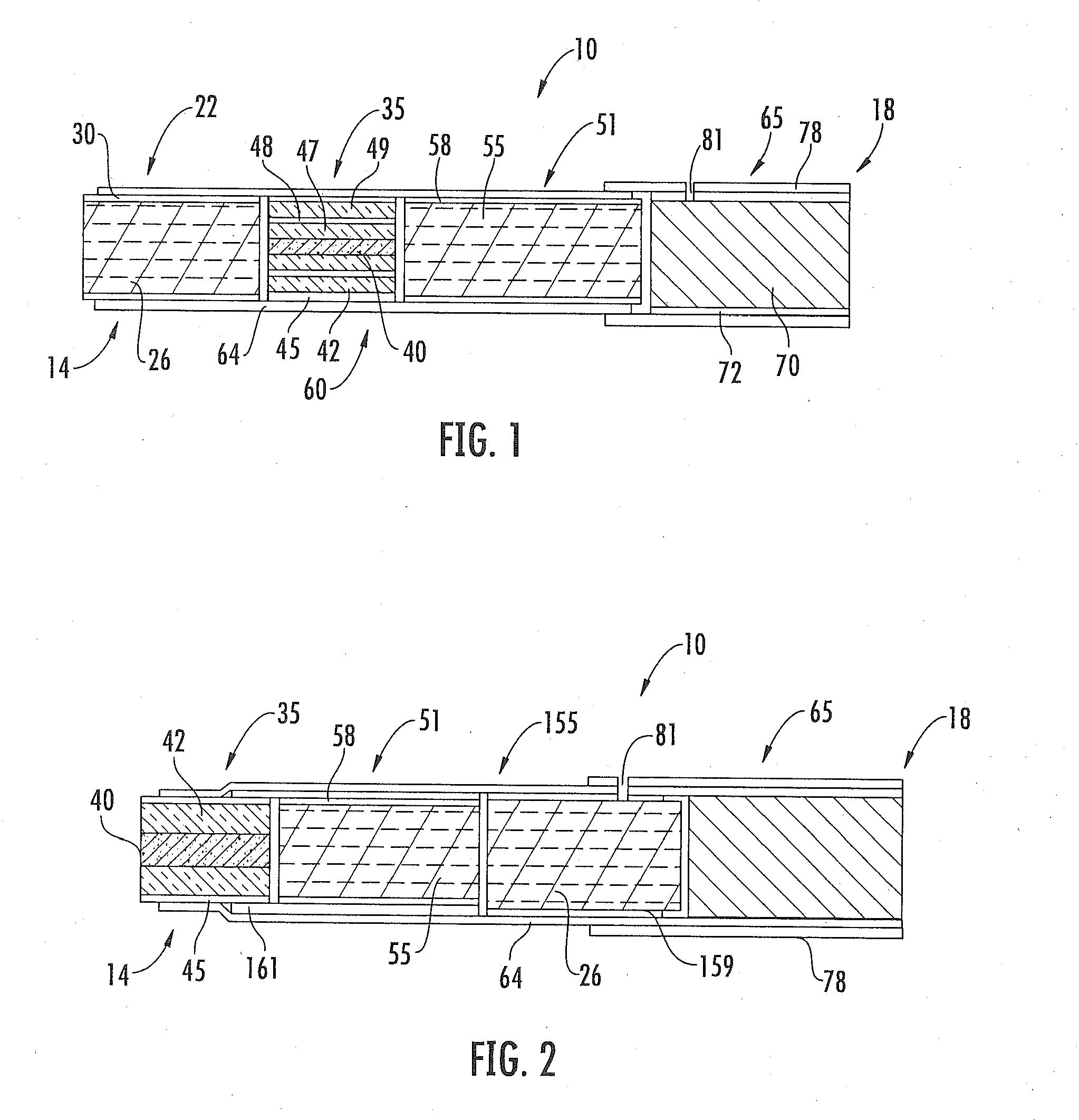
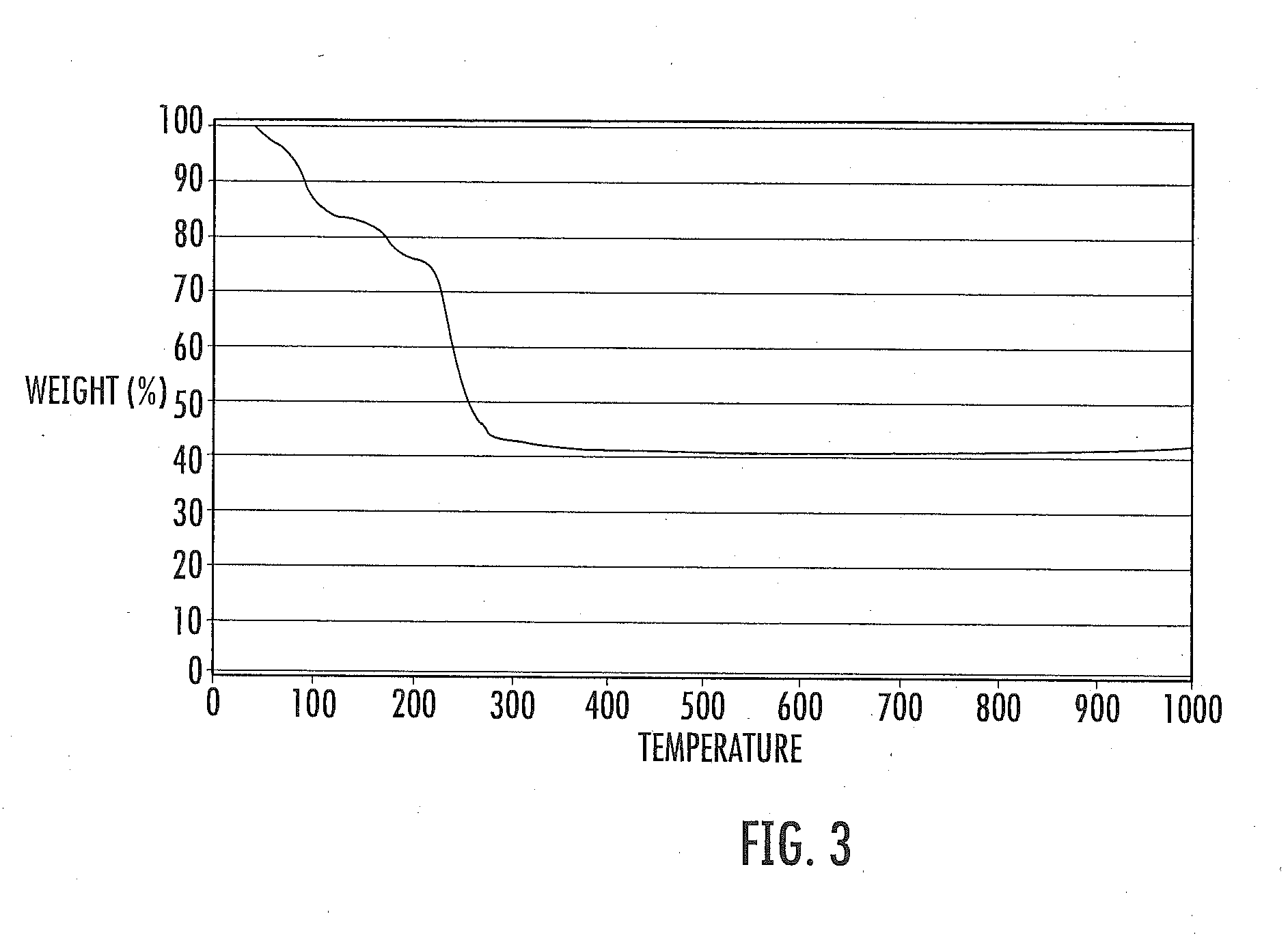
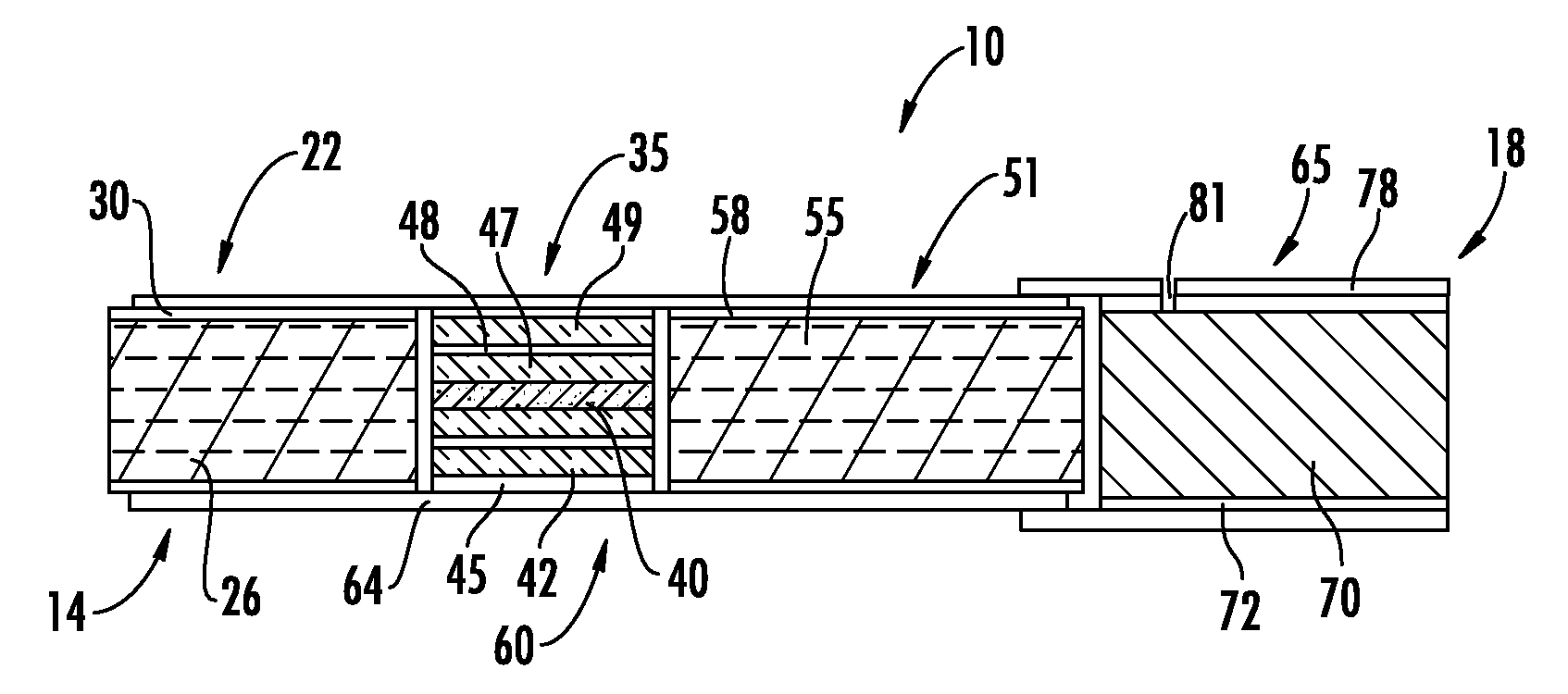
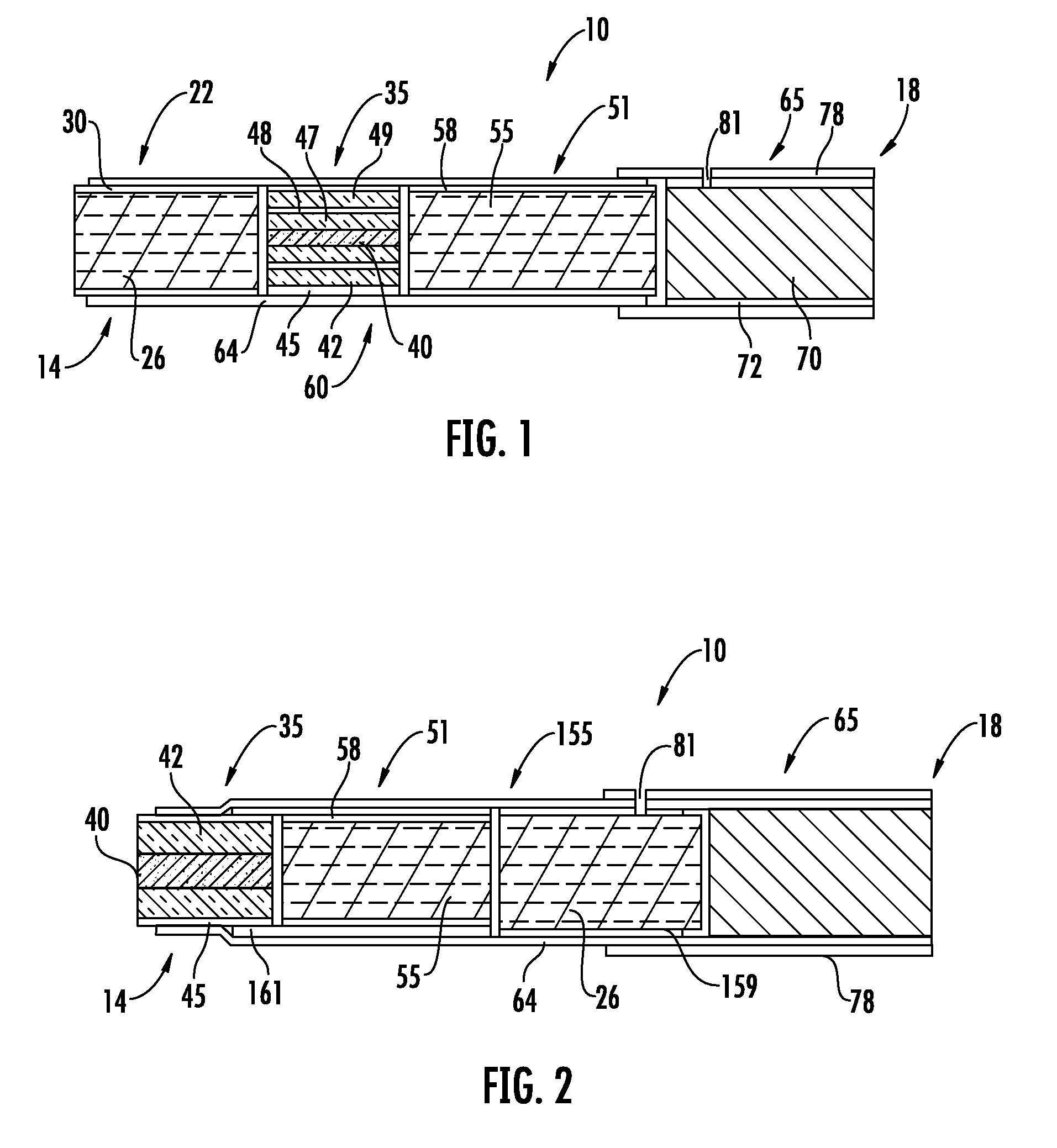
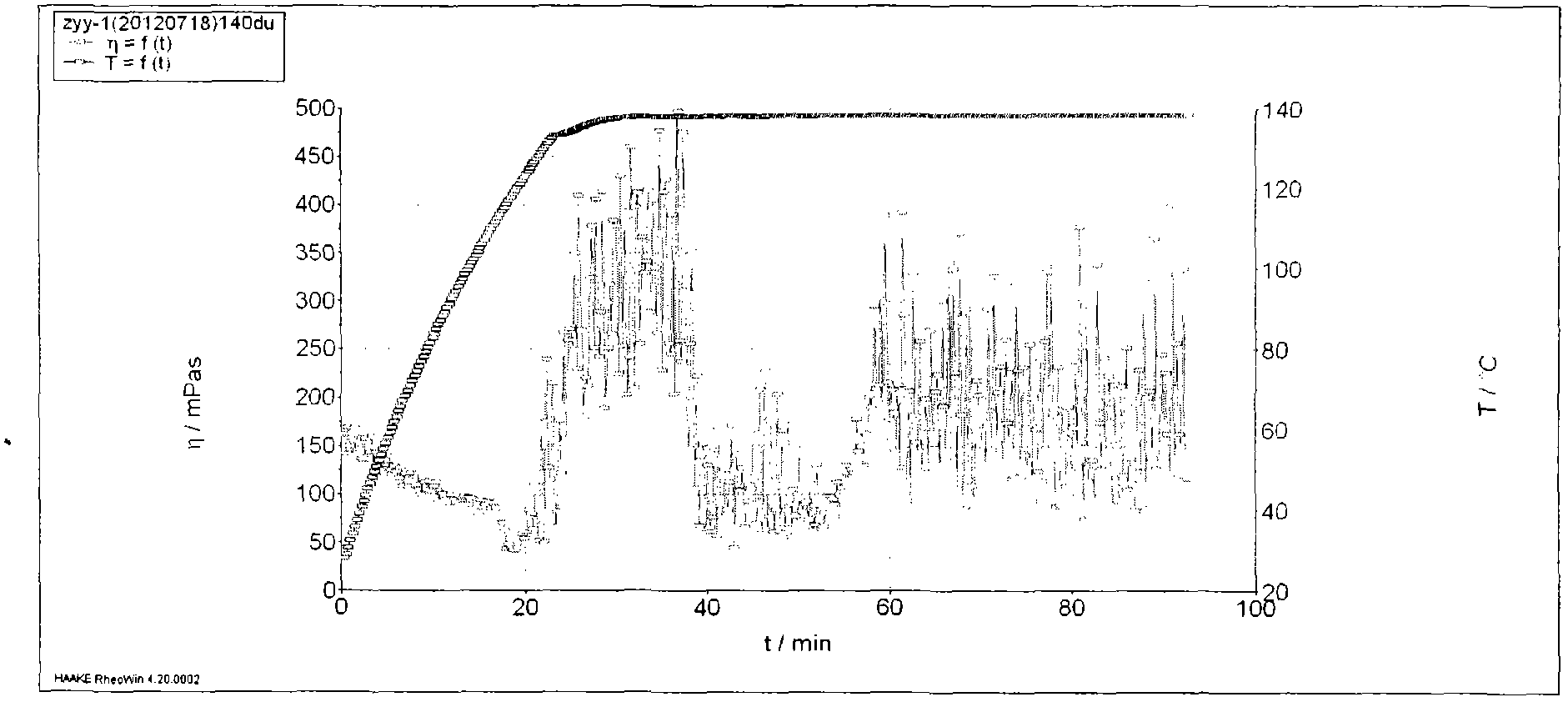
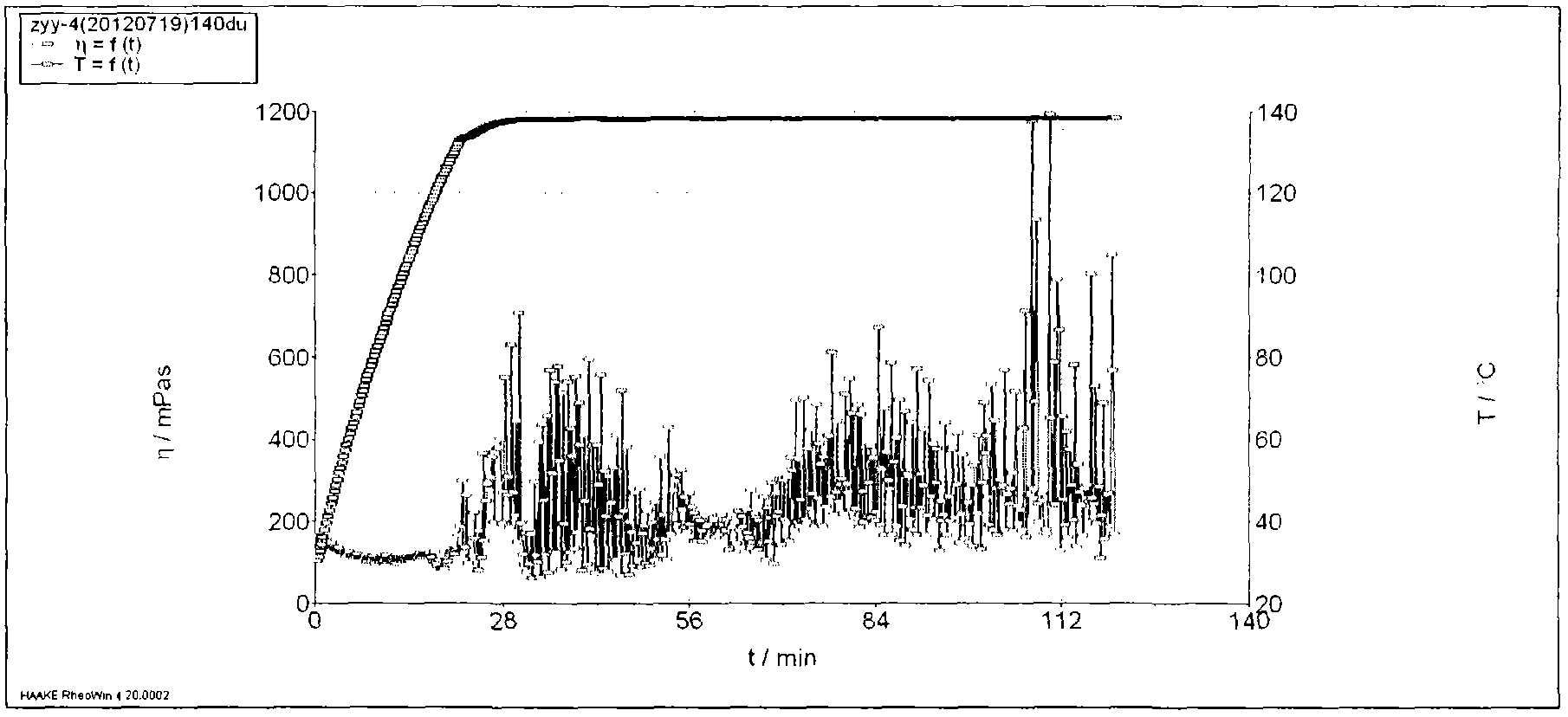
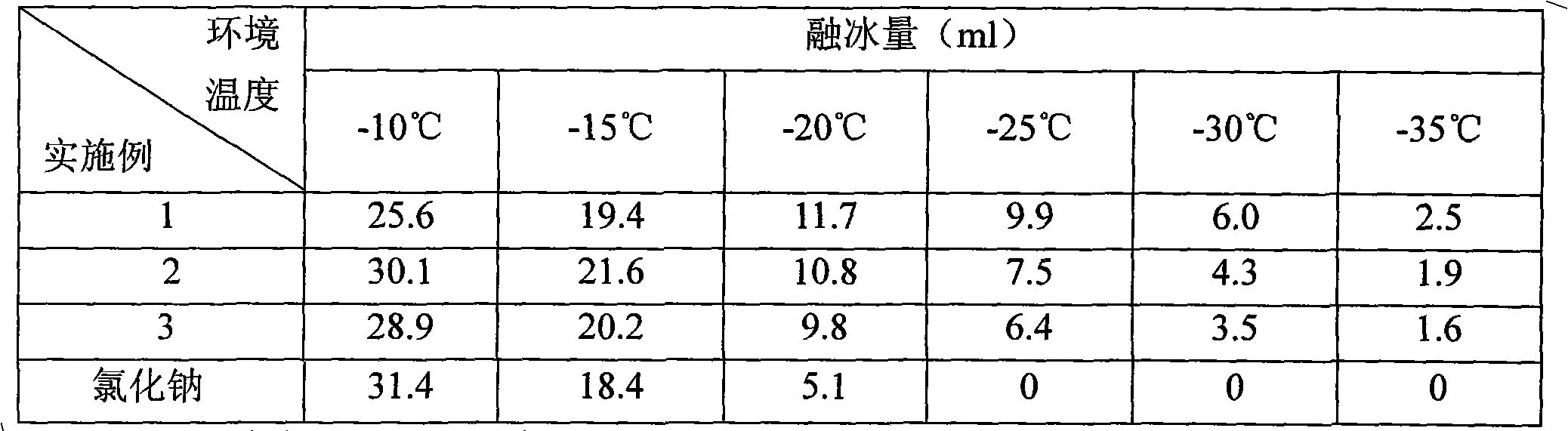
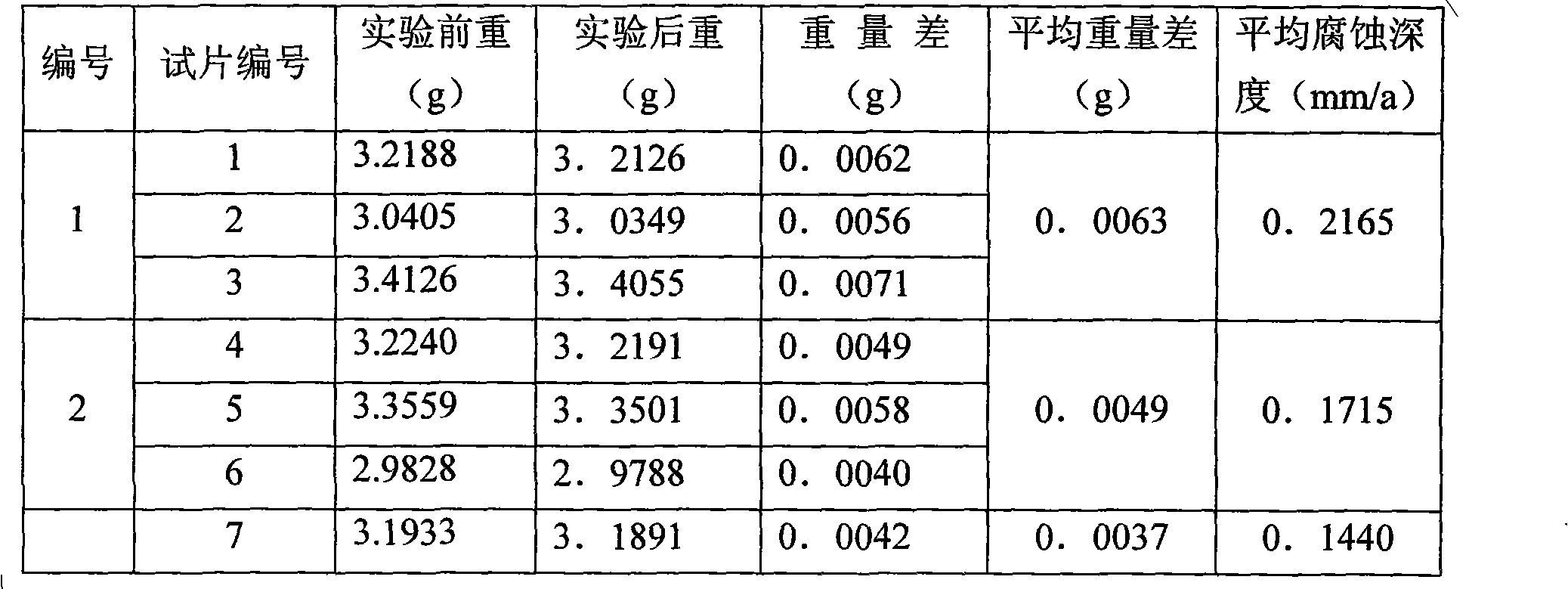
Patents
Literature
