Ruthenium-based ammonia synthetic catalyst and preparation thereof
A catalyst, a technology for ammonia synthesis, which is used in the preparation/separation of ammonia, chemical instruments and methods, catalysts for physical/chemical processes, etc., can solve the problems of unfavorable catalyst use and promotion, expensive and other problems, and achieves low energy consumption and easy operation. , the effect of strong catalytic activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] 25.6g magnesium nitrate and 0.43g ruthenium chloride (based on the mass of magnesium oxide carrier, containing 4% ruthenium, without special instructions, the percentages mentioned in the present invention are all percentages by weight) are mixed with 100ml mixed solution, after stirring for 10min , quickly add 100ml 2mol·L -1 In potassium hydroxide solution, stir for 5 minutes after adding, then wash with deionized water until there is no chloride ion, dry at 120°C for 24 hours, and then sinter at 450-550°C for 2 hours in a hydrogen-argon mixed gas atmosphere. The equal-volume impregnation method is used to impregnate the barium nitrate (M1) first, then the cesium nitrate (M2), and dry to prepare the Ru-M1-M2 / MgO catalyst.
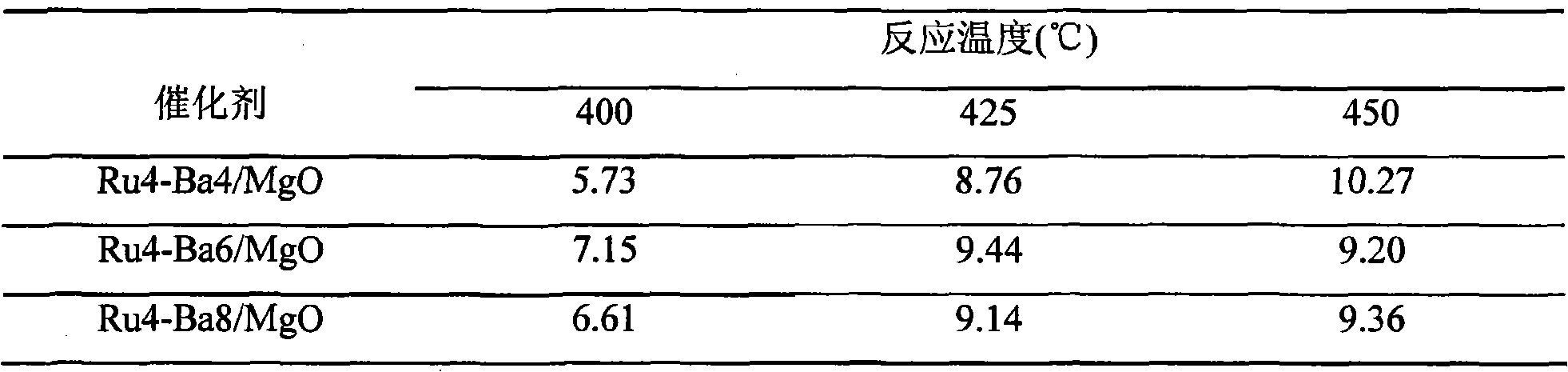
[0026] Different series of ammonia synthesis catalysts Ru-M1 / MgO, Ru-M2 / MgO, Ru-M1-M2 / MgO can be prepared by changing the addition amount of ruthenium trichloride, additive amount and impregnation order, sintering reduction temperature Or impregna...
Embodiment 2
[0028] Catalyst activity evaluation was carried out in a high-pressure activity testing device. The reactor is a fixed bed with an inner diameter of 14mm. The catalyst particles are 12-16 meshes, the bulk volume is 2ml, and the catalyst is packed in the isothermal zone of the reactor. The reaction gas is a mixture of nitrogen and hydrogen, and the ratio of hydrogen to nitrogen is 3:1.
