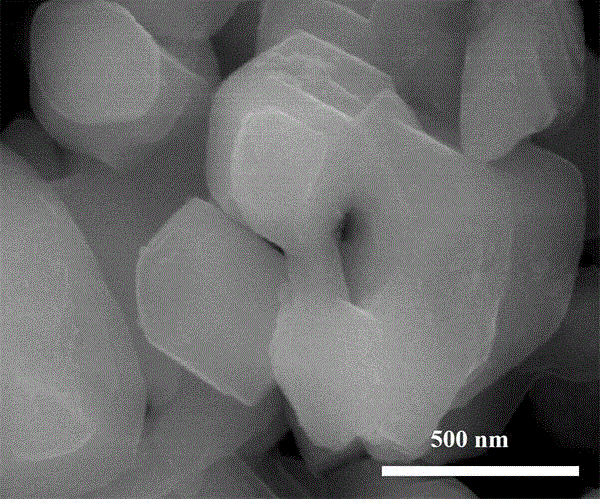
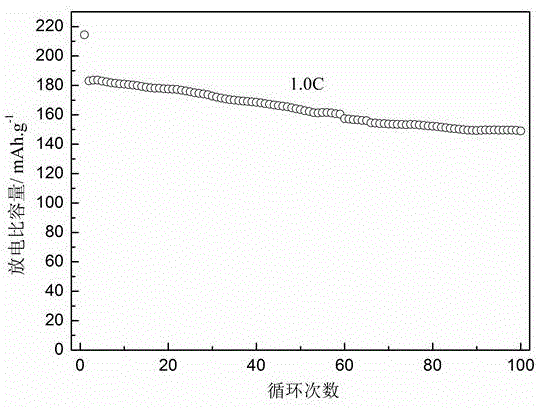
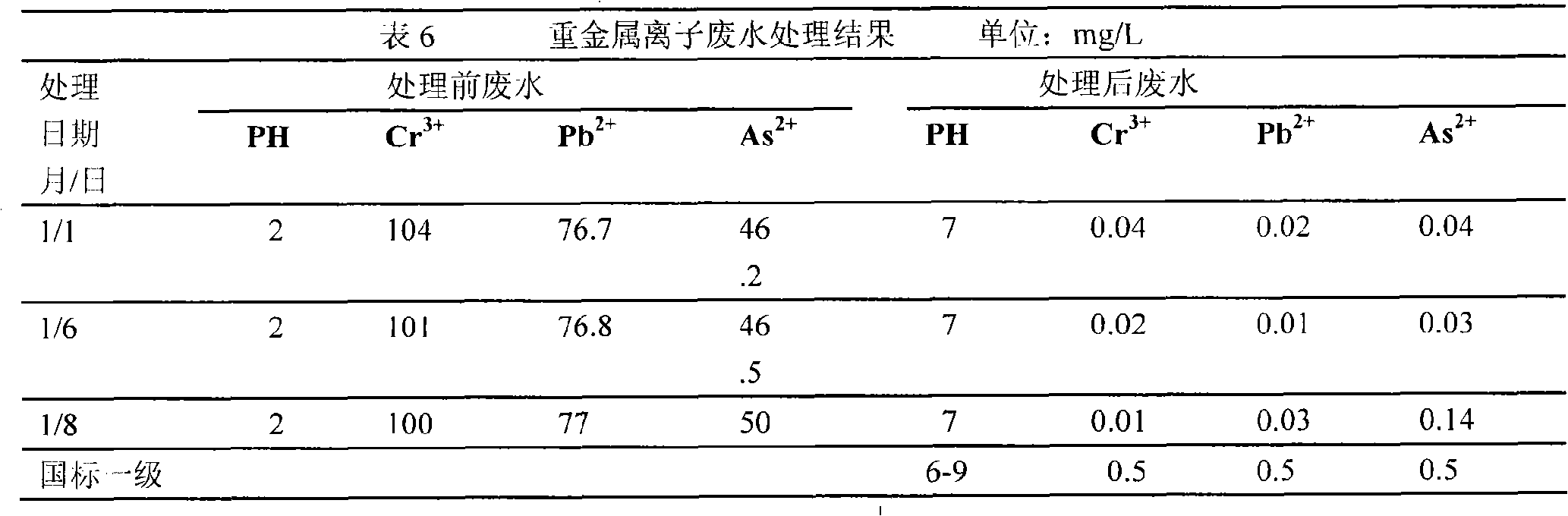
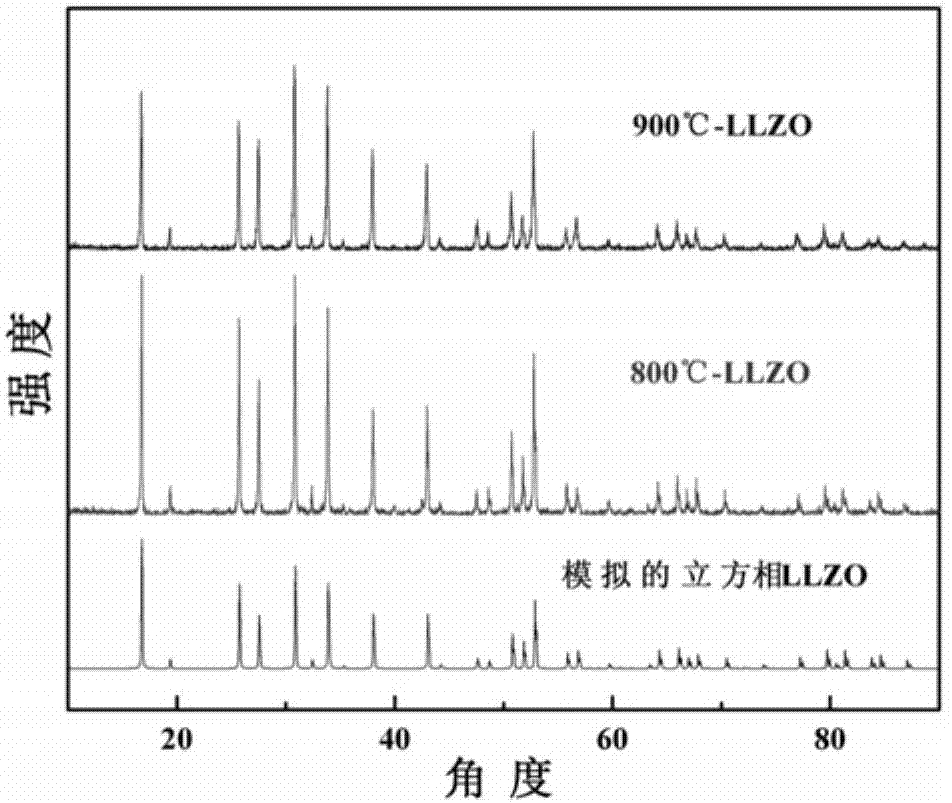
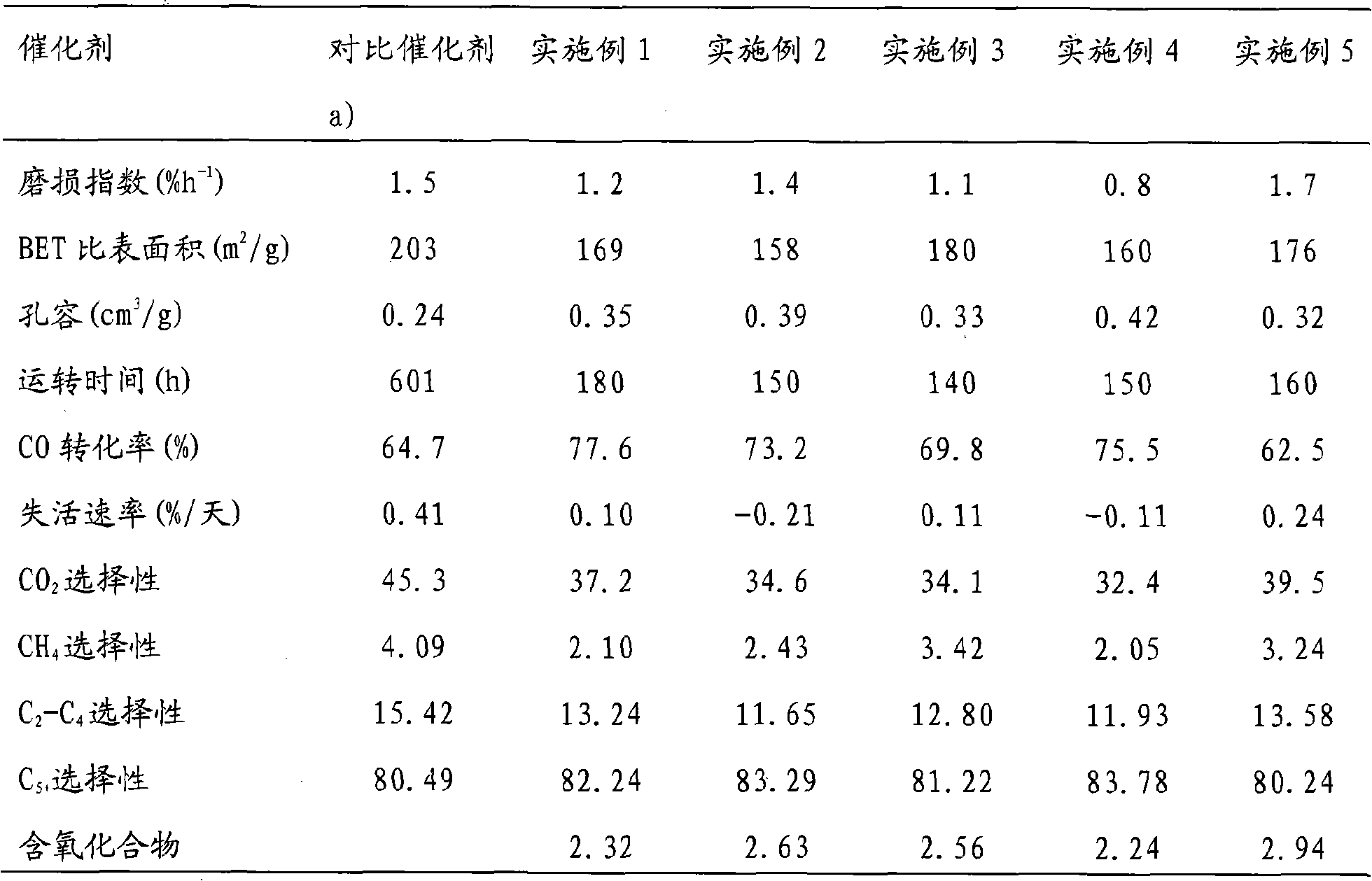
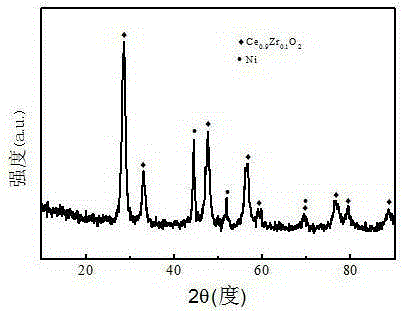
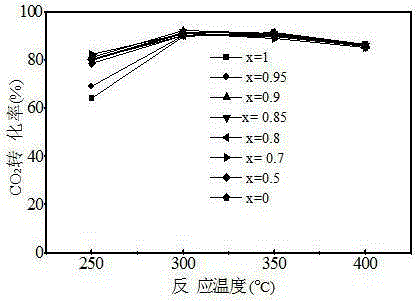
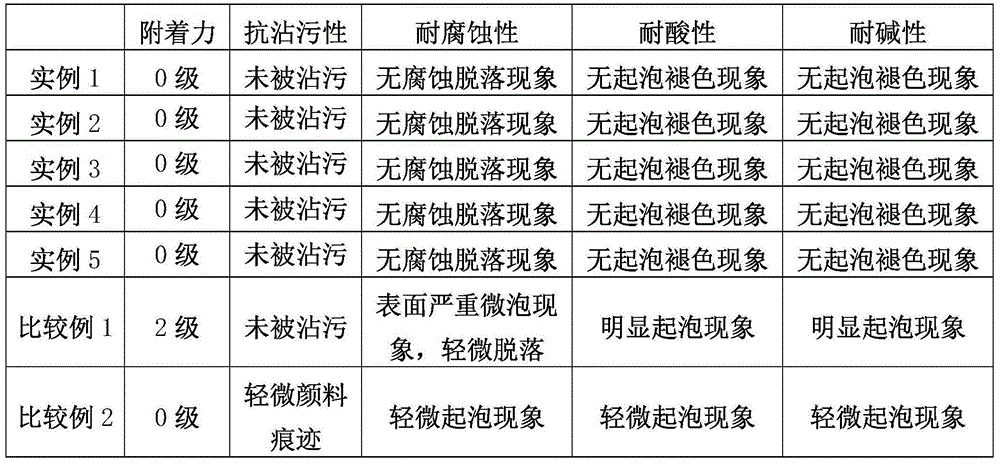
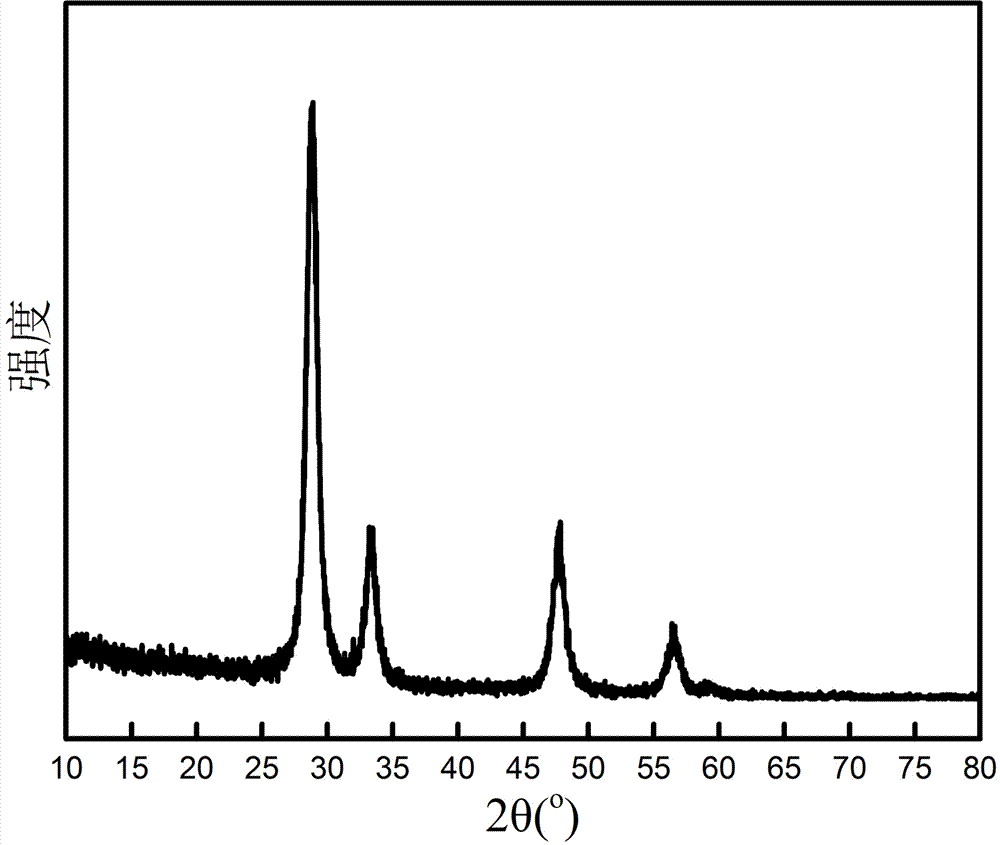
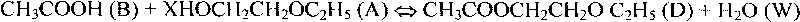Patents
Literature
442 results about "Zirconium nitrate" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Zirconium nitrate is a volatile anhydrous transition metal nitrate of