1263 results about "Magnesium nitrate" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
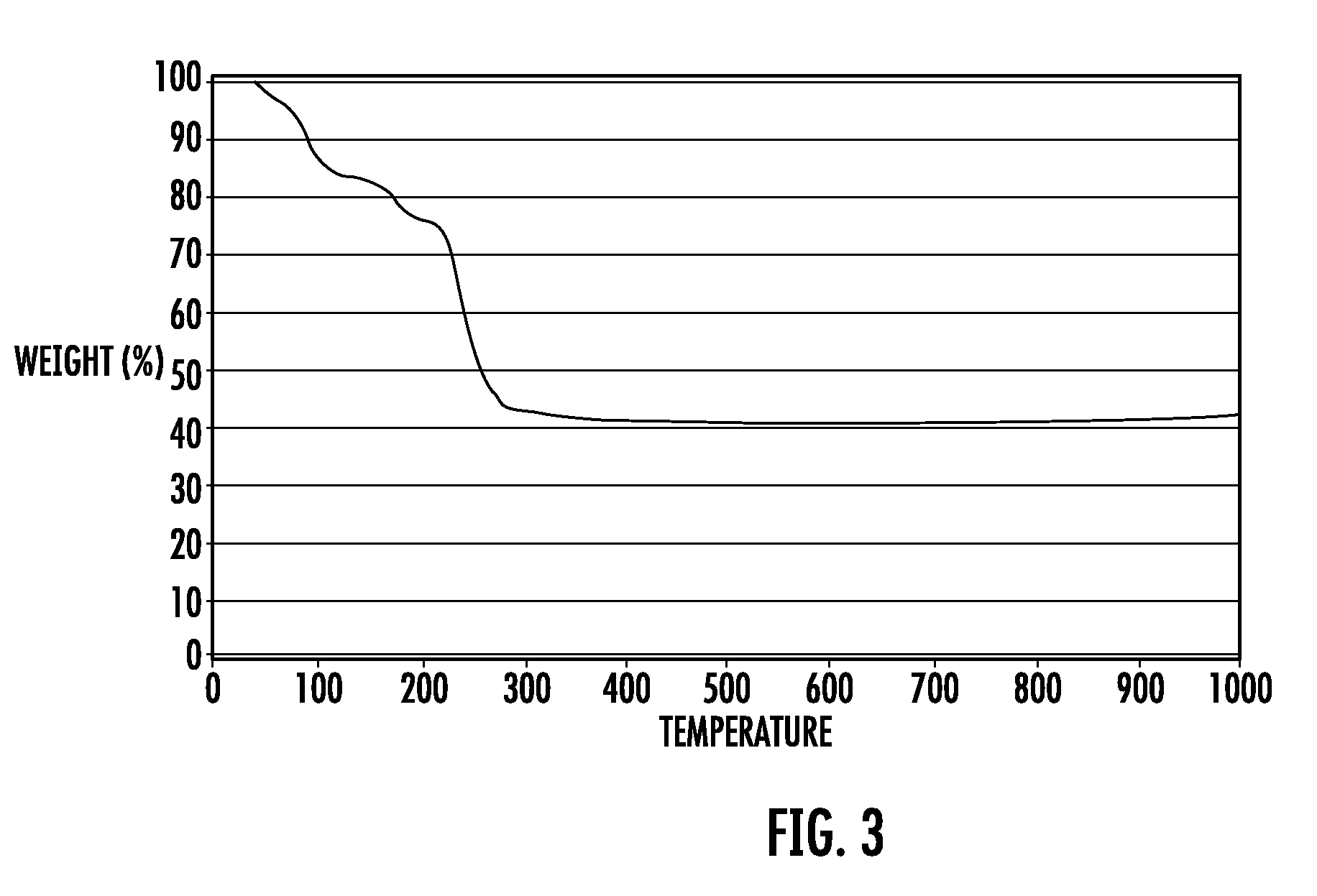
Magnesium nitrate refers to inorganic compounds with the formula Mg(NO₃)₂(H₂O)ₓ, where x = 6, 2, and 0. All are white solids. The anhydrous material is hygroscopic, quickly forming the hexahydrate upon standing in air. All of the salts are very soluble in both water and ethanol.