Embodiment 3
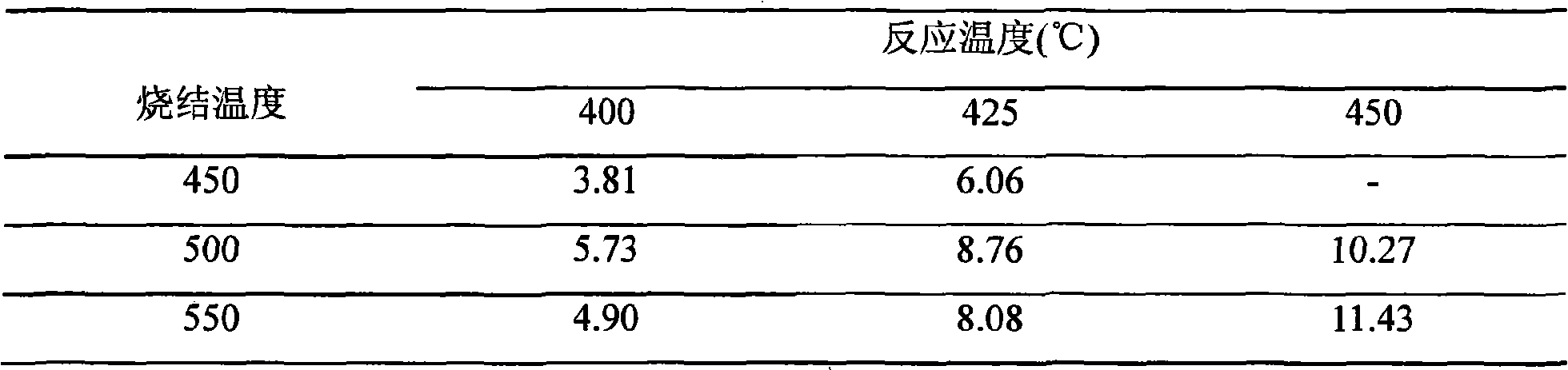
[0030] For Ru-Ba / MgO catalyst, the sintering temperature is 550°C, the sintering time is 2 hours, and the reaction gas space velocity is 10000h -1 , and the reaction pressure was 10MPa, the effect of reaction temperature on catalyst activity was investigated, and the results are shown in Table 1.
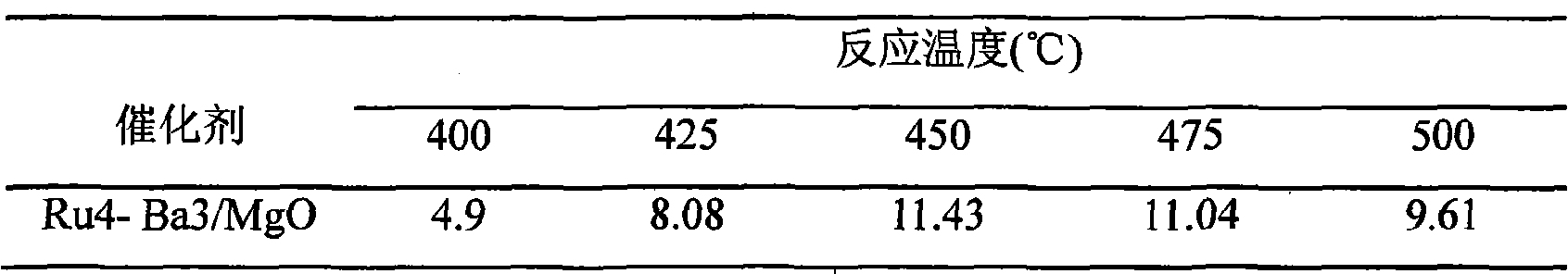
[0031] The influence (vol%) of table 1 reaction temperature on outlet ammonia concentration
[0032]
[0033] Note: The mark Ru4-Ba3 / MgO refers to the ruthenium catalyst containing 4% metal ruthenium and 3% metal barium on the ruthenium catalyst supported by magnesium oxide, and the following marks have the same meaning.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com