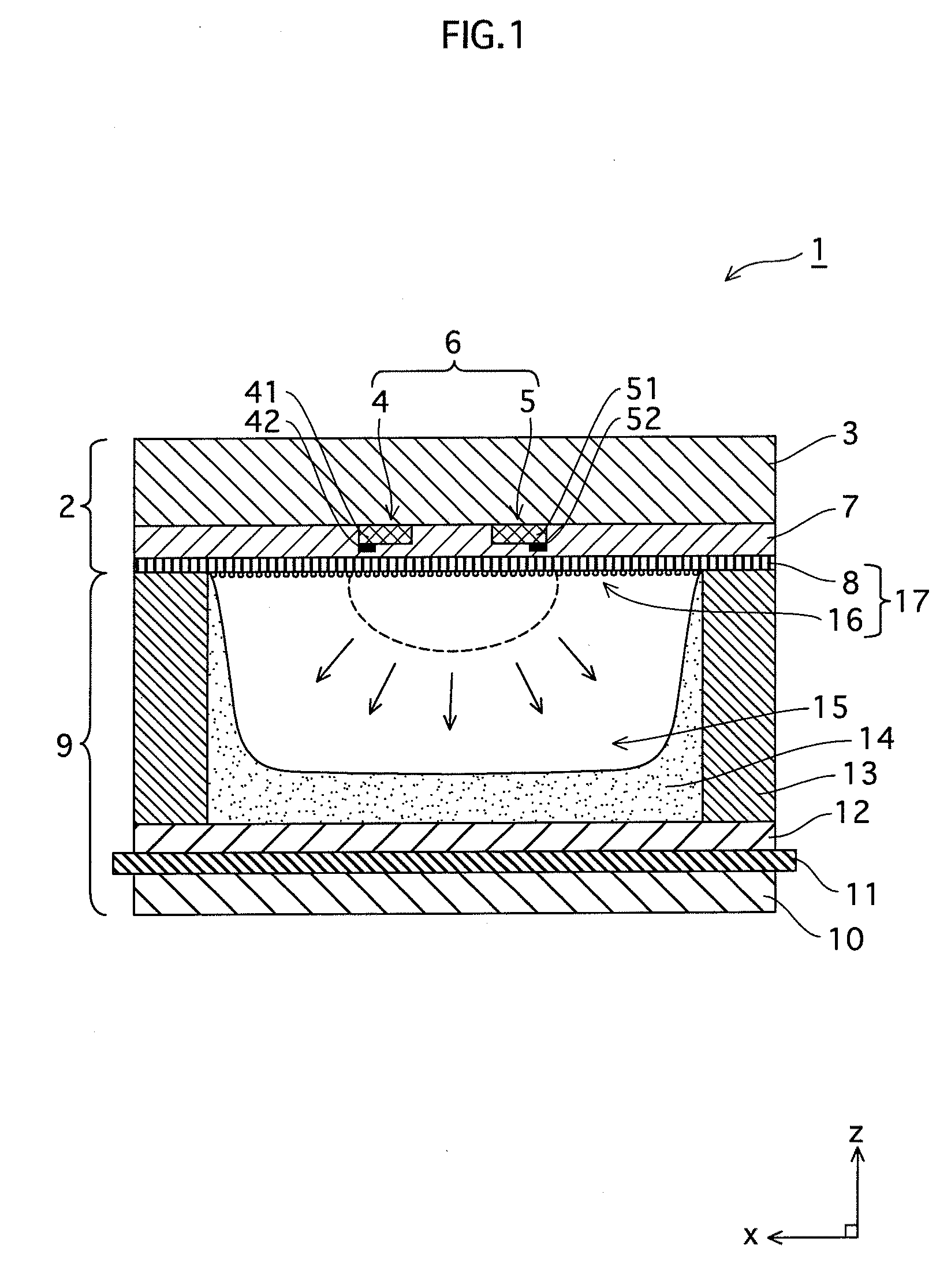
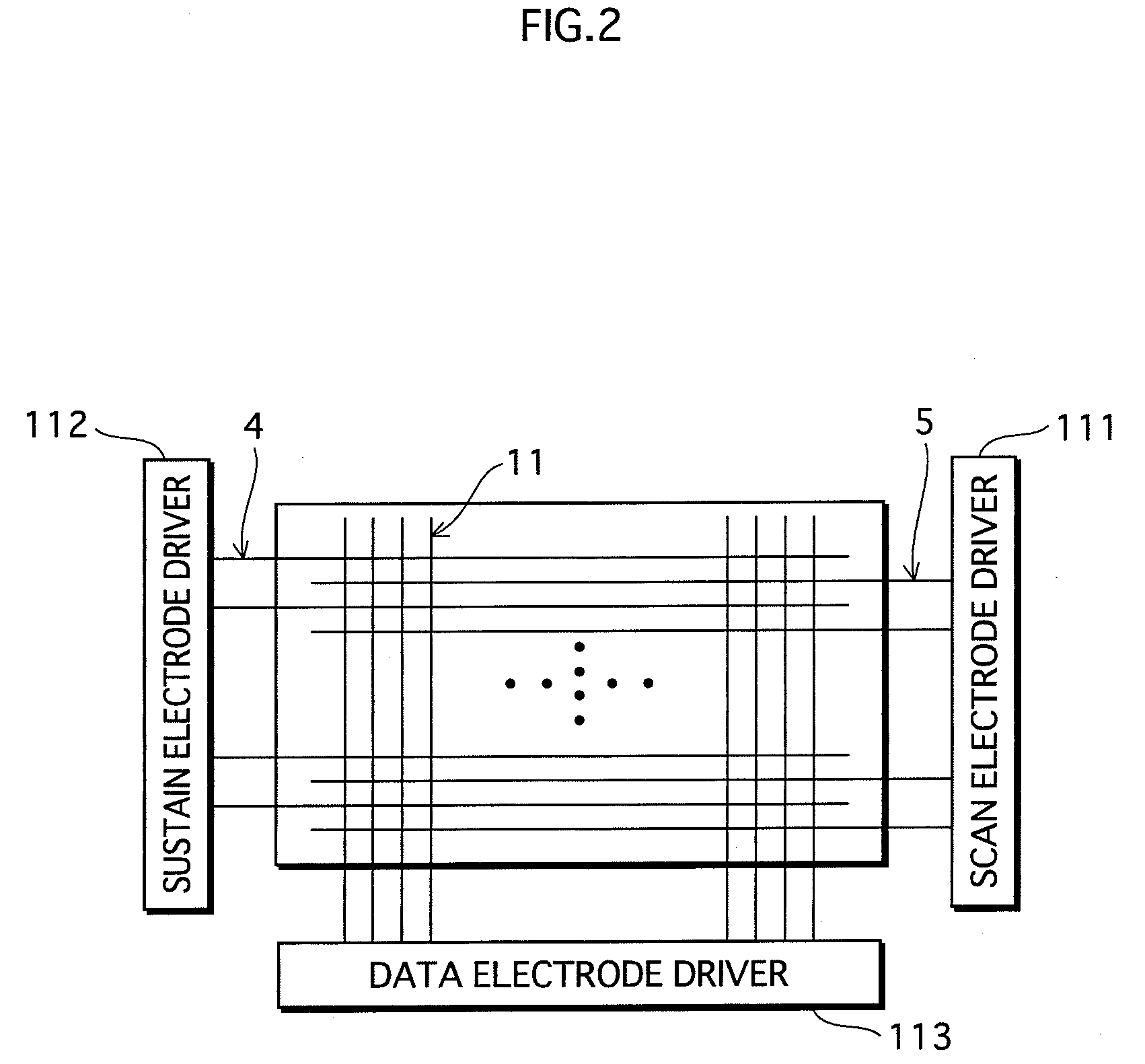
ActiveUS20090140652A1Large secondary electron emission coefficientLaunch evenlyTube/lamp screens manufactureAddress electrodesLow voltagePlasma display
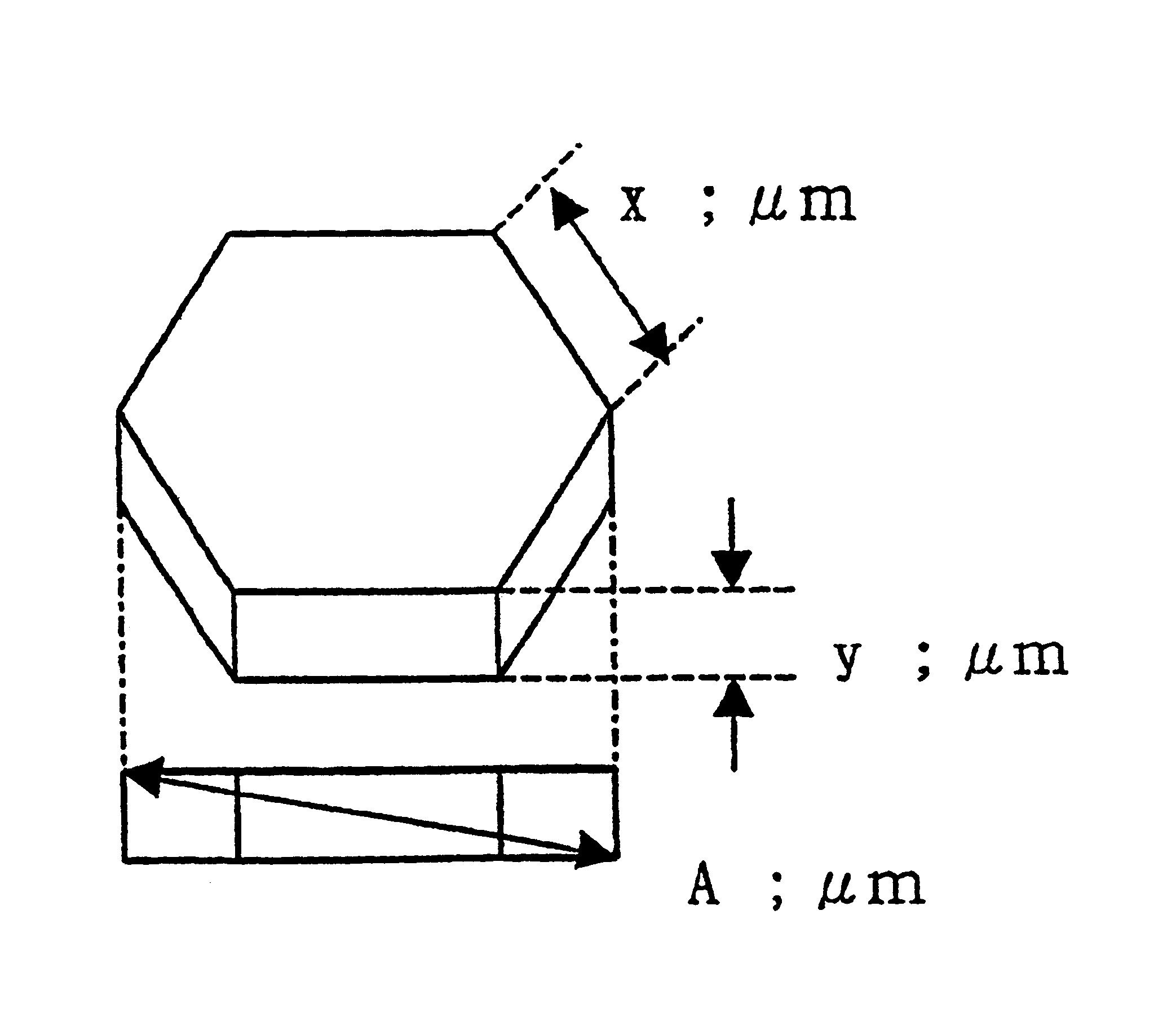
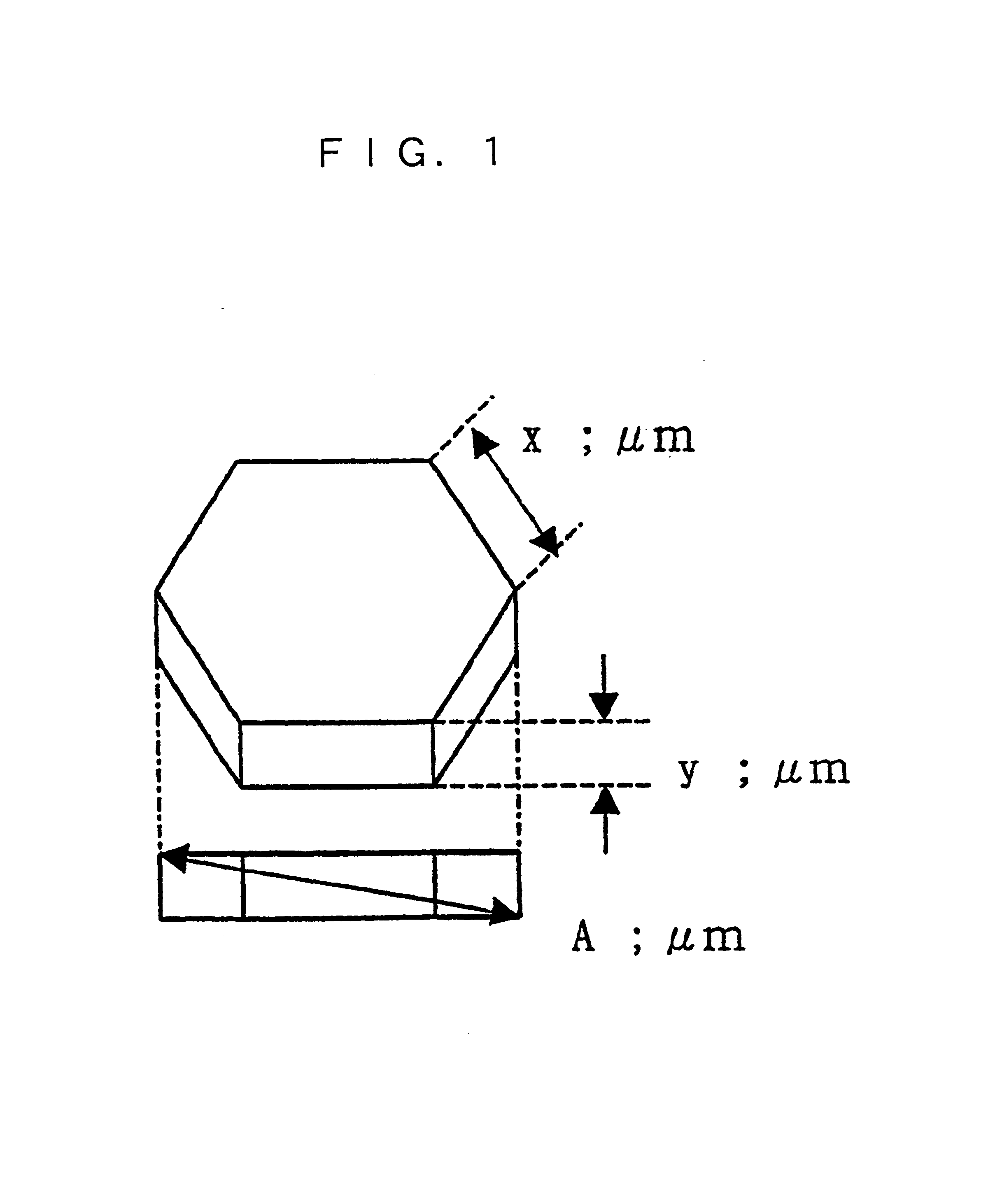
“Discharge delay” and “dependence of discharge delay on temperatures” are solved by improving a protective layer, thus a PDP can be driven at a low voltage. Furthermore, the PDP can display excellent images by suppressing “dependence of discharge delay on space charges.” Liquid-phase magnesium alkoxide (Mg (OR)2) or acetylacetone magnesium ate whose purity is 99.95% or more is prepared, and is hydrolyzed by adding a small amount of acids to the solution. Thus, a gel of magnesium hydroxide that is a magnesium oxide precursor is formed. Burning the gel in atmosphere at 700° C. or more produces powder containing MgO particles 16a-16d having the NaCl crystal structure with (100) and (111) crystal faces or with (100), (110) and (111) crystal faces. By pasting the powder on a dielectric layer 7 or a surface layer 8, the MgO powder 16 is formed so as to serve as the protective layer.
Owner:PANASONIC CORP
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com