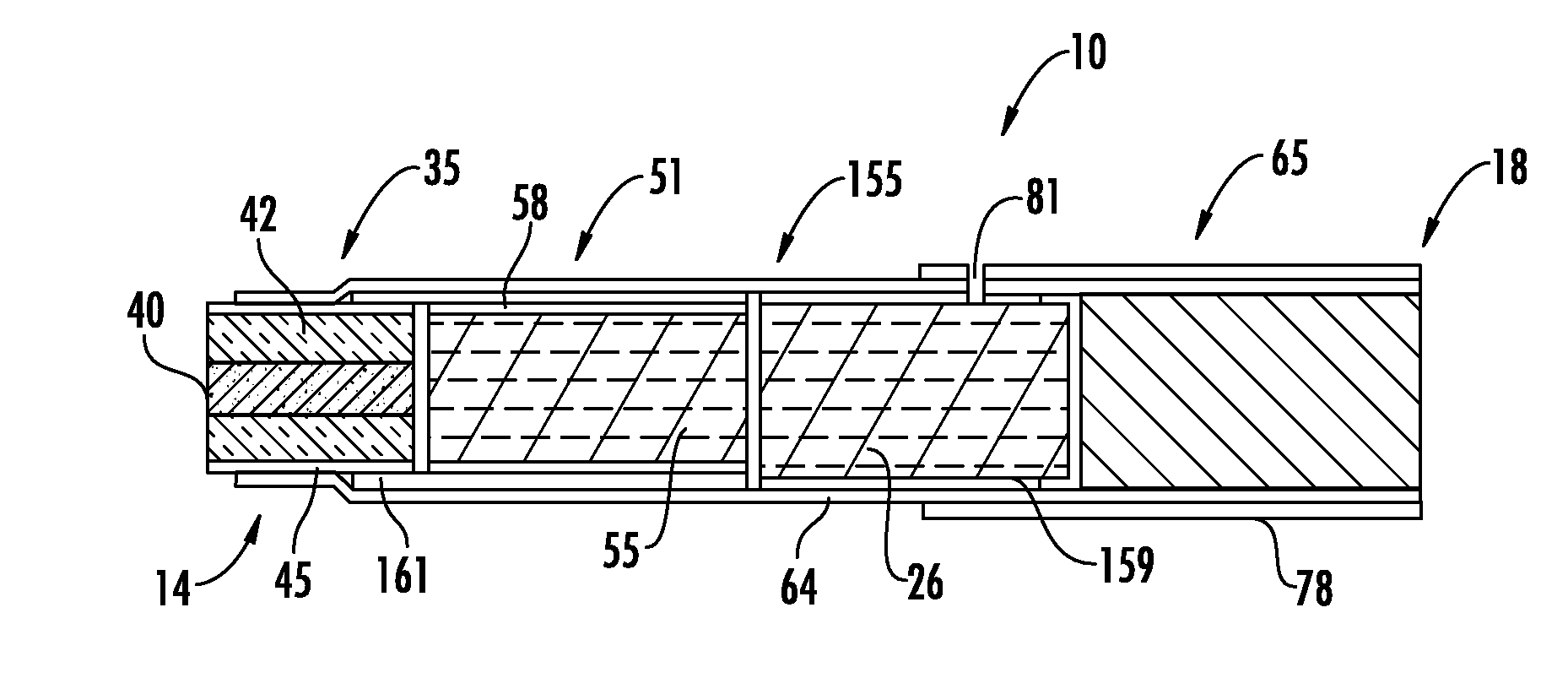
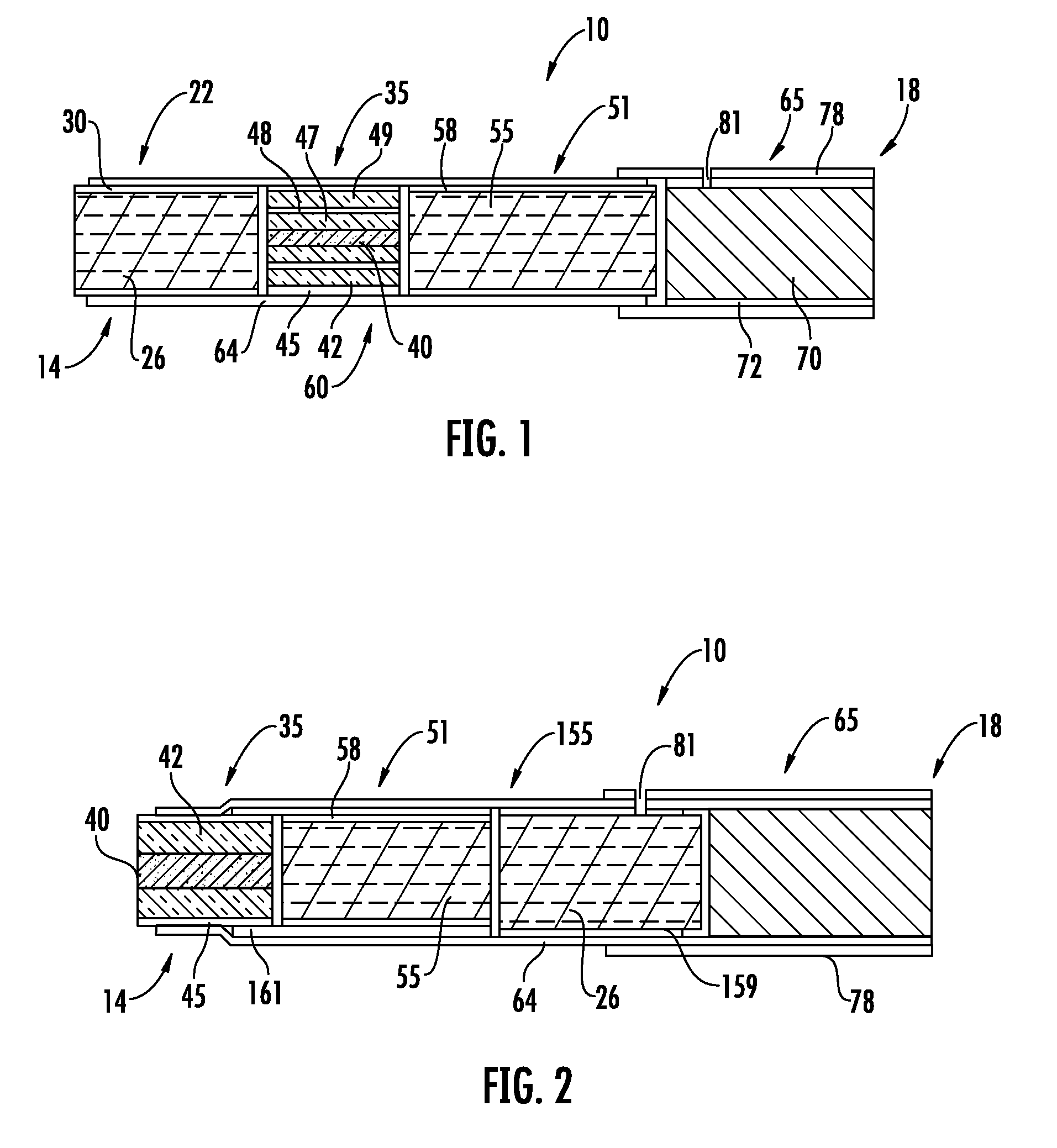
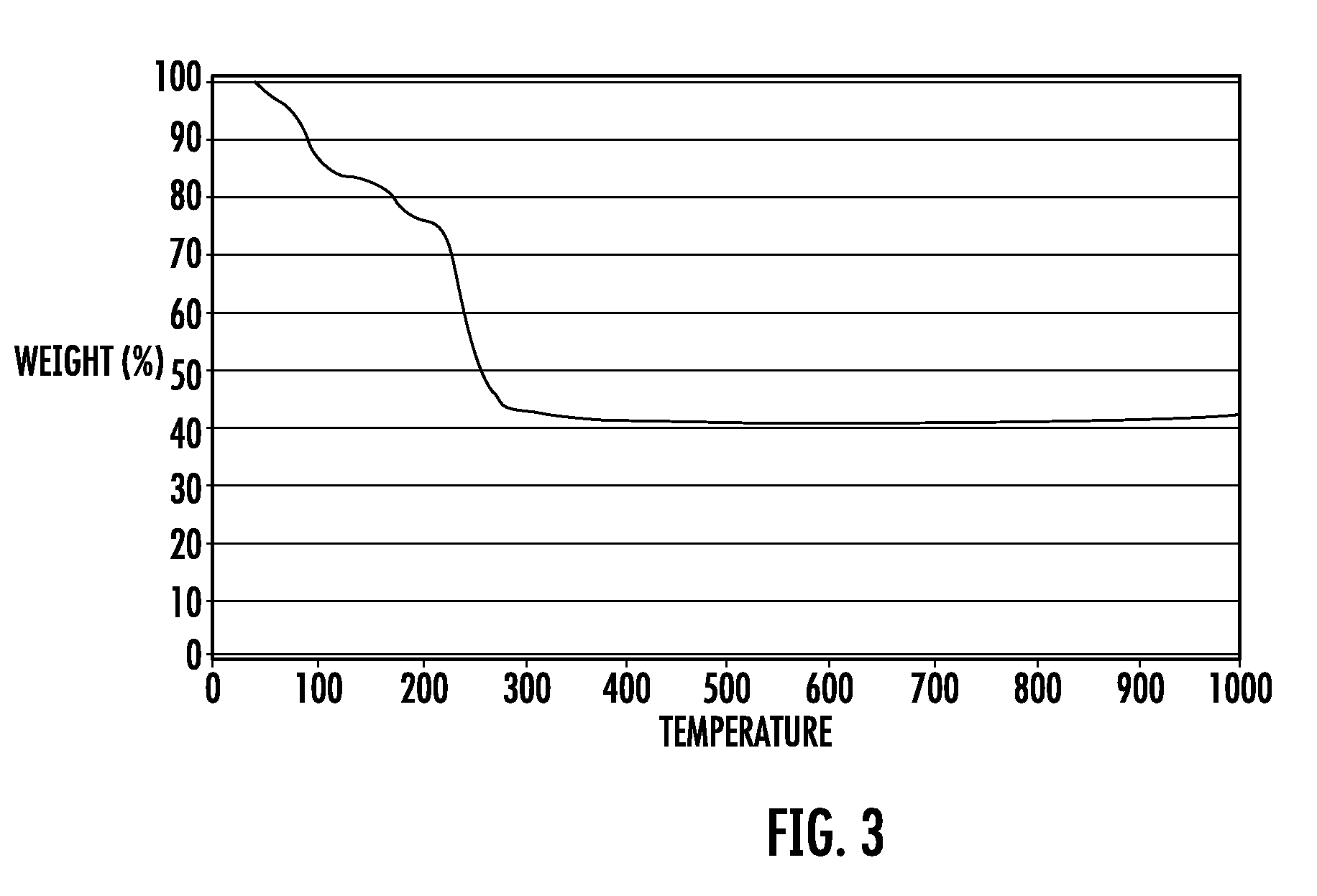
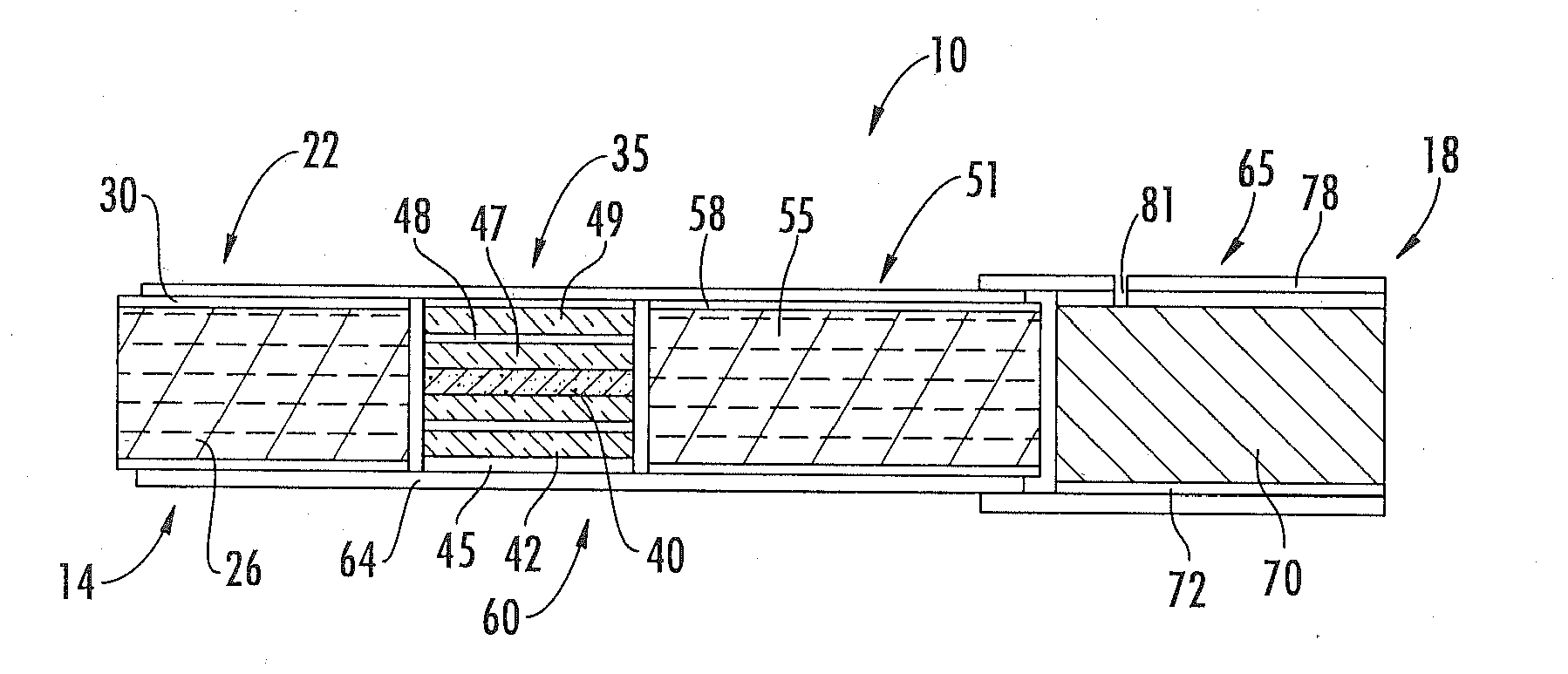
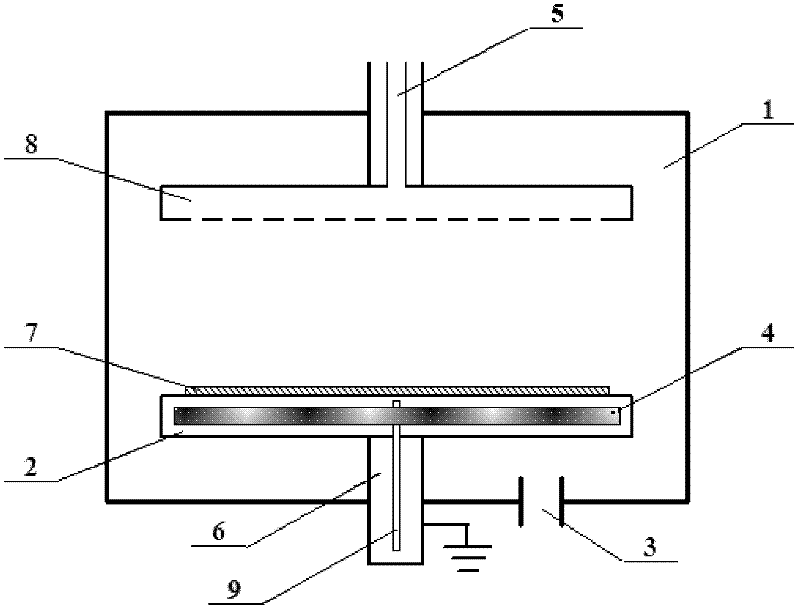
Method for Preparing Fuel Element For Smoking Article
ActiveUS20100065075A1Good water solubilityTobacco treatmentTobacco smoke filtersCerium nitrateCopper nitrate
The invention provides a method for making a fuel element for a smoking article comprising forming a combustible carbonaceous material into a fuel element adapted for use in a smoking article; incorporating a metal-containing catalyst precursor into the fuel element or onto the surface thereof to form a treated fuel element, the incorporating step occurring before, during, or after said forming step; and optionally heating or irradiating the treated fuel element at a temperature and for a time sufficient to convert the catalyst precursor to a catalytic metal compound. Examples of metal-containing catalyst precursors include iron nitrate, copper nitrate, cerium nitrate, cerium ammonium nitrate, manganese nitrate, magnesium nitrate, and zinc nitrate. Fuel elements treated according to the invention, and smoking articles including such fuel elements, are also provided.
Owner:R J REYNOLDS TOBACCO COMPANY
Method for preparing fuel element for smoking article
The invention provides a method for making a fuel element for a smoking article including the steps of mixing a metal-containing catalyst precursor with a filler material or graphite or a combination thereof to form a pre-treated fuel element component; optionally calcining the pre-treated fuel element component in order to convert the catalyst precursor to a catalytic metal compound; after the optional calcining step, combining the pre-treated fuel element component with a carbonaceous material and a binder to produce a fuel element composition; and forming the fuel element composition into a fuel element adapted for use in a smoking article. Examples of metal-containing catalyst precursors include iron nitrate, copper nitrate, cerium nitrate, cerium ammonium nitrate, manganese nitrate, magnesium nitrate, and zinc nitrate. Fuel elements treated according to the invention, and smoking articles including such fuel elements, are also provided.
Owner:R J REYNOLDS TOBACCO COMPANY
Method for preparing fuel element for smoking article
The invention provides a method for making a fuel element for a smoking article comprising forming a combustible carbonaceous material into a fuel element adapted for use in a smoking article; incorporating a metal-containing catalyst precursor into the fuel element or onto the surface thereof to form a treated fuel element, the incorporating step occurring before, during, or after said forming step; and optionally heating or irradiating the treated fuel element at a temperature and for a time sufficient to convert the catalyst precursor to a catalytic metal compound. Examples of metal-containing catalyst precursors include iron nitrate, copper nitrate, cerium nitrate, cerium ammonium nitrate, manganese nitrate, magnesium nitrate, and zinc nitrate. Fuel elements treated according to the invention, and smoking articles including such fuel elements, are also provided.