zirconium with formula Zr(NO₃)₄. It has alternate names of zirconium tetranitrate, or zirconium(IV) nitrate. It has a UN number of UN 2728 and is class 5.1, meaning oxidising substance.
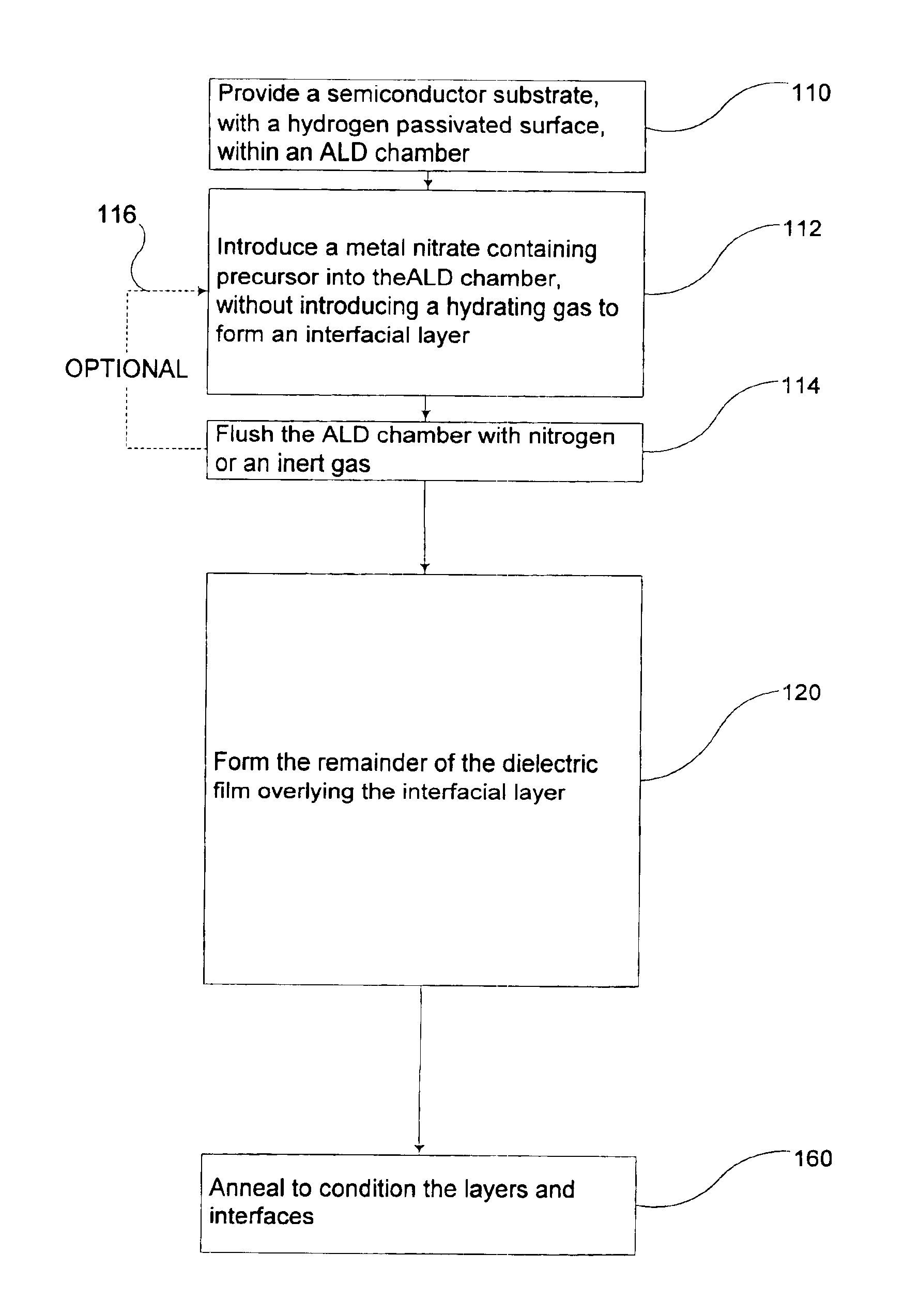
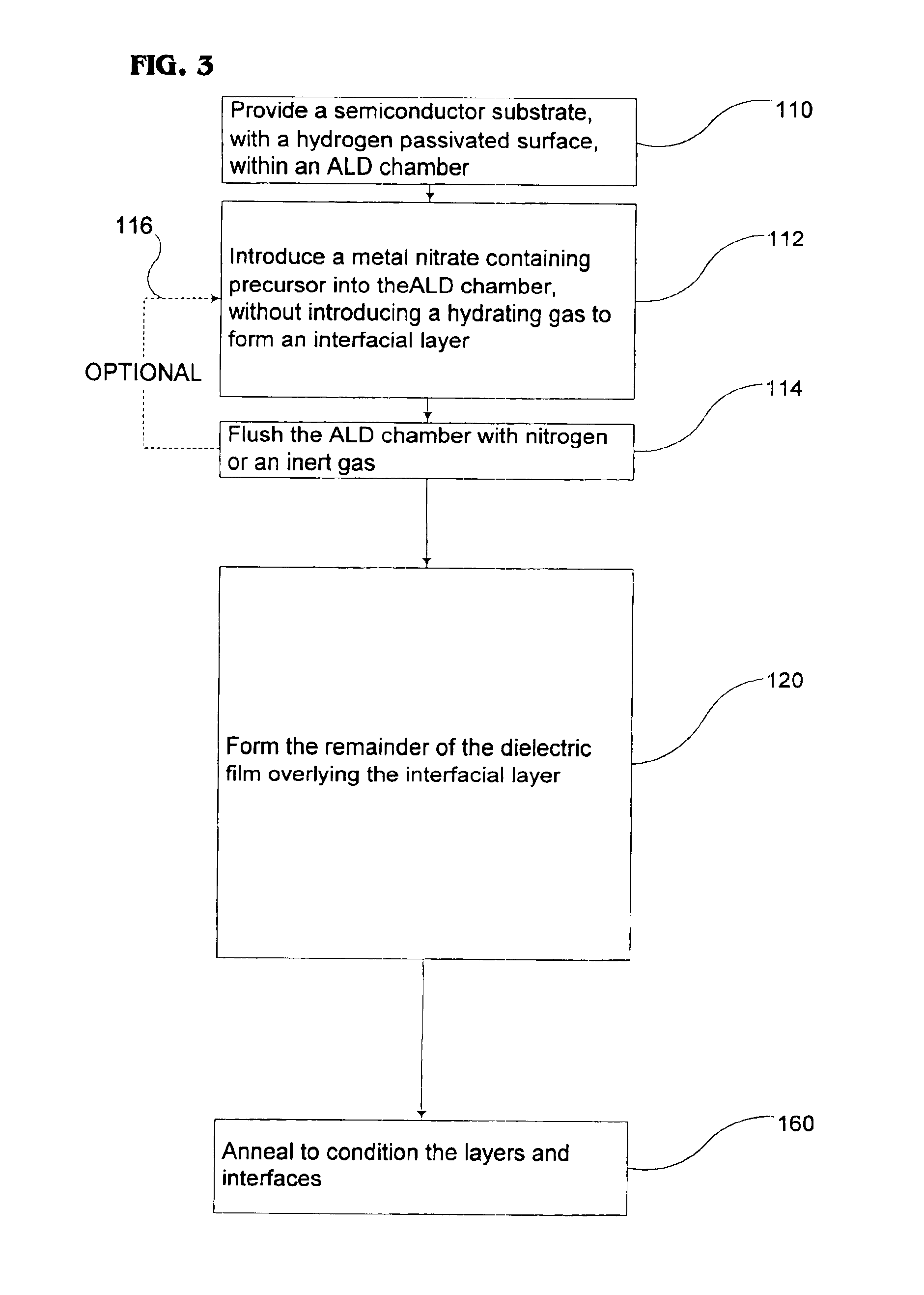
Method to control the interfacial layer for deposition of high dielectric constant films
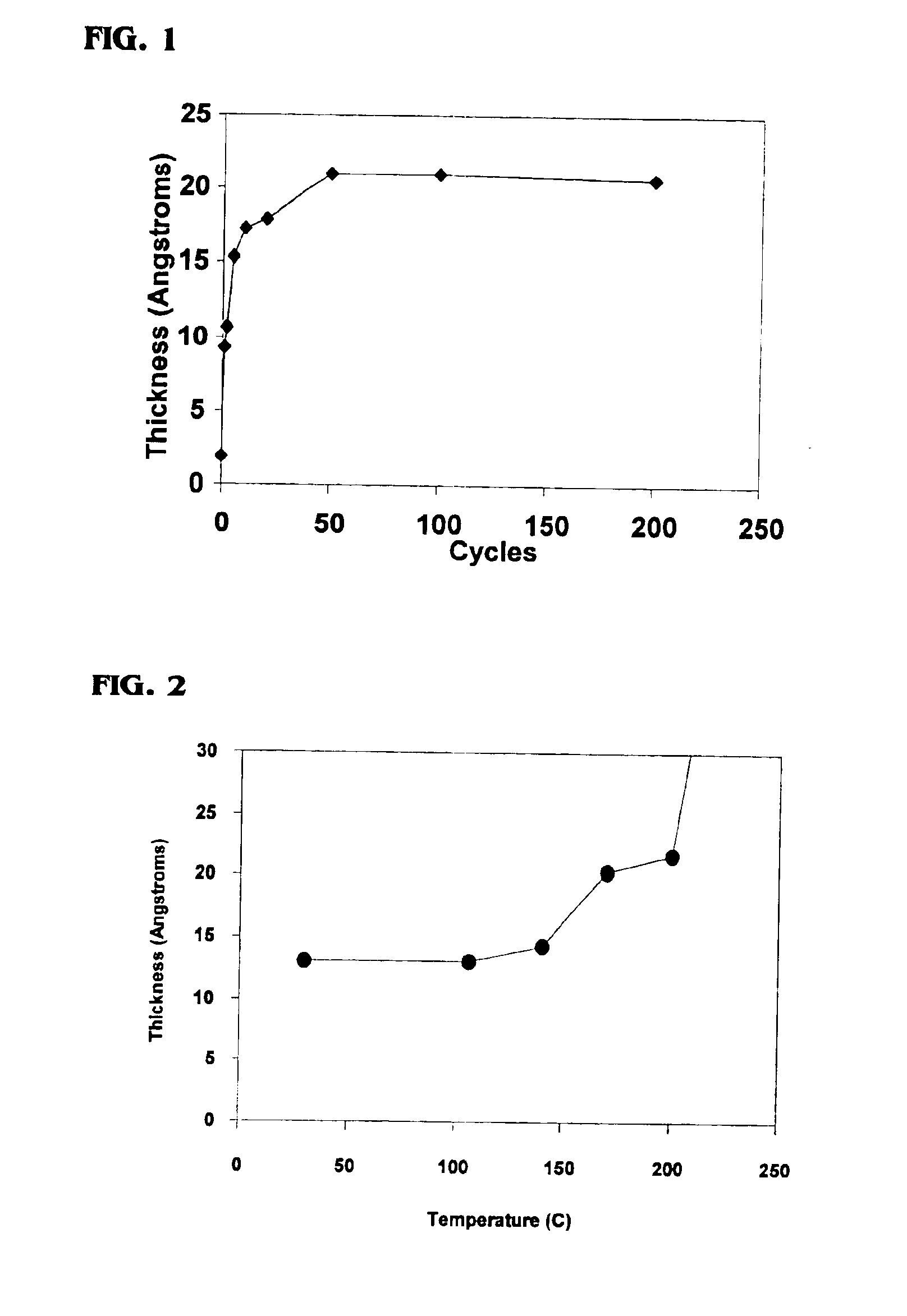
Methods of forming an interfacial layer on a hydrogen-passivated substrate are provided. These methods utilize atomic layer deposition techniques incorporating metal nitrate-based precursors, such