Metal oxide nanometer material for treating wastewater containing dyes or heavy metal ions, preparation method and application thereof
A technology of heavy metal ions and nanomaterials, which is applied in the field of metal oxide nanomaterials used to treat wastewater containing dyes or heavy metal ions and its preparation and application, can solve the problems of small dye adsorption, harsh conditions, and difficult regeneration, and achieve improved Degradation and adsorption rate, strong regenerability, and the effect of reducing operating costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
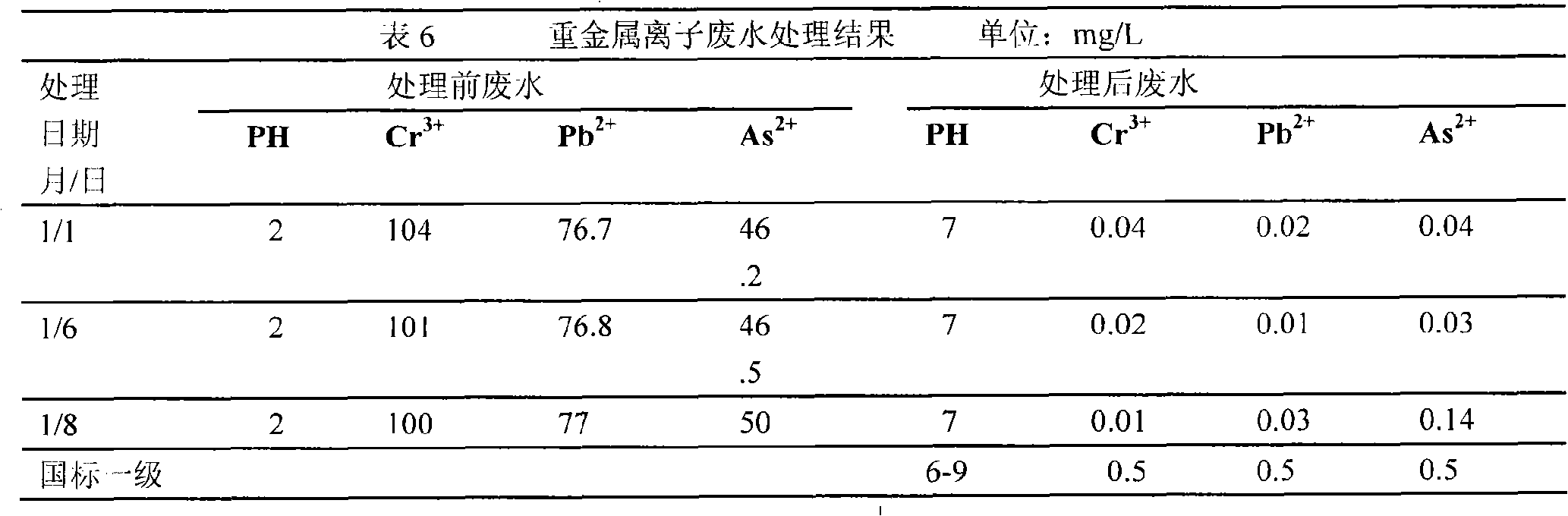
[0027]In the ethanol of the magnesium nitrate 100ml of 18g, add the benzyl alcohol structure directing agent of 13.5g, move in the autoclave after mixing evenly, in 10 6 Under the protection of Pa chlorine, heat to 200°C for 6 hours to completely alcoholyze the magnesium nitrate, then heat to 264°C and keep for 15h, and dry to obtain the flaky 111 magnesium oxide nanomaterial precursor, which is fired at 500°C to obtain Magnesium oxide nanocrystalline material with flaky 111 crystal plane; put 0.9g of the prepared metal oxide nanomaterial into 3.5L of Congo red dye wastewater, the initial concentration of Congo red in the dye wastewater is 75mg / L, and the temperature is 10°C , the rotating speed is 150r / min, after half an hour of reaction, the removal rate of Congo red dye is over 90%.
[0028] operating conditions
[0029] Waste water volume: 120ml
[0030] Initial concentration of wastewater: 100mg / L
[0031] Stirring method: the speed is 150r / min
[0032] Reaction tempe...
Embodiment 2
[0038] The magnesium nitrate of 18g, the ferric nitrate of 28.4g are dissolved in the ethanol of 200ml, add the structure directing agent of benzyl alcohol of 26.8g, move in the autoclave after mixing evenly, in 10 6 Under the protection of Pa chlorine, heat to 200 ° C for 6 hours to completely alcoholyze the nitrate, then heat to 265 ° C and keep it for 15 hours, dry to obtain the precursor of metal oxide nanomaterials, and burn at 500 ° C to obtain metal oxides nanomaterials. Put 0.09g of the prepared metal oxide nanomaterials into 120ml of Congo red dye wastewater. The initial concentration of Congo red in the dye wastewater is 100mg / L, the pH range is 3.29-11.03, the temperature is 10°C, and the rotation speed is 150r / min, after reacting for half an hour, the removal rate of Congo red dye was more than 90%.
[0039] operating conditions
[0040] Waste water volume: 120ml
[0041] Initial concentration of wastewater: 100mg / L
[0042] Stirring method: the speed is 150r / ...
Embodiment 3
[0054] Dissolve 18g of magnesium nitrate, 20.88g of zinc nitrate and 20.77g of calcium nitrate in 600ml of ethanol, add 40.2g of benzyl alcohol structure-directing agent, mix well and move into an autoclave, in a 1.5×10 6 Under the protection of Pa chlorine, heat to 200 ° C for 6 hours to completely alcoholyze the nitrate, then heat to 267 ° C and keep it for 15 hours, dry to obtain the precursor of metal oxide nanomaterials, and burn at a high temperature of 500 ° C to obtain metal oxides nanomaterials. Put 0.09g of metal oxide nanomaterials into 120ml of X3B dye wastewater. The initial concentration of dye wastewater is 100mg / L, the pH range is 3.29-11.03, the temperature is 10°C, and the rotation speed is 150r / min. After half an hour of reaction The removal rate is above 90%.
[0055] operating conditions
[0056] Waste water volume: 120ml
[0057] Initial concentration of wastewater: 100mg / L
[0058] Stirring method: the speed is 150r / min
[0059] PH value range: 3.29...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com