Owner:R J REYNOLDS TOBACCO COMPANY
Extinguishment combination with hot gas sol
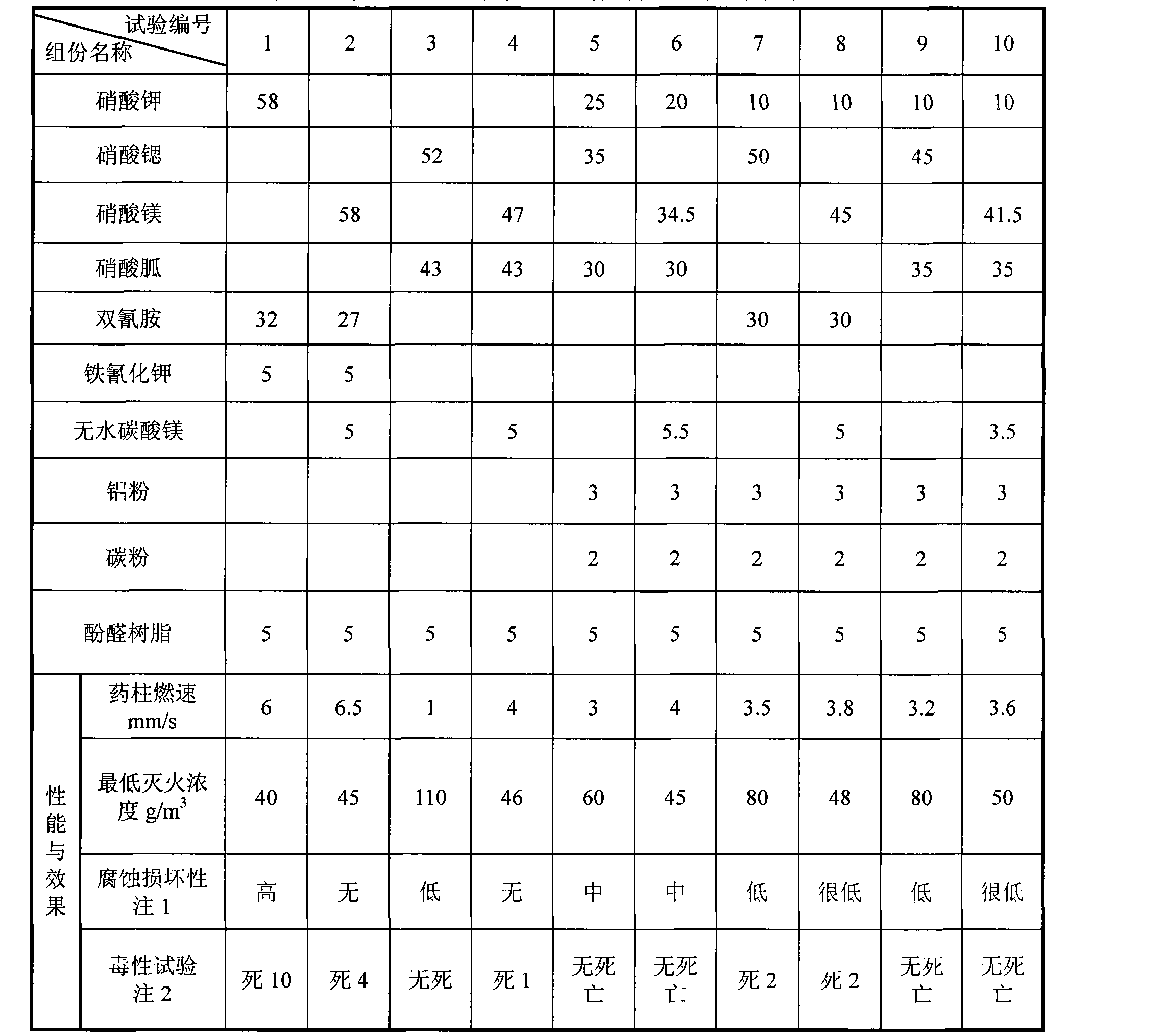
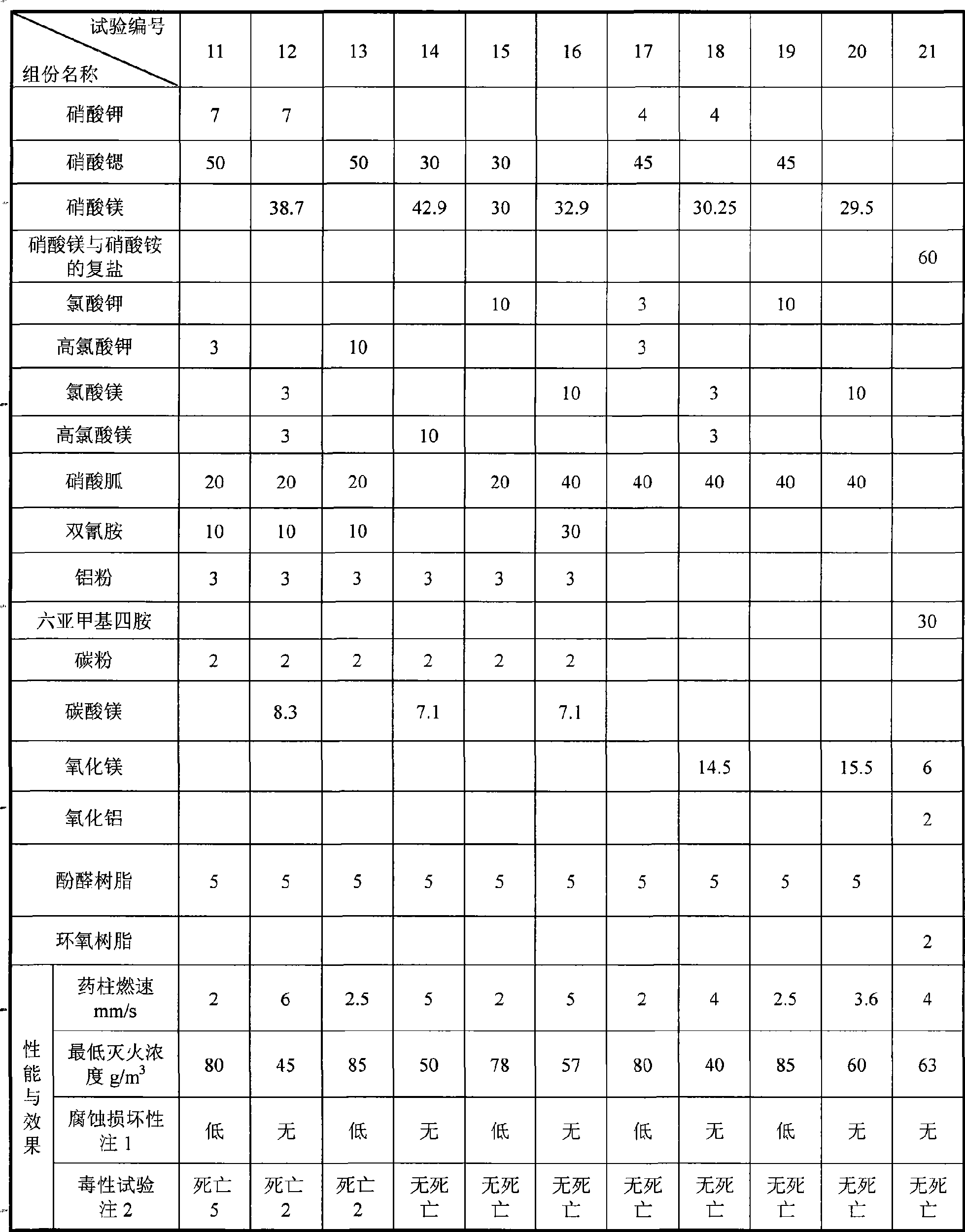
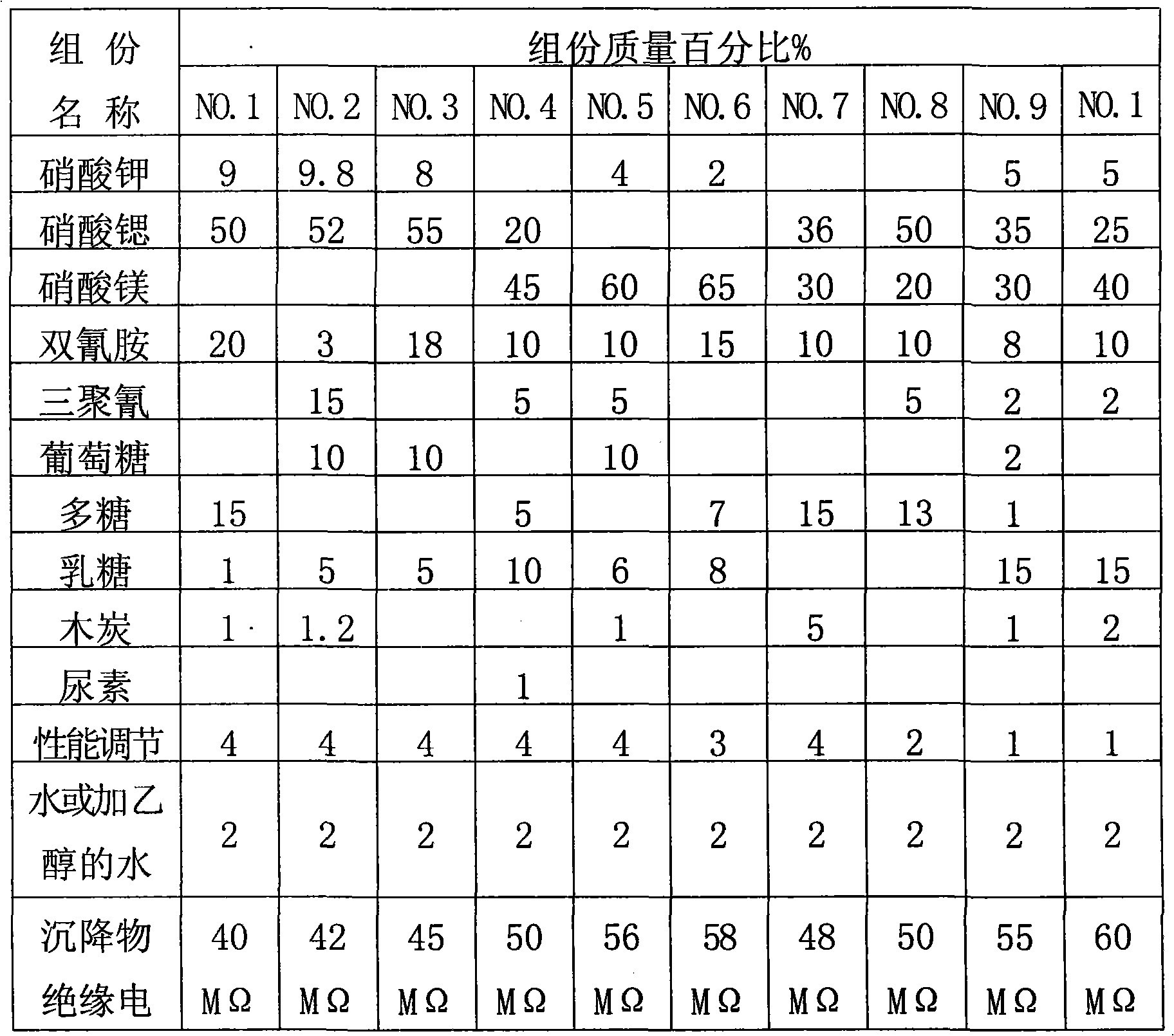
The invention provides ''a hot aerosol fire-extinguishing composition'' and relates to an oxidative magnesium salt hot aerosol fire-extinguishing composition represented by anhydrous magnesium nitrate. The composition is characterized in that the composition can be magnesium nitrate, magnesium carbonate, or other magnesium salt, and can also be a compound of magnesium nitrate, or other magnesium salt with potassium nitrate, strontium nitrate, or other potassium salt or strontium salt; a reducer can be one of or the combination of a plurality of ammonium carbamidine, dicyandiamide, red prussiate of potash, formamine, triazole, and tetrazole; a capability improver can be magnesium carbonate, manganous carbonate, aluminium powder, powdered carbon, magnesium hydrate, metal oxide, etc.; and the bond adopts phenolic resin, etc. The preparation of the magnesium salt comprises the continuous steps: medium temperature and low pressure dehydration, spray under the protection of nitrogen-oxygen flow or ultrafine grinding of grinded colloid, and microencapsulated hydrophobic treatment, etc. Compared with the prior art, the fire-extinguishing composition has the advantages of low price, extensive source, fire-extinguishing capability of K-type composition, and low causticity and toxicity of the ultimate product of combustion.
Owner:SHAANXI J&R FIRE FIGHTING CO LTD
Fume desulfurizing and denitrifying device based on magnesia and method
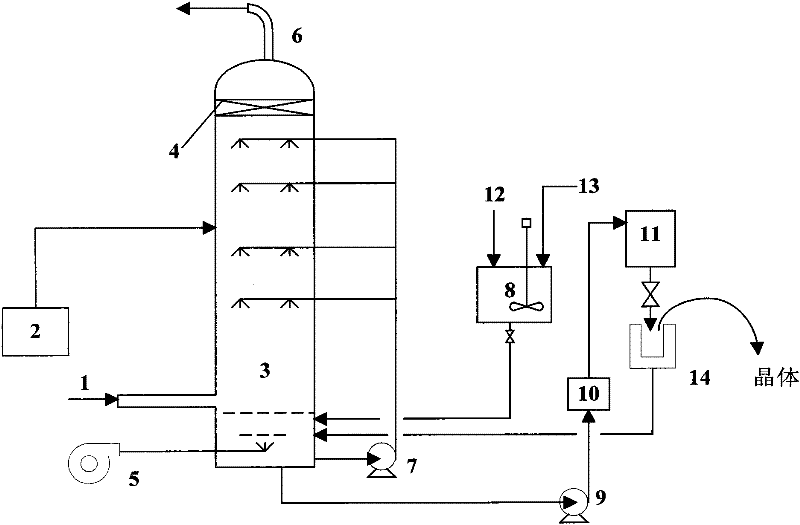
InactiveCN102350197AEfficient recyclingSimple processDispersed particle separationMagnesium nitratesEngineeringSlurry
The invention relates to technology for treating fume pollutants and aims at providing a fume desulfurizing and denitrifying device based on magnesia and a method. The fume desulfurizing and denitrifying device based on the magnesia and the method are technically characterized in that SO2 is desorbed at the lower part of a desulfurizing and denitrifying tower by using magnesia slurry, a metal M2+ catalyst is added into circular absorption liquid, and air is also blown into the desulfurizing and denitrifying tower so as to quickly oxidize a desulfurized product of MgSO3 into MgSO4; ozone is introduced into the middle part of the desulfurizing and denitrifying tower so as to oxidize NO in fume into NOx; and the magnesia slurry is sprayed into the upper part of the tower, the NOx is absorbed, and magnesium nitrate is generated. Magnesium sulfate heptahydrate and magnesium nitrate hexahydrate can be respectively obtained by the conventional crystal separation of magnesium sulfate and the magnesium nitrate in the absorption liquid. According to the fume desulfurizing and denitrifying device based on the magnesia and the method, the desulfurizing and denitrifying procedures are finished in one tower by using the magnesia, and the recycle of the by-products of the SO2 and the NO is also realized. The fume desulfurizing and denitrifying device based on the magnesia and the method have simple desulfurizing and denitrifying processes, low investment, low resistance and low running cost. By adopting the fume desulfurizing and denitrifying device based on the magnesia and the method, the desulfurizing rate of more than 96 percent and the denitrifying rate of more than 92 percent can be obtained.
Owner:EAST CHINA UNIV OF SCI & TECH
Method for preparing fuel element for smoking article
The invention provides a method for making a fuel element for a smoking article including the steps of mixing a metal-containing catalyst precursor with a filler material or graphite or a combination thereof to form a pre-treated fuel element component; optionally calcining the pre-treated fuel element component in order to convert the catalyst precursor to a catalytic metal compound; after the optional calcining step, combining the pre-treated fuel element component with a carbonaceous material and a binder to produce a fuel element composition; and forming the fuel element composition into a fuel element adapted for use in a smoking article. Examples of metal-containing catalyst precursors include iron nitrate, copper nitrate, cerium nitrate, cerium ammonium nitrate, manganese nitrate, magnesium nitrate, and zinc nitrate. Fuel elements treated according to the invention, and smoking articles including such fuel elements, are also provided.
Owner:R J REYNOLDS TOBACCO COMPANY
Method for preparing spherical nano silver powder
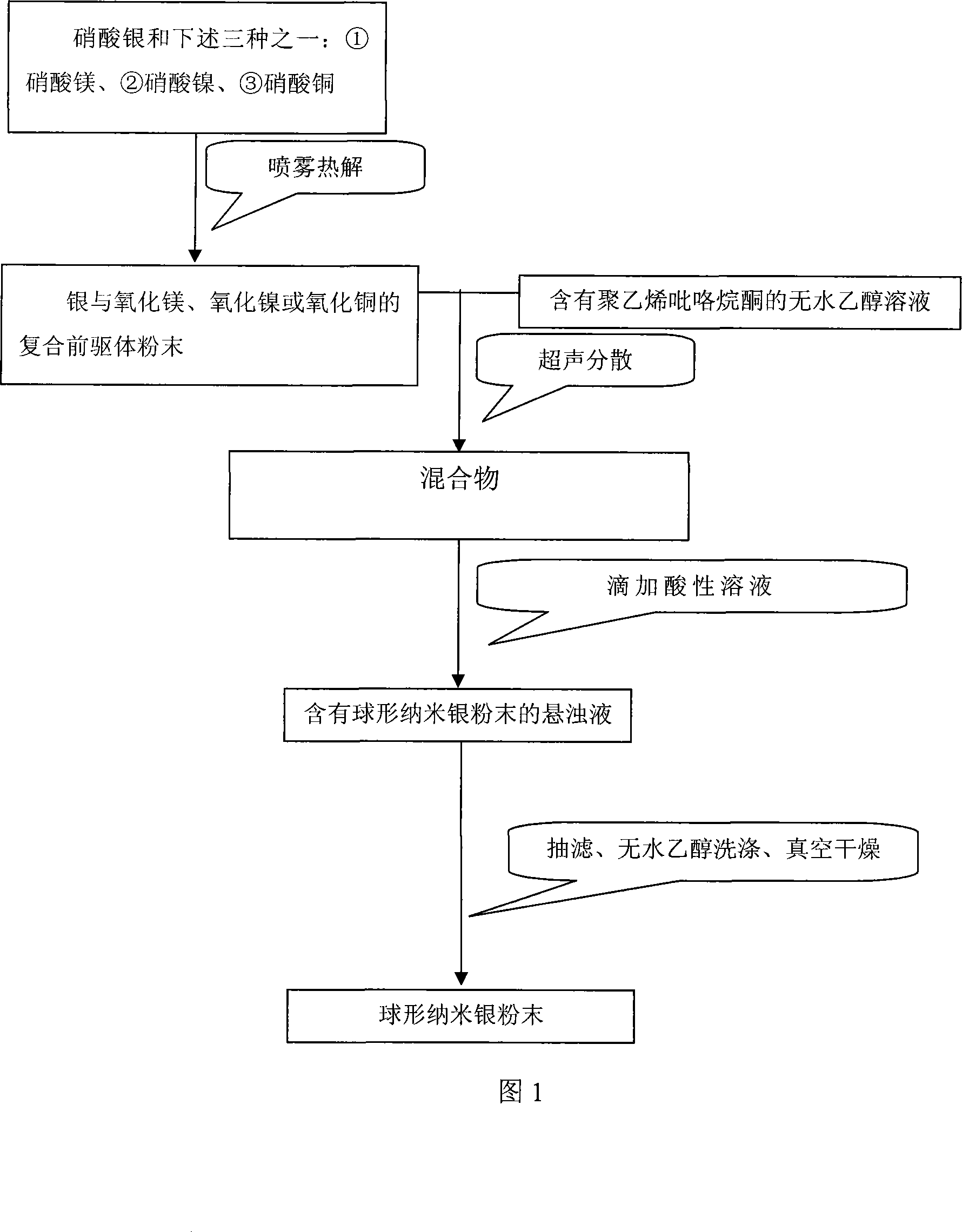
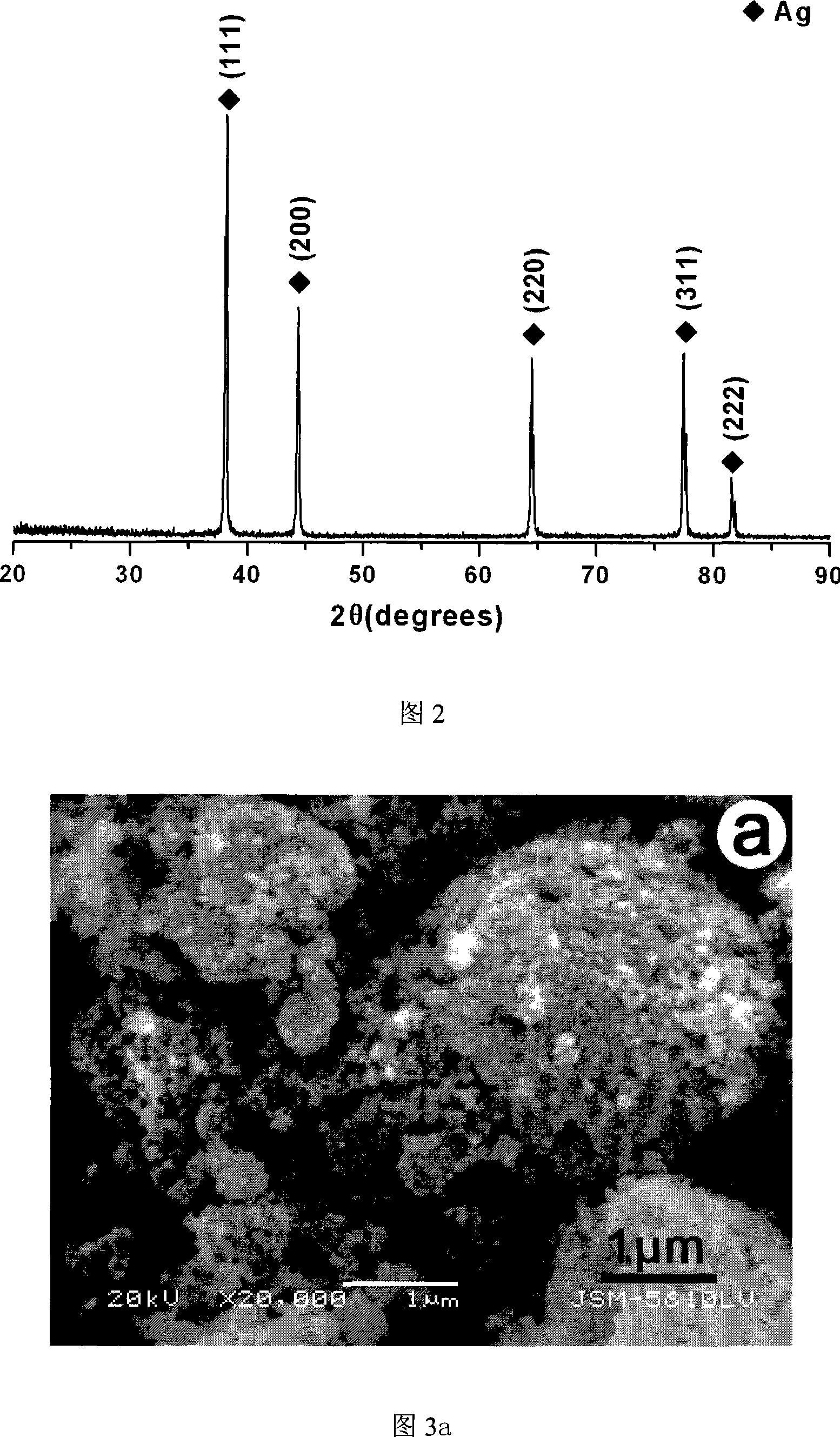
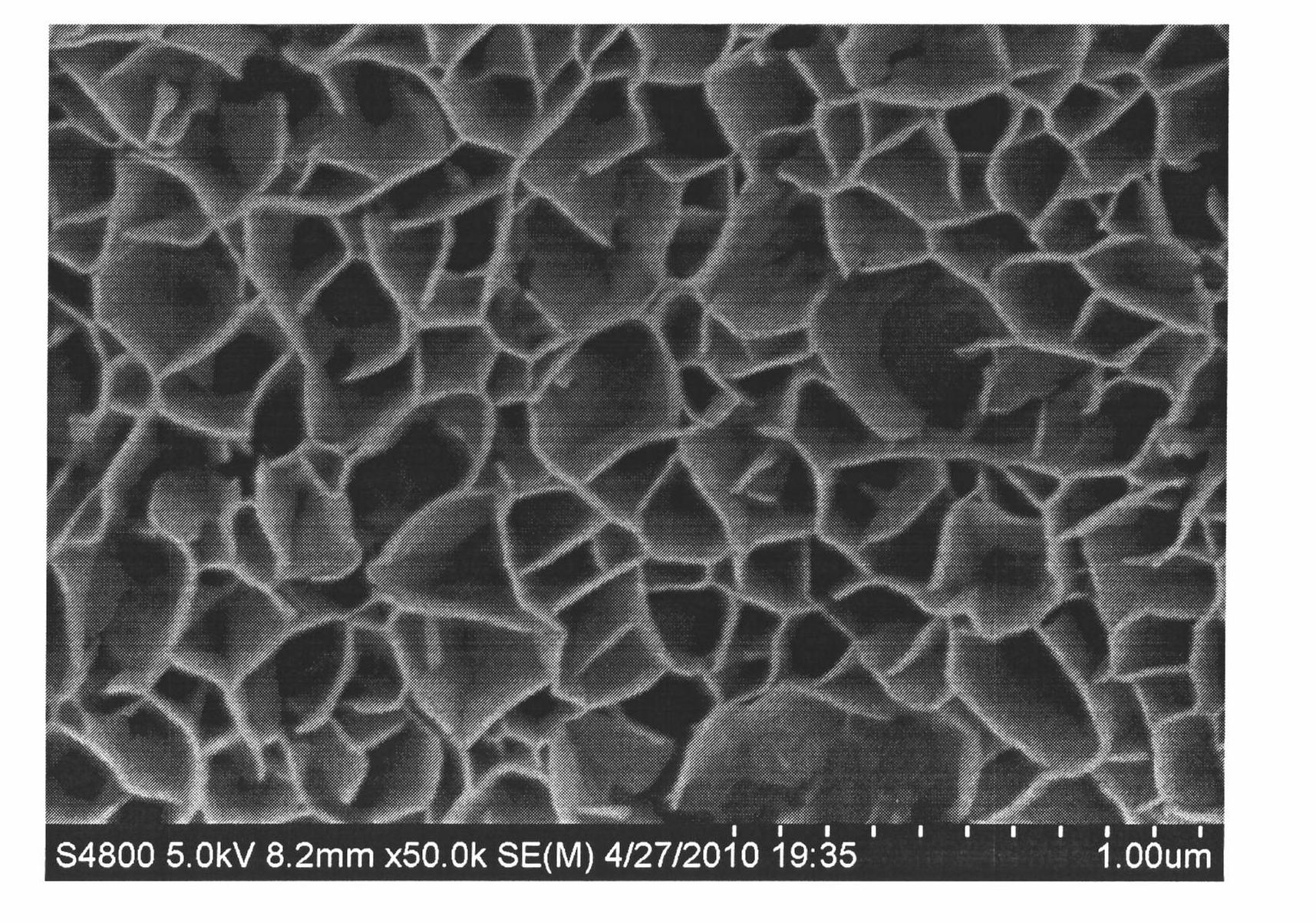
The invention relates to a method for preparing a spherical nanometer sliver powder. A method for preparing a spherical nanometer sliver powder is characterized in that the invention includes the following steps: 1) spraying and pyrolyzing: a, dissolving silver nitrate with one of the three: {1}magnesium nitrate, {2}nickel nitrate, {3}copper nitrate into water to prepare into a water liquor with a consistency of 10 to 40wt percent; the mol ratio of magnesium ion, nickel ion or copper ion in the liquor is 0.05 to 1 :4; then spraying for pyrogenation; b, under the existence of a dispersant and protecting agent, adding into a non-water ethanol to disperse to acquire a mixture; 2) preparing the spherical nanometer sliver powder: a, dripping acid liquor into the mixture and adjusting a PH value between 3 to 5 to acquire a suspension containing the spherical nanometer sliver powder; b, pumping filtration, washing and drying to acquire the spherical nanometer sliver powder. The preparing method is simple and easy to be controlled, has no pollution and low cost, is suitable for industrialization scale production. The prepared spherical nanometer sliver powder belongs to the nanometer grade and the powder has uniform distribution of the grain diameter, regular shape and high yield.