as hafnium nitrate or zirconium nitrate, without introducing a hydrating agent, or oxidizing agent, such as water, during the formation of the interfacial layer. Also provided are methods of forming high-k films, by first forming an interfacial layer on the surface of a hydrogen-passivated substrate, and then depositing one, or more, high-k dielectric films.
Owner:SHARP LAB OF AMERICA INC
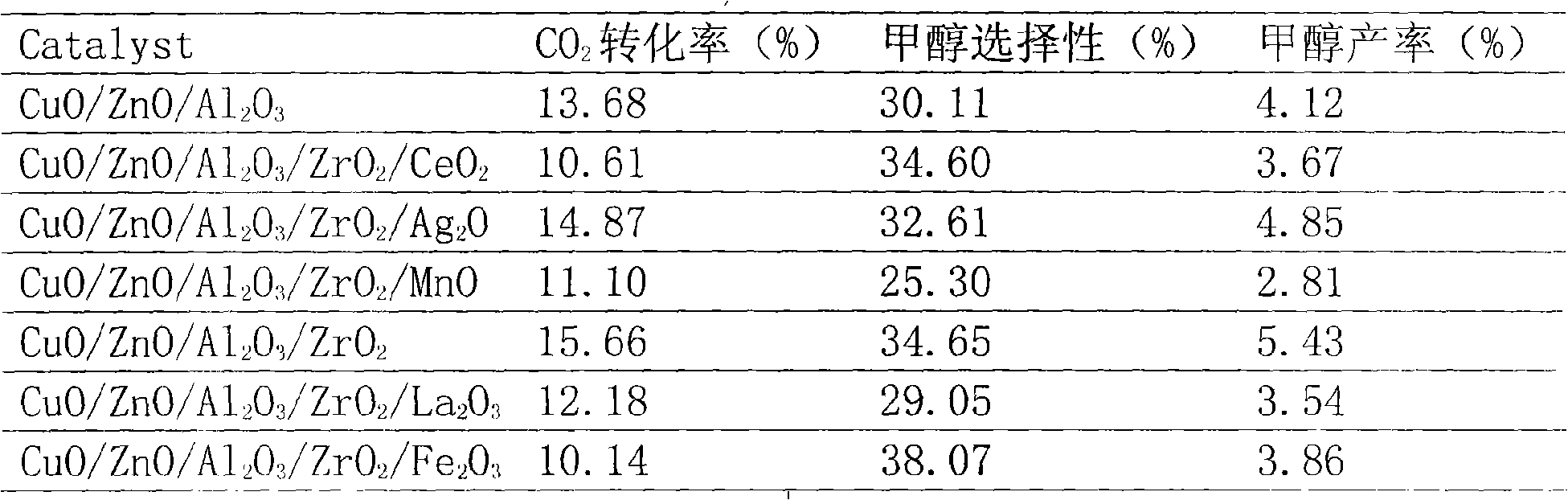
Carbon dioxide-synthesized methanol catalyst and preparation method thereof
ActiveCN101513615AImprove conversion rateHigh activityOrganic compound preparationHydroxy compound preparationCopperZinc
A carbon dioxide-synthesized methanol catalyst and a preparation method thereof are disclosed, belonging to the technical field of catalyst. The invention is characterized in that the carbon dioxide-synthesized methanol catalyst has the molar ratio of components of Ci to Zn to Al to Zr to M being 45 to 45 to 10 to 5 to 2, wherein M is MnO, CeO2, Ag2O, Fe2O3, La2O3, and is prepared by stepped co-deposition. The preparation steps are that: (1) a mixed liquid of aluminum nitrate solution and zirconium nitrate solution is in concurrent flow and co-deposition with carbonate solution to prepare a carrier precursor; (2) copper-zinc M mixed nitrate is in concurrent flow and co-deposition with the carbonate solution and added into (1), and then the synthesized methanol catalyst is prepared by aging, filtering, drying and roasting. The inventive effect and benefit are that the prepared catalyst is excellent in activity and thermal resistance and can effectively decompose carbon dioxide with high one-step conversion rate, therefore, the invention provides an effective catalyst for synthesizing methanol by hydrogenising the carbon dioxide.