Owner:WUHAN UNIV OF TECH
Film for graphene/porous nickel oxide composite super capacitor and preparation method thereof
InactiveCN102013330AIncrease contact areaShorten the diffusion distanceCapacitor electrodesCapacitanceElectron
The invention discloses a film for a graphene / porous nickel oxide composite super capacitor. The invention is characterized in that the film is of a disordered nano porous structure, the aperture range is 10 to 350nm, the thickness of the film is 1 to 5 mu m, and the weight ratio of graphene to nickel oxide is (0.5:100) to (2:100). The method for preparing the film comprises the following steps: dissolving graphite oxide sheets and magnesium nitrate into an isopropanol solution so as to form a positively-charged graphite oxide sheet sol, then carrying out electrophoretic deposition on the obtained sol so as to obtain a graphene film; and through taking the graphene film as a deposition carrier, preparing a three-dimensional porous film for the graphene / porous nickel oxide composite super capacitor by using a chemical bath film-coating method. The porous composite thin film prepared by the method has the advantages of good mechanical capacity and super-capacitance property, high-discharge specific capacitance, high-ratio charge-discharge properties, high cycle life and the like, and has wide application prospects in the fields such as electric automobiles, communication, consumer electronics, signal control and the like.
Owner:ZHEJIANG UNIV
Compound type aerosol extinguishing agent
InactiveCN101862517ALow fire extinguishing concentrationImprove corrosion resistanceFire extinguisherSucroseNuclear chemistry
The invention discloses a compound type aerosol extinguishing agent comprising the following components in percentage by weight: 30-75 percent of oxidizing agent, 20-50 percent of reducing agent, 2-15 percent of performance regulating agent and no more than 2 percent of binding agent. The oxidizing agent is prepared by matching any two or three matched components selected from potassium nitrate, strontium nitrate and magnesium nitrate; the reducing agent is prepared by matching two or more components selected from polyhexose, glucose, starch, sorbitol, xylitol, lactose, dicyandiamide, melamine, carbamide and sucrose; the performance regulating agent is prepared by matching one or more component selected from carbon powder, light magnesium carbonate, magnesium stearate, aluminium nitrate, hexamethylenetetramine, aluminium oxide, magnesium oxide and light metal oxide; and the binding agent is prepared by matching water or 20-30 percent of ethanol added into the water. In the invention, production raw materials have a wide source and low cost, can be constantly obtained and are safe and reliable, and burnt residues have no toxicity and good environmental protection performance.
Owner:湖南省金鼎消防器材有限公司
A low-temperature preparation method of carbon nanotubes
A low-temperature preparation method of carbon nanotubes by a chemical vapor deposition method with Ni / MgO as a catalyst comprises the following steps: dissolving nicdel nitrate hexahydrate and magnesium nitrate hexahydrate in ethanol according to different ratios and concentrations to obtain a mixed solution of nicdel nitrate and magnesium nitrate which is used as a catalyst precursor for carbonnanotube growth; spraying the catalyst precursor solution on a substrate such as silicon, glass, and the like, placing the substrate on a sample stage of a chemical vapor deposition system, heating and decomposing to generate nickel oxide and magnesium oxide, introducing hydrogen, performing reduction in hydrogen plasma of the plasma chemical vapor deposition system to generate nickel nanometer metal particles and to obtain a Ni / MgO catalyst system; introducing hydrocarbons, preparing carbon nanotubes for various demands under different process conditions. The advantages of the invention are that the carbon nanotube preparation process is simple, high in yield, and low in synthetic temperature, and the obtained carbon nanotubes have good quality, high purity, uniform tube diameter distribution, and microelectronic process compatibility.
Owner:TIANJIN UNIVERSITY OF TECHNOLOGY
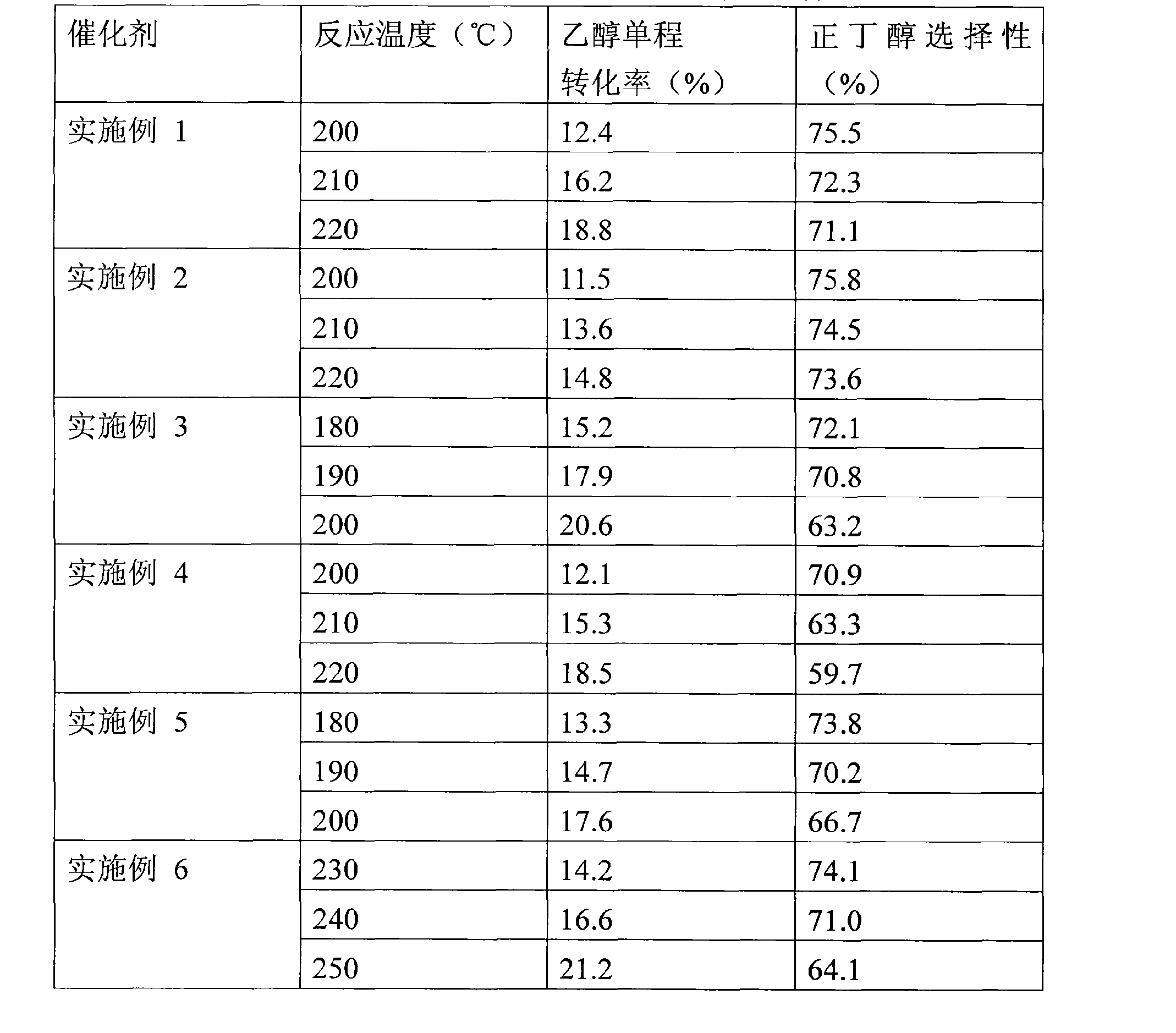
Bi-component supported catalyst of ethanol condensed n-butanol and a method of preparing thereof
InactiveCN101530802ASimple methodLow costOrganic compound preparationHydroxy compound preparationPotassium fluorideGram
The invention discloses a bi-component supported catalyst of ethanol condensed n-butanol and a method of preparing thereof. The main active ingredients of the catalyst are as follows by weight percentage content: 2%-10% nickel, 0.5%-9% cocatalyst MgO or KF. The preparing method comprises the steps of: directly dipping 30-50 grams of granulose gamma-Al2O3 into nickel acetate or nickel nitrate aqueous solution with consistency being 0.5-1.0 mol / L, dipping for 2 to 4 days, occasionally stirring, evaporating, drying under 130 degrees centigrade, then dipping into magnesium nitrate or potassium fluoride aqueous solution with consistency being 0.1-1.0 mol / L for 2 to 3 days, evaporating, drying under 130 degrees centigrade to prepare the bi-component supported catalyst of ethanol condensed n-butanol. The method of preparing bi-component supported catalyst in the invention is simple and has low cost; the catalytic activity is higher than that of the single component nickel supported catalyst; the selectivity of the n-butanol in the products can achieve above 70% and has wildly industrialized application prospect.
Owner:ZHEJIANG UNIV
Nickel-based catalyst for hydrogen production by ethanol steam reforming and preparation method thereof
InactiveCN101444737AHigh mechanical strengthHigh catalytic activityHydrogenMetal/metal-oxides/metal-hydroxide catalystsSteam reformingNanowire
The invention discloses a nickel-based catalyst for hydrogen production by ethanol steam reforming and a preparation method thereof. The catalyst is made by taking nanoporous silicon dioxide aerogel as a catalyst carrier, a metal elementary substance nickel nanowire as an active component, and nano-particles of MgO or CaO or ZrO2 or TiO2 or CeO2 or compound nano-particles thereof as an adjuvant. The preparation method comprises the following steps: preparing a sol from silanolate, an alcohol solvent, nickel nitrate or magnesium nitrate or calcium nitrate or zirconium nitrate or cerous nitrate or complex nitrate thereof and an acidic catalyst at certain proportions; forming a wet gel complex, and then performing supercritical fluid drying. The catalyst has strong catalytic activity and selectivity for the hydrogen production by the ethanol steam reforming, has higher hydrogen yield and stronger CO2 selectivity at a lower temperature, and limits the selectivity of byproducts CH4 and CO at a lower level. Meanwhile, the preparation method has simple process, low cost and certain mechanical strength.
Owner:HUNAN SHANGYIFENG NEW MATERIAL TECH CO LTD
Compositions comprising ammonium nitrate double salts
ActiveUS20070199357A1Reduce detonation sensitivityReduce sensitivityAlkali orthophosphate fertiliserAmmonium orthophosphate fertilisersPotassium nitrateDetonation
Preferred aspects of the present invention provide ammonium nitrate compositions comprising ammonium nitrate and at least a second compound, said second compound being present under conditions and in amounts effective to substantially reduce the detonation sensitivity of the composition and / or to otherwise improve a desired property of the composition. In certain embodiments, the second compound is selected from the group consisting of ammonium sulfate, ammonium phosphate, calcium nitrate, potassium nitrate, magnesium nitrate, ammonium molybdenate, ammonium hexaflouralsilicate, neodymium hydroxynitrate, and combinations of two or more of these. In preferred embodiments, at least a substantial portion of the ammonium nitrate in the composition is in the form of a double salt with one or more of said second compounds. In highly preferred embodiments, the present compositions consist essentially of one or more double salts of ammonium nitrate and a second compound as described herein.
Owner:ADVANSIX RESINS & CHEM LLC
Metal oxide nanometer material for treating wastewater containing dyes or heavy metal ions, preparation method and application thereof
InactiveCN101591044ARaising the temperature can increase the active pointIncrease active pointOther chemical processesWater/sewage treatment by sorptionHigh pressureZinc nitrate
The invention relates to a metal oxide nanometer material for treating wastewater containing dyes or heavy metal ions, a preparation method and application thereof. Compositions of the metal oxide nanometer material is one or a mixture of more than two of CaO, ZrO2, SiO, ZnO, TiO2, MgO, Fe2O3 and NiO. The preparation method comprises the following steps: dissolving one or a mixture of more than two of calcium nitrate containing crystallization water, zirconium nitrate, ethyl orthosilicate, zinc nitrate, butyl titanate, magnesium nitrate, ferric nitrate and nickel nitrate into methanol, ethanol or propanol, adding a phenylcarbinol or benzylcarbinol structure-directing agent into the obtained solution, moving the mixture into a high-pressure kettle after the mixture is mixed evenly, heating the mixture to between 120 and 200 DEG C for 2 to 6h to ensure that nitrate is completely alcoholized under the protection of chlorine gas with the pressure of between 10 and 1.5*10 Pa, then heating the mixture to between 261 and 269 DEG C for 15h, drying the obtained product, and then performing high-temperature roasting at a temperature of between 300 and 500 DEG C to obtain the metal oxide nanometer material. The metal oxide nanometer material is added into the wastewater containing the dyes or the heavy metal ions to ensure that the metal oxide nanometer material and the wastewater are fully contacted to decolorize, absorb or degrade the wastewater containing the dyes.
Owner:SOUTH CENTRAL UNIVERSITY FOR NATIONALITIES
Catalyst for synthesizing para diethyl benzene by ethanol and ethyl benzene combination reaction and its preparation method
InactiveCN1605390AHigh yieldGood choiceOrganic chemistryMolecular sieve catalystsMolecular sievePtru catalyst
The present invention relates to catalyst for alkylating ethanol and ethyl benzene into p-diethylbenzene and its preparation process. The industrial catalyst for efficient synthesis of p-diethylbenzene is prepared with the H-ZSM-5 molecular sieve with Si / Al ratio of 50 as basic matter and through surface acidity and tunnel regulation with B, Mg and Co, and has ideal pore size distribution and in-tunnel acidity distribution and strong coking resisting capacity. The precursor of B is boric acid, that of Mg is magnesium nitrate and that of Co is cobalt nitrate. The mass ratio between B and H-ZSM-5 molecular sieve is 1-3 %, that between Mg and H-ZSM-5 molecular sieve is 0.1-1 % and that between Co and H-ZSM-5 molecular sieve is 1-3 %. Compared with available similar catalyst, the present invention has the features one simple preparation process, low cost, high selectivity and yield of p-diethylbenzene, etc.
Owner:NANKAI UNIV
Preparation method of catalyst for coproducing isopropanol and methyl isobutyl ketone by acetone hydrogenation
ActiveCN102806086ALow content of active metal componentsExtended service lifeOxygen-containing compound preparationOrganic compound preparationNitrateMethyl isobutyl ketone
The invention relates to a preparation method of a catalyst for coproducing isopropanol and methyl isobutyl ketone by acetone hydrogenation. The catalyst comprises an alumina carrier and Ni and Mg loaded on the gamma-Al2O3 carrier, and particularly comprises, by total weight, 10-18% of the Ni, 3-8% of the Mg and the balance alumina; and the BET specific surface area of the catalyst ranges from 100m<2> / g to 180m<2> / g, and the pore volume of the catalyst ranges from 0.35mL / g to 0.55mL / g. The preparation method includes steps of (1), preparing the carrier by details of weighing pseudo-boehmite powder, adding binders and extrusion assistants into the pseudo-boehmite powder, forming by strip extrusion or granulating by rotation, drying and calcining, cooling to reduce temperature so as to obtain the stripped or spherical carrier; (2), impregnating the carrier into magnesium nitrate by details of loading a magnesium additive onto the carrier by means of saturated impregnation, drying and then calcining for 2-6 hours at the temperature ranging from 400 DEG C to 500 DEG C; (3), loading the actively metallic nickel by details of impregnating for 10-24 hours and calcining for 2-6 hours at the temperature ranging from 350 DEG C to 480 DEG C; and (4), loading the actively metallic nickel again by details of drying a semi-finished product, impregnating the dried semi-finished product into impregnation liquid of nickel nitrate again, repeating the step (3) once, and calcining the semi-finished product for 2-4 hours at the temperature ranging from 400 DEG C to 450 DEG C so as to obtain the finished catalyst.
Owner:CHINA NAT OFFSHORE OIL CORP +1
Preparation method of LDHs (magnesium-based layered double hydroxides)
ActiveCN105753022ARich sourcesLow priceAluminium-carbonate compoundsZinc compoundsMagnesium saltCalcium magnesium
The invention provides a preparation method of LDHs (magnesium-based layered double hydroxides). According to the method, calcium oxides or calcium magnesium oxides are used as an alkali source, calcium oxides or calcium magnesium oxides and a magnesium salt are subjected to a precipitation reaction, magnesium hydroxide precipitates are produced, then the magnesium hydroxide precipitates and divalent and trivalent soluble metal nitrates or chlorides are used as raw materials, LDHs are prepared through a liquid-solid phase reaction, and byproducts, namely, magnesium chlorides or magnesium nitrates, can be used as raw materials. The method has the advantages that the raw materials are rich in source, the price is low, the preparation process is simple, reaction conditions are mild, industrial production is facilitated and the like, and prepared LDHs can be widely applied to fields of catalysis, adsorption, environmental protection, high-polymer plastic and the like.
Owner:BEIJING UNIV OF CHEM TECH
Sponge composite metallic organic framework material for adsorption separation
ActiveCN110038540AStrong loadHigh load rateGas treatmentOther chemical processesFragilityMetal-organic framework
The invention relates to a sponge composite metallic organic framework material for adsorption separation and belongs to the technical field of materials. According to the invention, a two-step in-situ growth method is adopted for loading MOFs (metallic organic framework material) into a duct in a sponge carrier. The MOFs is synthesized from following organic ligands and metal salts, wherein the organic ligands include pyrazine, trimesic acid, terephthalic acid, 2,5-dihydroxyterephthalic acid, and the metal salts include cupric fluosilicate, magnesium nitrate, zirconium chloride, cupric nitrate, chromic nitrate, ferric nitrate, aluminum nitrate and hydrates thereof. According to a preparation method provided by the invention, a large amount of MOFs loaded in the duct in the sponge carriercan be realized; MOFs load rate is high; load is stable; the defects of fragility and easiness in loss of MOFs powder can be overcome; a breakthrough experiment proves that the material provided by the invention has the characteristics of high CO2-absorbing capacity, high adsorption separation selectivity, stable adsorption separation property, and the like.
Owner:BEIJING INSTITUTE OF TECHNOLOGYGY
Ruthenium-based ammonia synthetic catalyst and preparation thereof
InactiveCN101322938ASimple processImprove production efficiencyMetal/metal-oxides/metal-hydroxide catalystsBulk chemical productionAlkaline earth metalRare earth
The invention provides a Ru-based ammonia synthesis catalyst and a preparation method thereof. The catalyst takes magnesium oxide as a carrier, metal ruthenium as an active constituent and at least one of alkali metal, alkaline earth metal or rare earth metal as an assistant; the composition is Ru-M1 / MgO, Ru-M2 / MgO, Ru-M1-M2 / MgO or Ru-(M1+M2) / MgO and the preparation method is as follows: the precursor, Ru-magnesium hydroxide catalyst, is prepared by a coprecipitation and dipping method: magnesium nitrate and ruthenium chloride are dissolved in water; the mixed solution is added into an alkaline solution to generate a precipitation; the precipitation is centrifugally washed, dried, sintered by reduction or soaked by equivoluminal soaking auxiliary after sintering, thus obtaining the catalyst that is required. The catalyst prepared by the synthesis method of the invention has good performance, relatively cheap raw material that is required, low energy consumption as well as simple devices and flow; besides, the catalyst is beneficial to reducing the cost and realizing mass production.
Owner:FUZHOU UNIV
Formula and preparation process of concrete sulfate resistance agent
The invention relates to a formula of a concrete sulfate resistance agent, which comprises the following ingredients by weight percentage: one or more of 30% to 50% ferric nitrate, ferrous nitrate and magnesium nitrate, 20% to 40% barium nitrate, 0.5% to 10% sodium silicate, 5% to 10% lithium hydroxide, 1% to 5% sodium benzoate and 30% to 60% coal ash or grounded mineral powder. A preparation process of the concrete sulfate resistance agent comprises the following steps of: mixing one or more of 30% to 50% ferric nitrate, ferrous nitrate and magnesium nitrate, 20% to 40% barium nitrate, 0.5% to 10% sodium silicate, 5% to 10% lithium hydroxide, 1% to 5% sodium benzoate and 30% to 60% coal ash or grounded mineral powder in a mixer or a mixing tank for 5 to 30 minutes, discharging the materials, sealing and preserving. The sulfate resistance coefficient of the concrete sulfate resistance agent can reach 95% to 98%, so that the impervious and the freeze proof levels of the concrete are greatly improved, and the service durability of the concrete is greatly enhanced.
Owner:WATER RESOURCES RES INST OF SHANDONG PROVINCE
Method and system for agricultural fertigation
ActiveUS8568506B1High fertilizer-nutrient contentEasy to customizeCalcareous fertilisersMagnesium fertilisersPotassium hydroxidePhosphoric acid
An agricultural fertigation method includes the in situ manufacture of one or more fertilizers within the irrigation upstream of the agricultural field being irrigated. Co-reactant raw materials, namely sulfuric acid, phosphoric acid, nitric acid, potassium hydroxide, urea, ammonium hydroxide, ammonia, calcium nitrate and / or magnesium nitrate, are intermixed with each other and with the stream of the flowing irrigation water. The stream of flowing irrigation water dampens the resultant dissolution and reaction exotherms. A system wherein raw materials are efficiently fed to the irrigation system main line or a side-arm reactor efficiently implements the method.
Owner:MILLER JOHN C +1
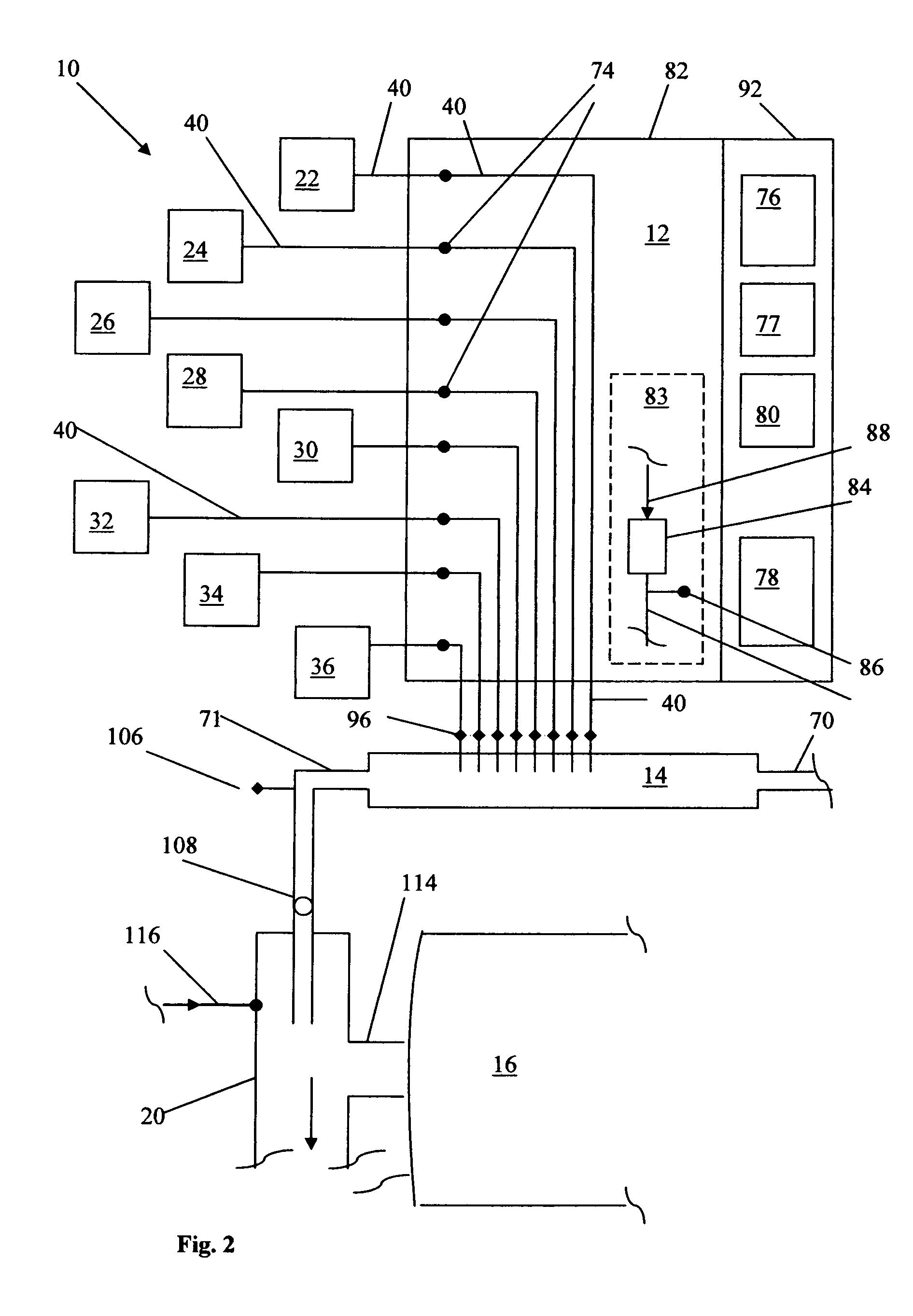
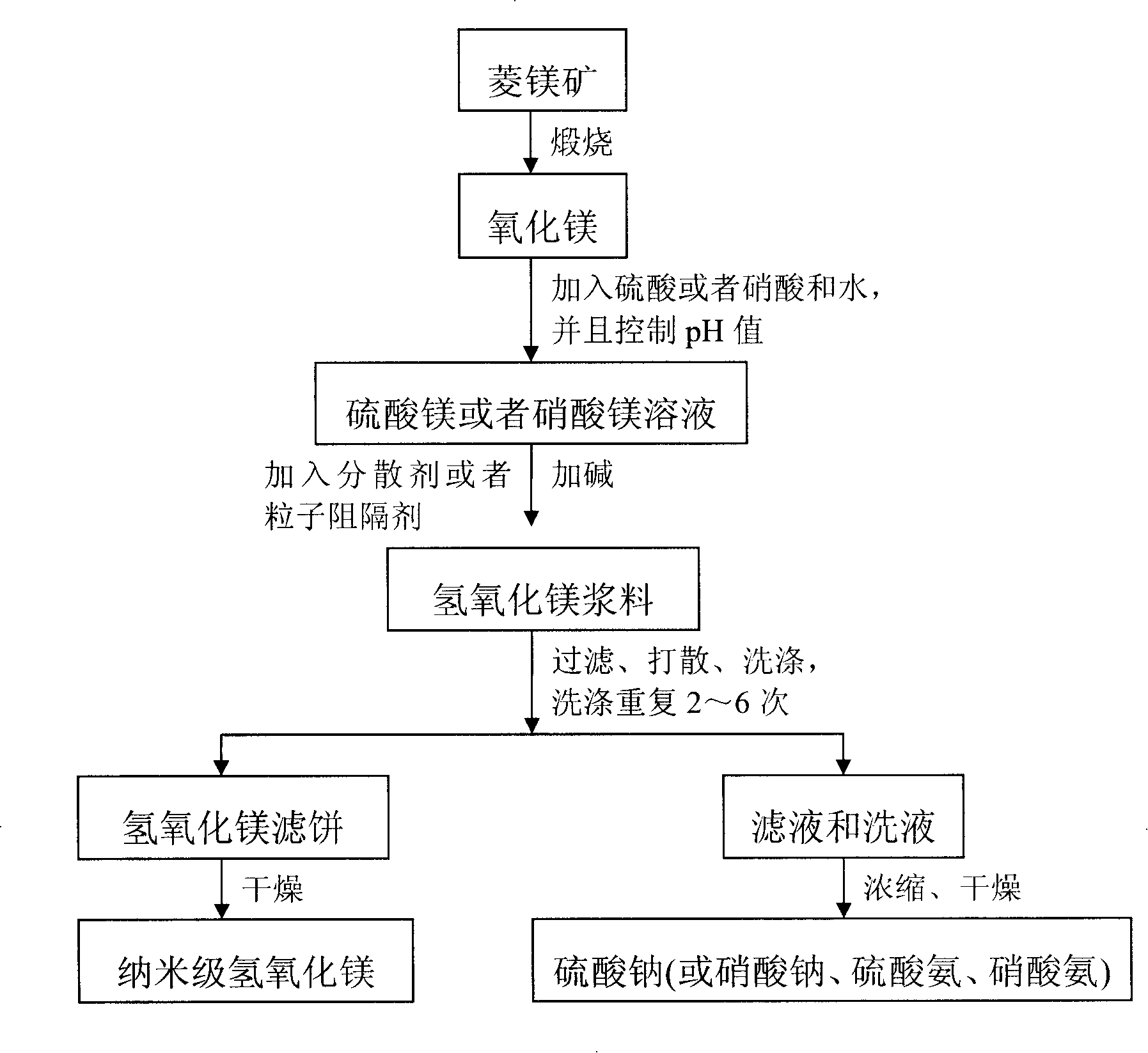
Method for producing nano-magnesium hydroxide by using low-level magnesite
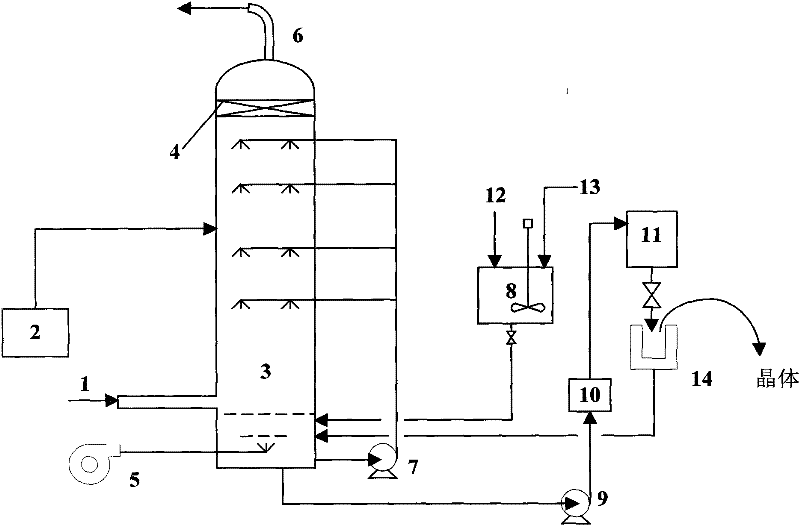
InactiveCN101219800ASmall specific surface areaHigh purityMagnesium hydroxideMagnesium saltEvaporation
The invention relates to a method for comprehensive utilizing low-taste magnesite, which belongs to a mineral process and environmental protection field. The magnesite powder is calcinated under a temperature ranging from 800 DEG C to 1200 DEG C to prepare magnesia; the magnesia is reacted with sulfuric acid or nitric acid to prepare dissolvable magnesium salt (magnesium sulfate or magnesium nitrate) and the pH value of the system is regulated to 3-9; the reacted slurry is filtered to get filtrate; the filtrate is reacted with alkali (sodium hydroxide or ammonia) and the technological condition is controlled to prepare the slurry of magnesium hydroxide with a granularity of nanometer and a shape of flake; nanometer magnesium hydroxide product is prepared by filtering, aging, drying and scattering the slurry. The filtrate and washings are substantially composed of sodium sulfate, sodium nitrate, ammonium sulfate or ammonium nitrate, from which products of the sodium sulfate, the sodium nitrate, the ammonium sulfate or the ammonium nitrate can be acquired after high efficient evaporation and drying process.