Owner:DALIAN UNIV OF TECH
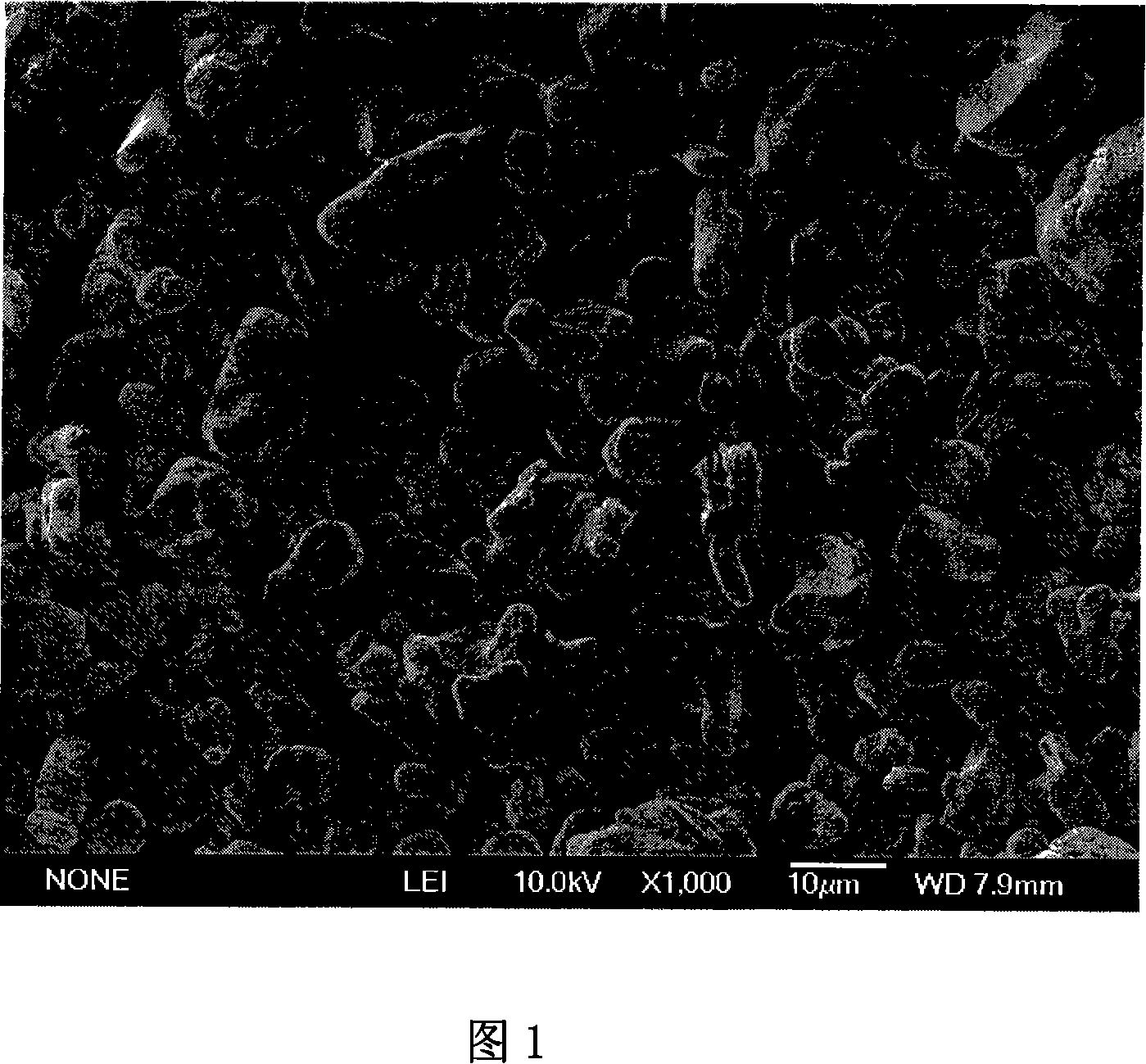
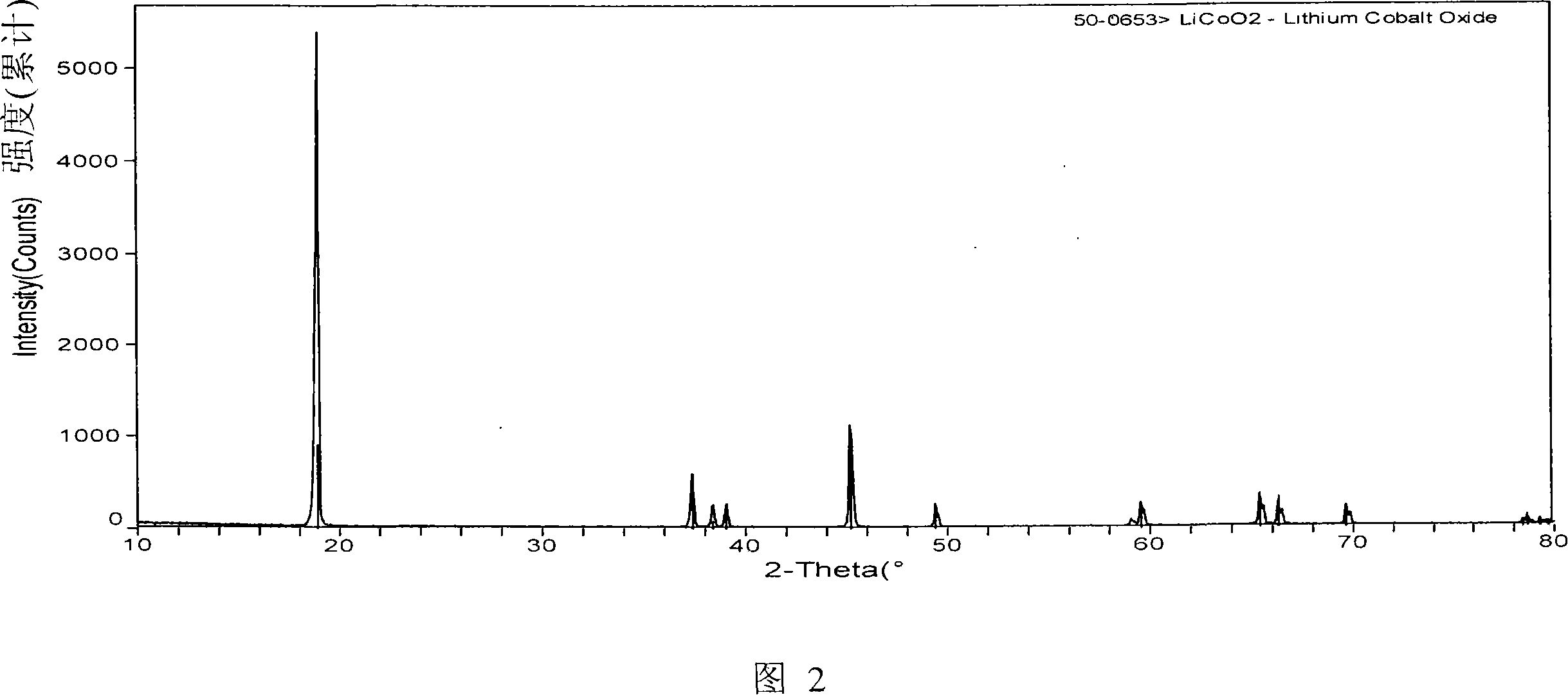
Anode material zirconium and phosphor adulterated lithium cobaltate of lithium ion secondary battery and its making method
ActiveCN101150190AImprove cycle performanceImprove securityCell electrodesCobalt compoundsLithium hydroxidePhosphate
This invention relates to LiCoO2 doped with Zr and P, positive material of Li ionic secondary cells and its preparation method characterizing that the chemical formula is: LiZrxCo(1-x-y)PyO2, in which, it is a laminated structure, and x=0.001-0.003, y=0.02-06, the preparation steps are as folow: 1, mixing Co3O4, CoCO3 or CoC2O4 with Li2CO3 or LiOH in the atomic ratio of Li and Co(0.9801.05) :1.00, 2, baking it for 6-24h under 600-1000deg.C, 3, crushing LiCoO2 to particles of 6-15mum, 4, mixing the LiCoO2 and water 1-3times weight into pulp, 5, adding ZrNO3 and phosphate at the same time, 6, spraying and drying it, 7, baking it for 4-12h under 600-1000deg.C, 8, crushing the doped LiCoO2 to particles of 6-15mum.