Owner:杜高翔
Preparation method of polyacrylamide nano composite fracturing fluid
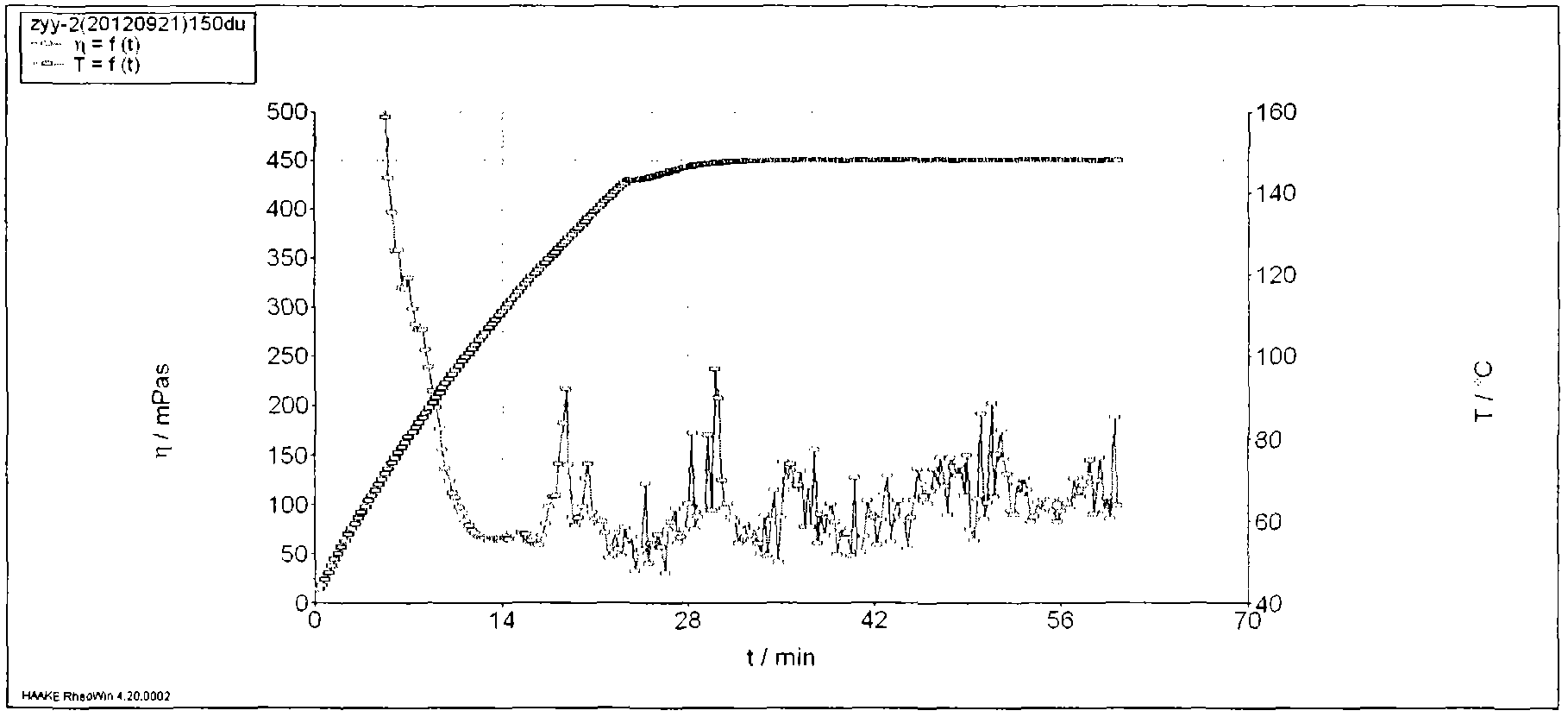
ActiveCN104109525AHigh temperature resistantSalt resistantDrilling compositionIn situ polymerizationFracturing fluid
The invention adopts polyacrylamide and a nano inorganic phase to prepare a polyacrylamide nano composite material through an in-situ polymerization method and then forms a fracturing fluid thickening agent. The nano inorganic phase is prepared by mixing the products of intercalation reactions between an organic long-chain intercalator and layered silicate with magnesium nitrate and aluminum nitrate. The nano inorganic phase, acrylamide monomer, a coupling agent, a complexing agent, an initiator, an oxidant, a reductant, a cosolvent, an auxiliary agent, and deionized water form a suspension fluid reaction system, and the polyacrylamide nano composite material is formed after the polymerization-intercalation composite reactions. The polyacrylamide nano composite material with a mass percentage of 0.25% is taken as the thickening agent, and then is mixed with a crosslinking agent with a mass percentage of 0.20%, a gel breaker with a mass percentage of 0.20%, and other auxiliary agents to form a fracturing fluid system. The system is sheared for 70 minutes under a shearing speed of 170 s<-1> at a temperature of 150 DEG C so as to form a fracturing fluid with a viscosity larger than 50 mPa.s, and the fracturing fluid has the characteristics of high temperature resistance, shearing resistance, low frictional resistance, complete glue breaking effect, and good compatibility with the formation fluid.
Owner:CHINA UNIV OF PETROLEUM (BEIJING)
Preparation method and application of a compound carrier metal nanometer catalyst
InactiveCN101143326AHigh activityLarge specific surface areaCarbonyl compound preparation by oxidationMetal/metal-oxides/metal-hydroxide catalystsCyclohexanoneNano catalyst
The invention relates to a preparation method of composite carrier metal nanometer catalyst and the application. Copper is used as the main activity catalytic component; one or a plurality of the elements, cobalt, nickel, iron, cerium and lanthanum is / are used as an auxiliary activity catalytic component or components; alumina is used as the main carrier; one or a plurality of the compounds, magnesium oxide or magnesium nitrate, silica, calcium oxide or calcium nitrate, zirconia, molecular sieve is / are used as composite carrier or carriers. The composite carrier metal nanometer catalyst is prepared in the following procedures: 1.the preparation of the catalyst solution; 2. catalyst gel preparation; 3. extruded catalyst preparation. When applied in catalytic dehydrogenation method to prepare cyclohexanone, the catalyst of the invention has the advantages that: the roasted catalyst has greater specific surface area, thereby improving the activity of the catalyst; that high temperature activity and stability is greatly improved; that cyclohexanone yield reaches above 90 percent; that the conversion rate and the selectivity reaches the highest point reported in documents; and that the catalyst performs good effects in service life experiment.
Owner:HEBEI UNIVERSITY
Environment protection snow-dissolved agent and preparation method thereof
The invention relates to an environment protection snow-dissolved agent, which is composed of magnesium nitrate and sodium acetate. Each reactant is fully mixed and smashed according to ratio; then, the reactant is fully mixed with slip proofing agent to be filled into a plastic film for sealing; and a woven bag is arranged outside for sealing, so that the environment protection snow-dissolved agent of the invention is obtained. The snow-dissolved agent overcomes the damages in the traditional snow-dissolved agent, such as corrosion to roads and bridges and influence on farmland crops and green plants on two sides of roads and the like. The invention has fast snow dissolving speed, favourable effect, low cost and wide using temperature range, and the applicable temperature range is minus 5 DEG C-minus 35 DEG C, so the invention has favourable promotion and application prospect.
Owner:SOUTH CHINA UNIV OF TECH
Preparation method of modified carbon dioxide calcium-based absorbent
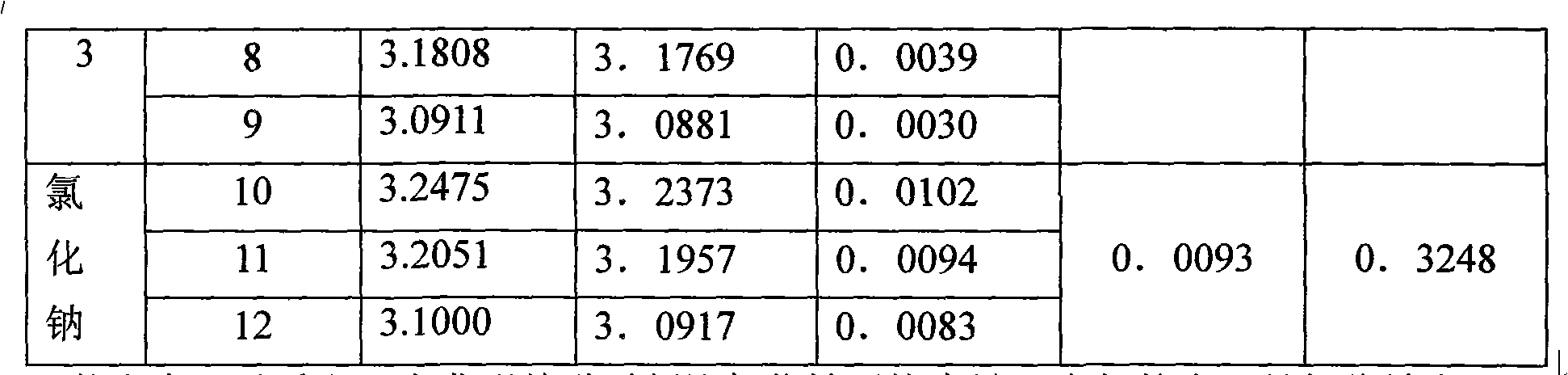
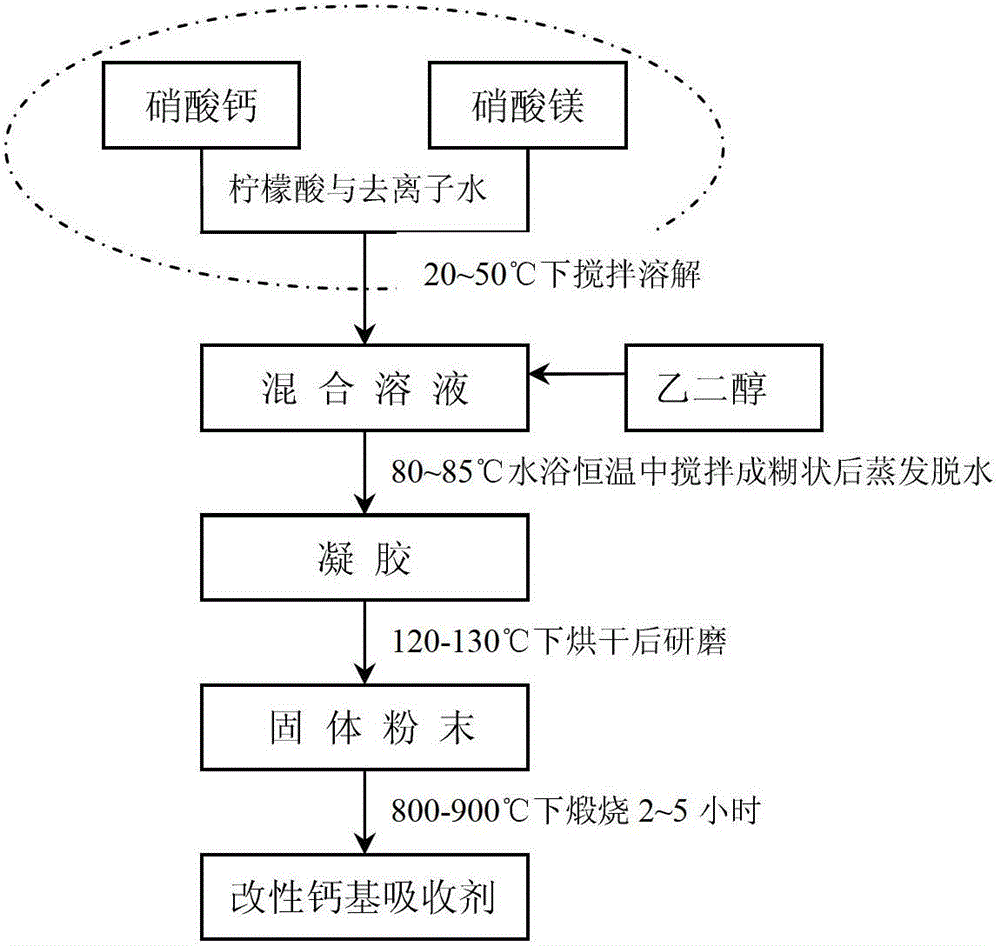
ActiveCN103331096AHigh cycle absorption efficiencyImprove anti-sintering performanceDispersed particle separationAir quality improvementWater bathsPorosity
The invention relates to a preparation method of a modified carbon dioxide calcium-based absorbent. The method comprises the steps of dissolving calcium nitrate, magnesium nitrate and citric acid in deionized water at the temperature of 20-50 DEG C to form a mixed solution, adding ethylene glycol into the mixed solution, carrying out evaporating dehydration in water bath at the temperature of 80-85 DEG C to form gel, drying the gel in a drying oven at the temperature of 120-130 DEG C, grinding the dried gel to obtain solid powder, calcining the solid powder at the temperature of 800-900 DEG C for 2-5 hours and obtaining the modified carbon dioxide calcium-based absorbent. The carbon dioxide calcium-based absorbent prepared through two modifications (the magnesium nitrate is the primary modifier and the ethylene glycol is the secondary modifier) has the advantages of high carbon dioxide cyclic absorption efficiency, strong sintering resistance and good pore structure distribution at the same time, so that the carbon dioxide calcium-based absorbent still has high carbon dioxide collecting efficiency in the multiple cyclic calcination / carbonation reaction processes and the defects of the existing carbon dioxide calcium-based absorbents, that the superficial area is decreased and the porosity is reduced after multiple cyclic calcinations, are overcome.
Owner:JIANGHAN UNIVERSITY
Production method of fire retardant type magnesium hydroxide
ActiveCN102417196AGood dispersionImprove the mixing effectMagnesium hydroxideMagnesium saltDistillation
The invention provides a production method of fire retardant type magnesium hydroxide and relates to a production method of magnesium hydroxide. In the invention, a hexagonal flaky fire retardant type magnesium hydroxide product is obtained in one step under the condition that any crystal form regulating agent is not used by applying special technology, process and equipment based on magnesium chloride or magnesium nitrate or magnesium salt obtained by ammonia distillation reaction of light calcined powder and ammonium chloride or ammonium nitrate as a raw material. The production method provided by the invention has the characteristics of short process route, less operation steps, low temperature and pressure condition requirements, continuous and large-scale production and stable and reliable product quality; and by using the production method provided by the invention, the production cost of the current fire retardant type magnesium hydroxide product can be obviously reduced. Magnesium hydroxide produced by using the method provided by the invention can be used for producing high polymer material products such as electric wires, cable jackets and the like.