Owner:TIANJIN B&M SCI & TECH
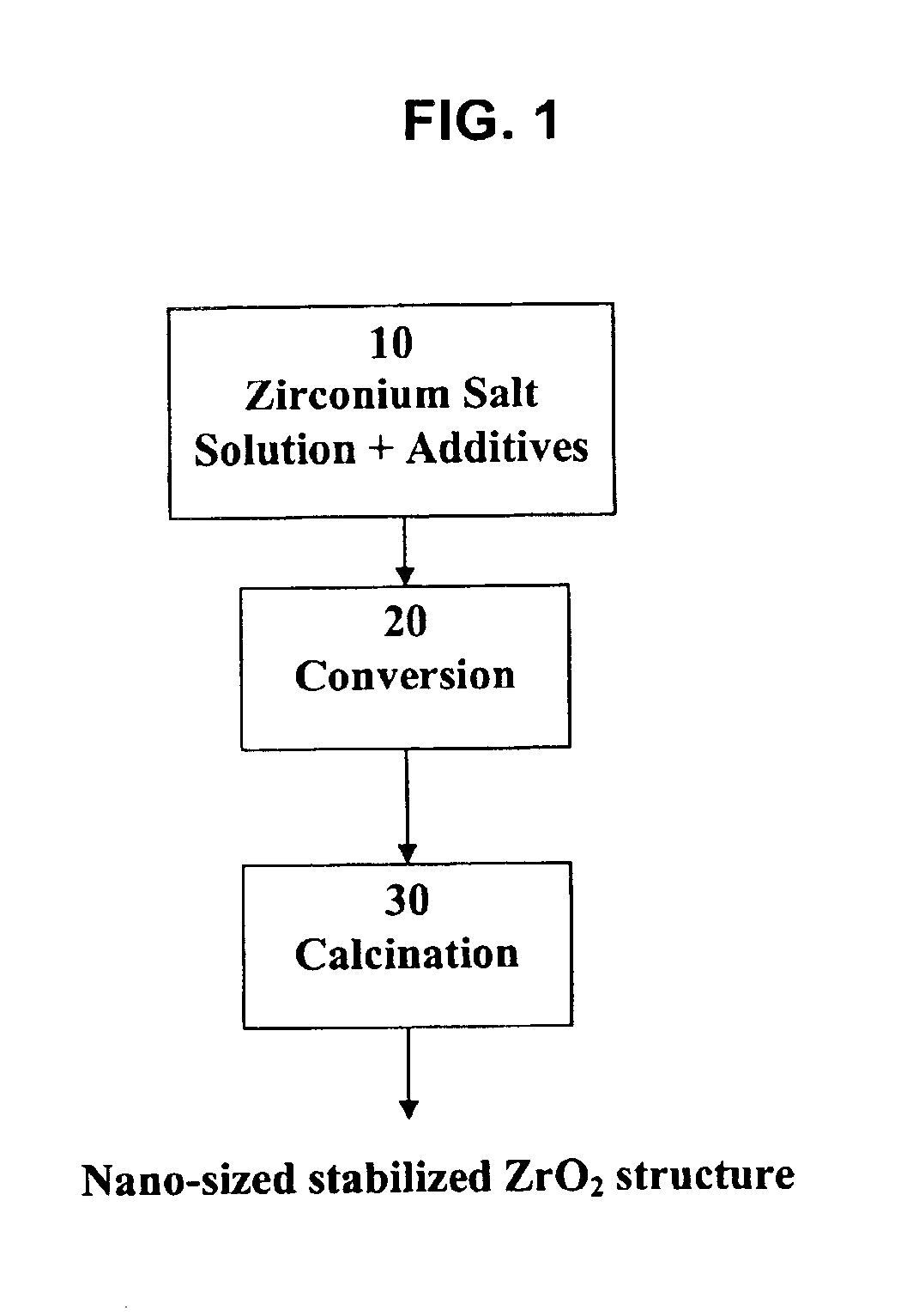
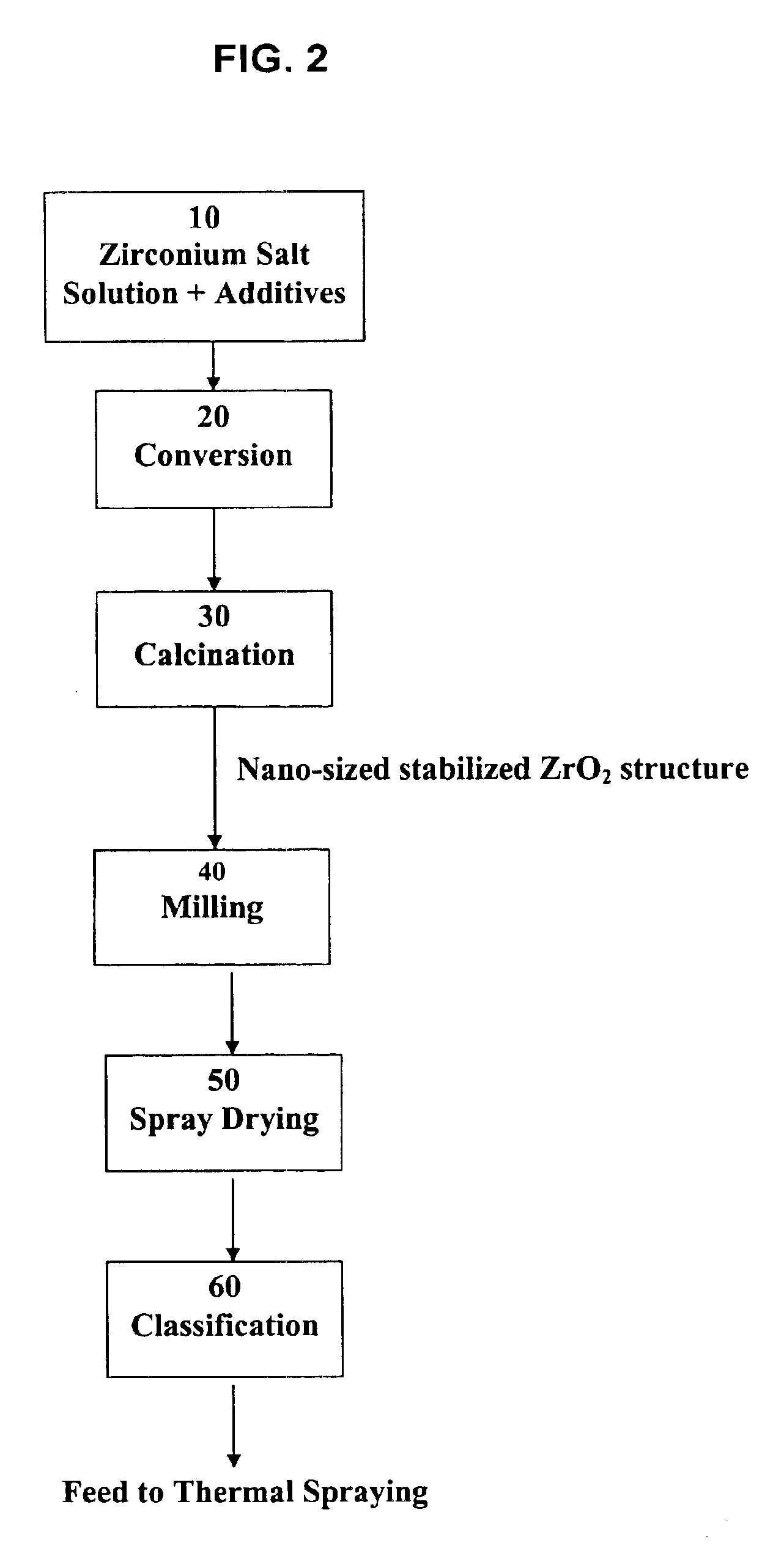
Process for making nano-sized stabilized zirconia
A process to produce stabilized zirconia from a solution of zirconium salt and a stabilizing agent. The zirconium salt may include zirconium oxysulfate, zirconium oxychloride, zirconium oxynitrate, zirconium nitrate, and other water-soluble zirconium salts. The stabilizing agent may include calcium, magnesium, yttrium salts of oxides and rare earth oxides. The process is conducted by evaporation of the solution above the boiling point of the solution but below the temperature where there is significant crystal growth. The evaporation step is followed by calcination to produce the desired nano-sized structure. Further processing by sintering may be applied to produce solid structures or by milling and classification to produce material for thermal spray coating.
Owner:ALTAIR NANOMATERIALS INC
Anti-carbon-deposition Ni-based catalyst for hydrogen production by methane steam reforming and preparation method thereof
ActiveCN103752319AMeet activityMeet service life requirementsHydrogenMetal/metal-oxides/metal-hydroxide catalystsSteam reformingWater vapor
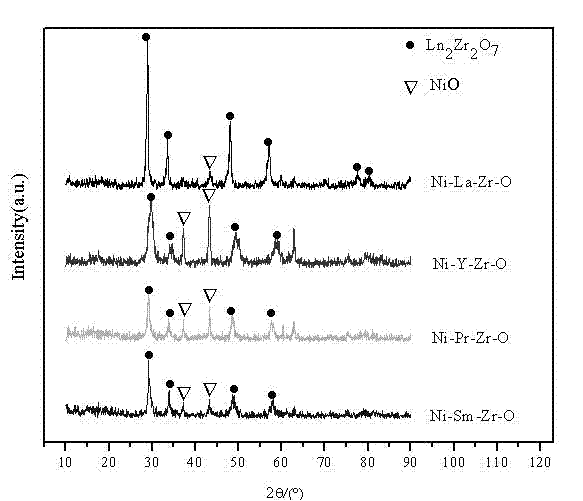
The invention relates to an anti-carbon-deposition Ni-based catalyst for hydrogen production by methane steam reforming and a preparation method thereof. By taking lanthanum nitrate, praseodymium nitrate, samarium nitrate, yttrium nitrate, zirconium nitrate, zirconium