Owner:辽宁麦格尼科技有限公司
Method for preparing dual-perovskite type methyl hydride combustion catalyst containing titanium
InactiveCN101293200ASimple structureHigh catalytic activityGaseous fuelsMetal/metal-oxides/metal-hydroxide catalystsEvaporationCoordination complex
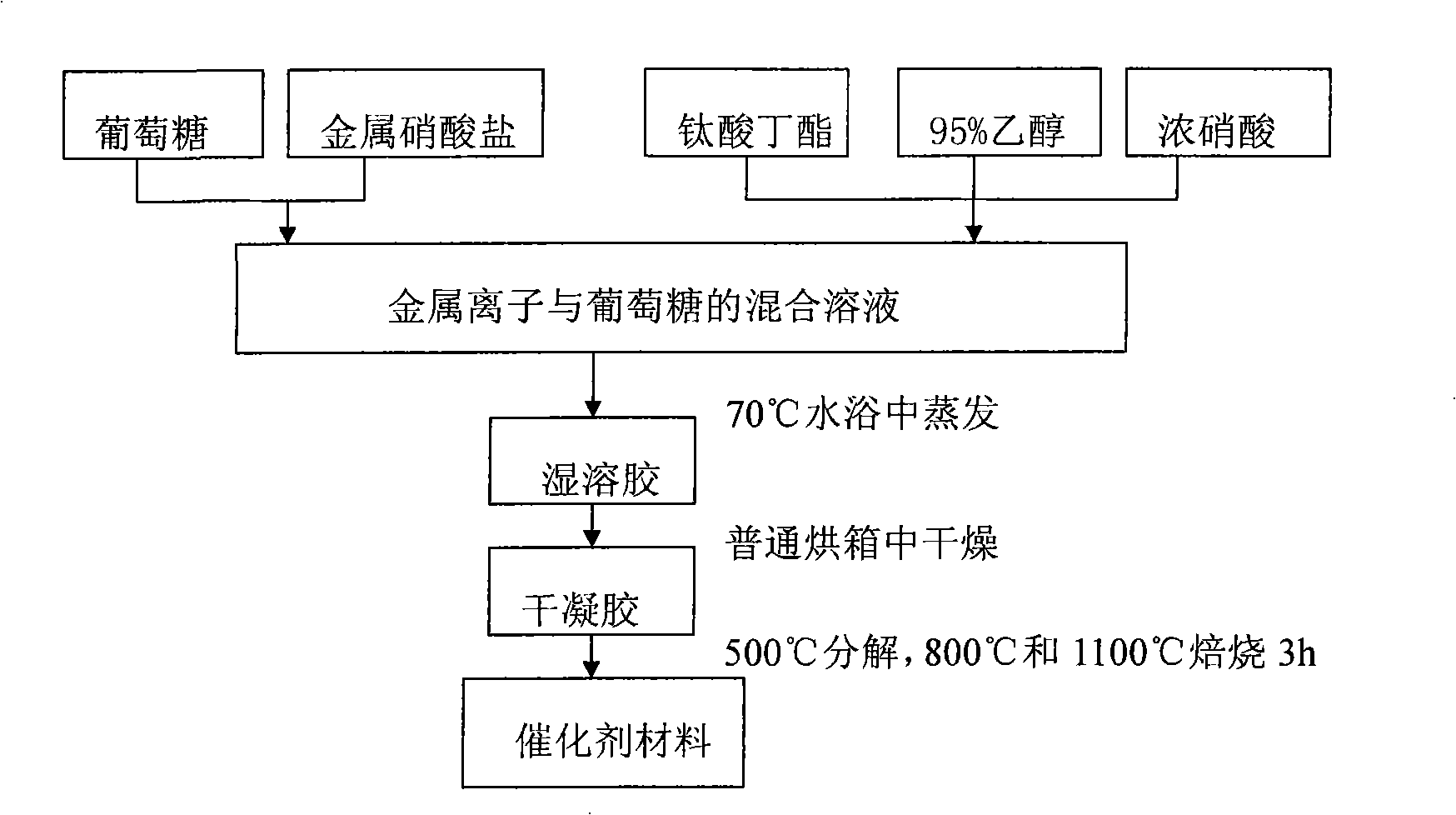
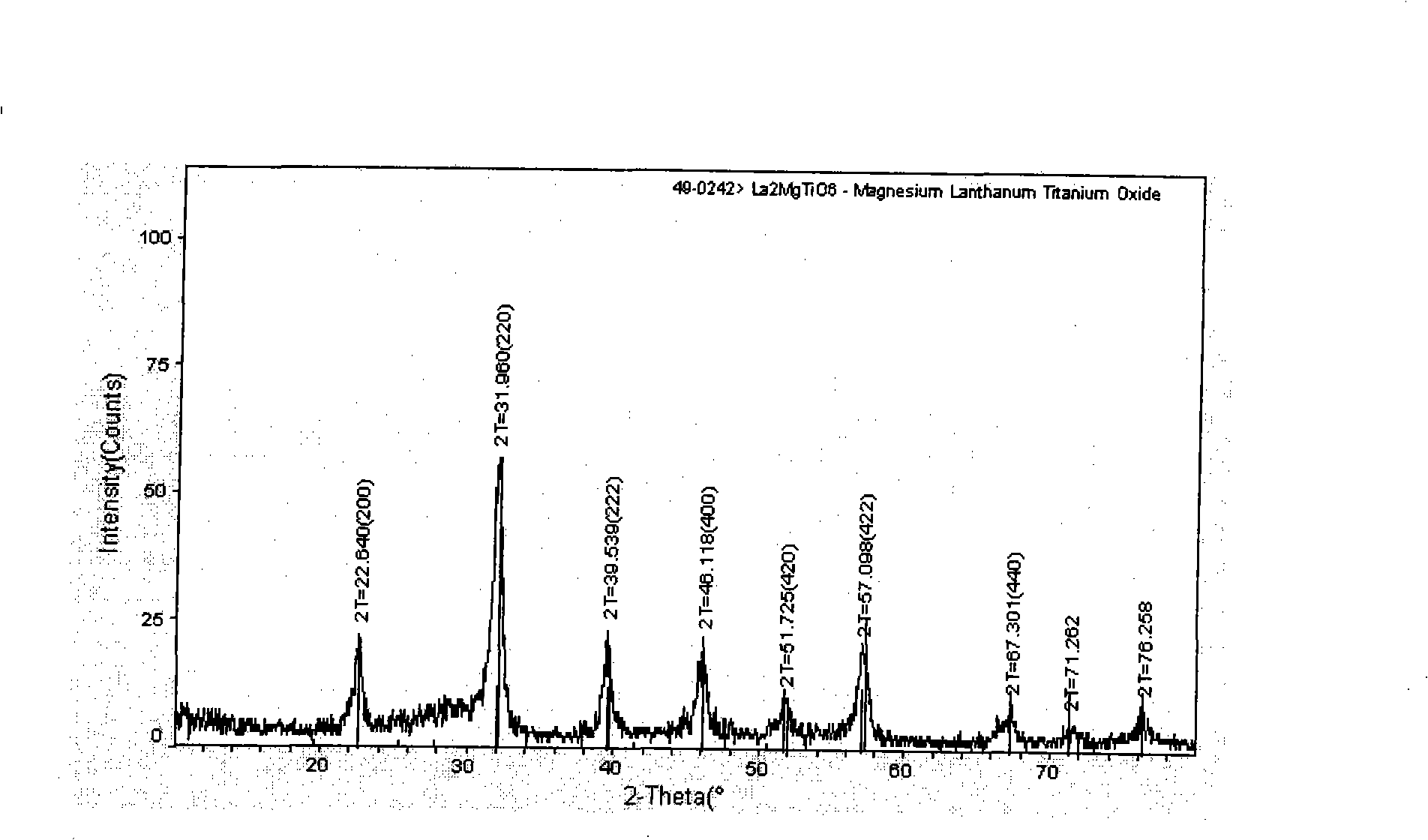
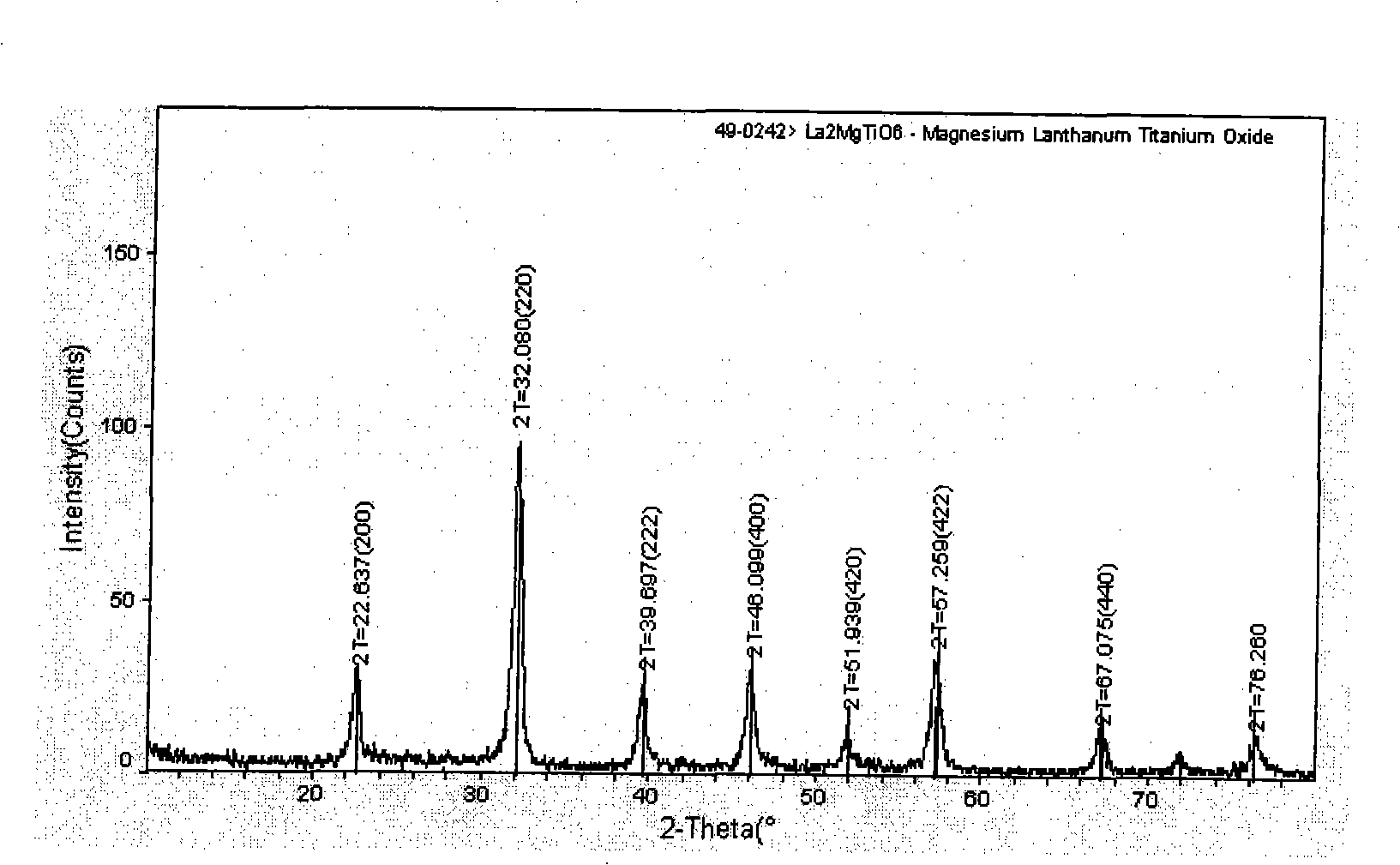
The present invention relates to a preparation method of titanium-containing double perovskite type methane combustion catalyst. The process is characterized in that a certain amount of butyl titanate is added into ethanol to produce white sediment; concentrated nitric acid is added until the sediment is totally dissolved and transparent solution is obtained; lanthanum nitrate and magnesium nitrate are weighed and dissolved in the deionized water and a certain amount of dextrose is added, thus completely forming composition solution; then gel is formed by constant-temperature stirring and evaporation; the gel obtained is dried and sintered at the temperature of 500 DEG C, 800 DEG C and 1,100 DEG C in the air for three hours to obtain the catalyst La2MgTiO6. The present invention is characterized in that the Sol-Gel Preparation Method with the dextrose as the complexing agent is adopted and the single-phase double layer perovskite type composite oxide catalyst is prepared at lower temperature. The preparation method is simple and low in cost. The catalyst used in methane catalytic combustion reaction has the characteristics of low initiation temperature and low whole transformation temperature.
Owner:INNER MONGOLIA UNIVERSITY
Method for preparing efficient adsorbent through in-situ reaction
InactiveCN102908978AReduce manufacturing costOther chemical processesAlkali metal oxides/hydroxidesMagnesium saltSorbent
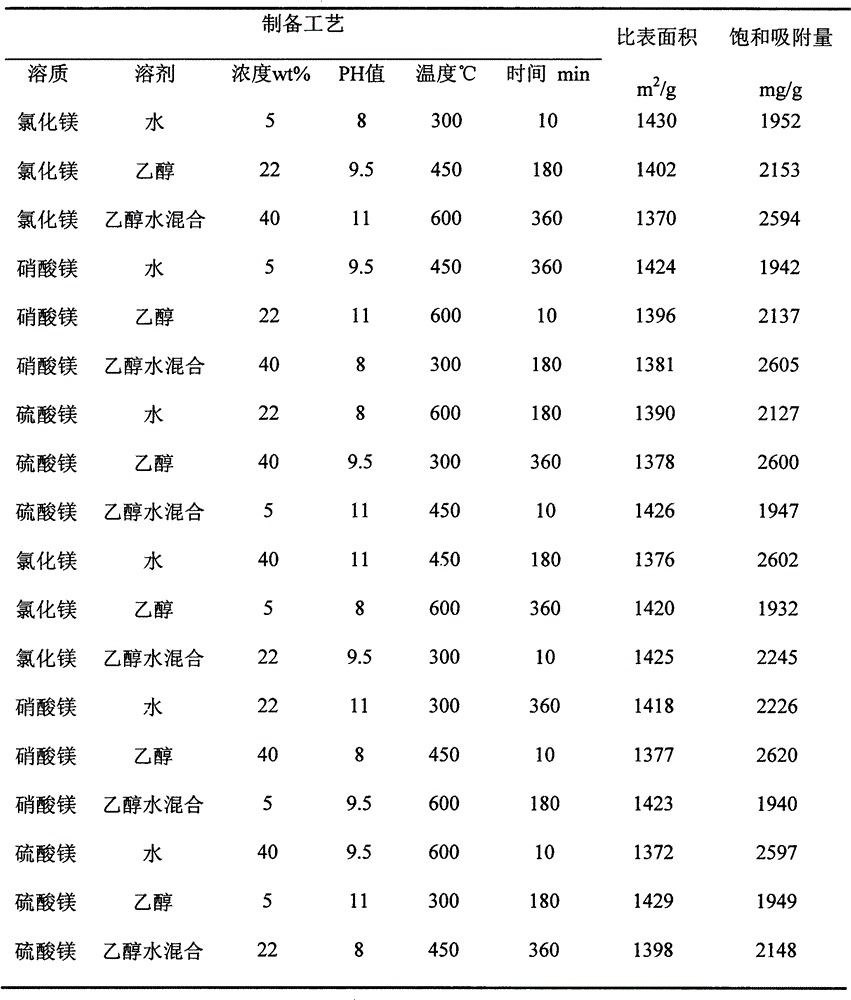
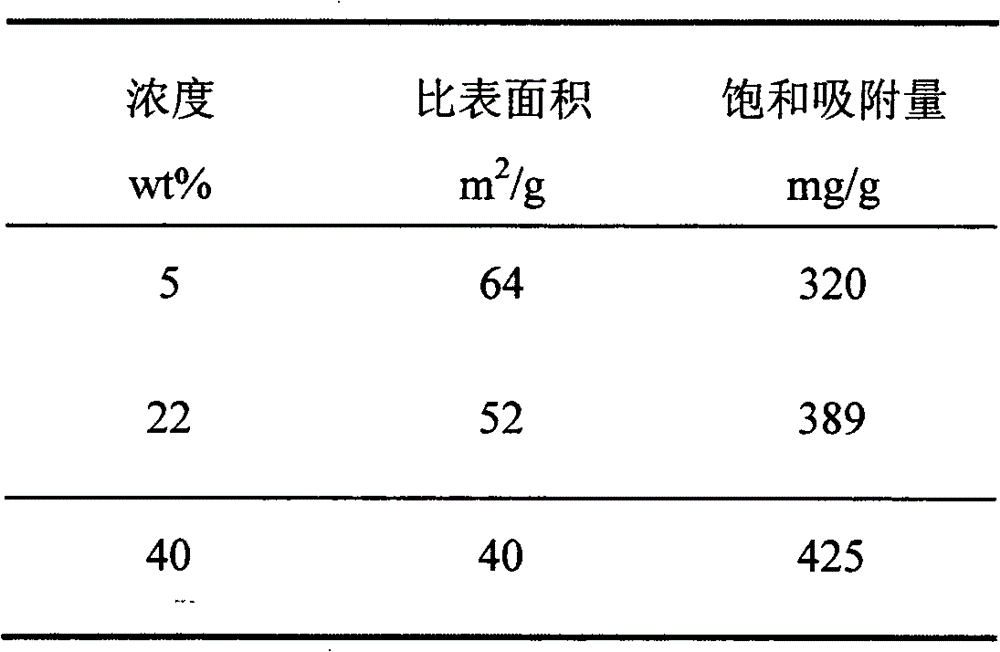
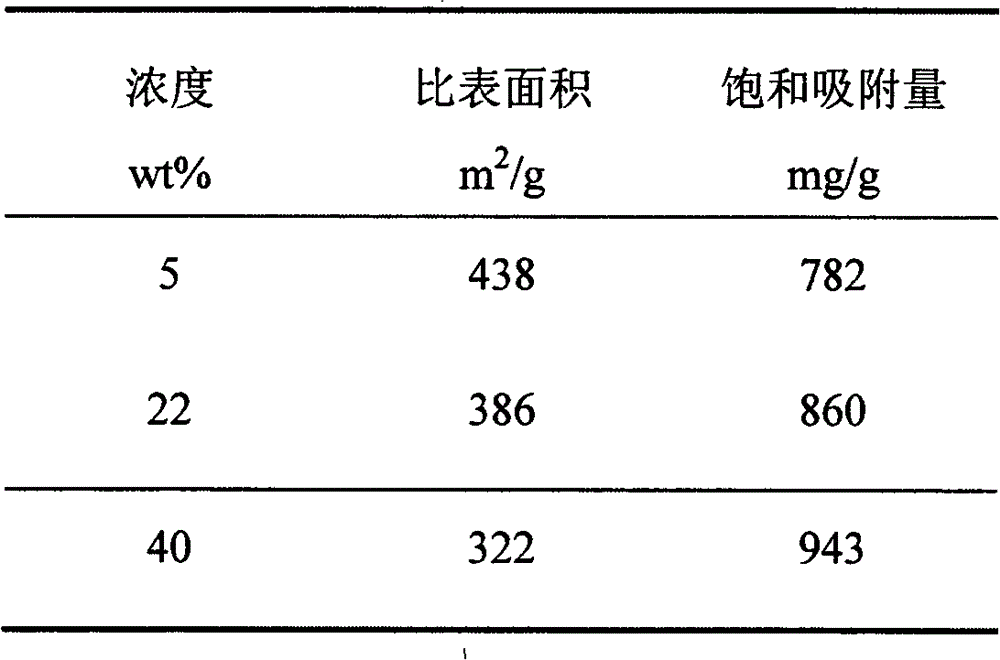
The invention relates to a method for preparing an efficient adsorbent through in-situ reaction. The method is characterized by firstly chemically absorbing magnesium ions in ducts and surfaces of porous materials, then adjusting the pH value to 8-11, generating magnesium hydroxide in situ and obtaining the efficient adsorbent porous magnesium oxide through calcining, wherein the adopted magnesium salts are at least one of magnesium chloride, magnesium nitrate, magnesium acetate and magnesium sulfate; the solvents of the magnesium salts are at least one of water and ethanol; the concentration of the magnesium salt solution is 5-40wt%; the calcination temperature is 300-600 DEG C; and the calcination time is 10-360min. The method has the advantages of simple needed equipment, short process flow, high preparation efficiency and low preparation cost. The prepared porous magnesium oxide has the advantages of large specific surface area, strong heavy metal ion adsorption capacity and high saturation adsorption, and has good industrial application prospects.
Owner:JIANGXI UNIV OF SCI & TECH
Fire coal combustion improver
InactiveCN102191110AImprove operational safetyExtended service lifeSolid fuelsChromium trioxideDolomite
The invention relates to an improvement of a fire coal combustion improver. The improved fire coal combustion improver is characterized by being prepared by compounding and mixing the following components: 20 to 30 weight percent of dolomite powder, 5 to 15 weight percent of dicyclopentadieny iron, 3 to 10 weight percent of chromium trioxide, 3 to 10 weight percent of manganese dioxide, 3 to 15 weight percent of magnesium nitrate, 3 to 15 weight percent of sodium dichromate and / or potassium dichromate, 3 to 18 weight percent of zinc oxide, 15 to 30 weight percent of active clay. The fire coal combustion improver has a plurality of functions of combustion acceleration, sulfur solidification, smoke abatement and the like; the thermal efficiency of a boiler can be improved by 3 to 8 percent; the coal-saving efficiency is up to 5 to 15 percent; the Ringelmann smoke blackness is less than 1; the total removal rate of SO2, NOx and the like is 25 to 60 percent, and the combustion improver has remarkable coal-saving and environmental-protection effect. Since the combustion effect is improved, the inferior coal (such as coal gangue), which is difficult to combust normally, is combusted and comes into play. After the long use, the boiler is not corroded, the scaling of the calcium sulfate in the boiler can be reduced, the operation safety of the boiler can be improved and the service life of the boiler can be prolonged.
Owner:YIXING KEQI CHEM
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com