carbonate, zirconium oxychloride, and the like as precursors and taking ammonia as a precipitant, a pyrochlore composite oxide is prepared through using a coprecipitation method; and then the pyrochlore composite oxide is mixed with alumina by using a mechanical mixing method so as to obtain a pyrochlore alumina composite carrier. Nickel nitrate, nickel chloride, nickel sulfate, nickel oxalate and the like serving as nickel sources are loaded on the pyrochlore alumina composite carrier through direct immersion. The loading capacity of nickel in the catalyst accounts for 5-30% of the weight of the catalyst, the pyrochlore content of the catalyst is 5-50%, and the alumina content of the catalyst is 20-90%. By taking the pyrochlore alumina composite oxide as a carrier, the reaction activity and anti-carbon-deposition performance of the catalyst can be greatly increased; the preparation method of the catalyst is simple; and the catalyst has excellent catalytic activity and stability to methane steam reforming in a stationary bed.
Owner:NANCHANG UNIV +1
Cobalt-nickel lithium manganate composite positive electrode material with surface wrapped by lithium zirconate and preparation method
InactiveCN105140492ASimple preparation processImprove cycle performanceSecondary cellsPositive electrodesElectrical batteryManganate
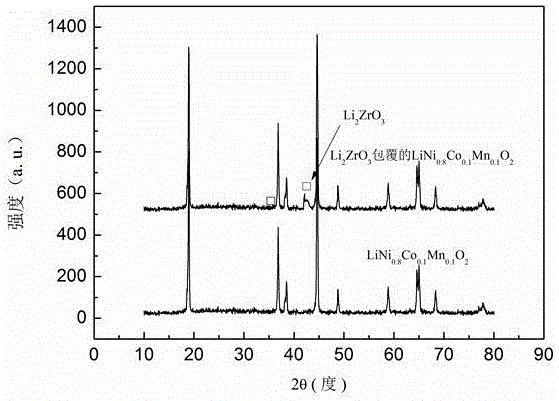
The invention discloses a cobalt-nickel lithium manganate composite positive electrode material with the surface wrapped by lithium zirconate and a preparation method. The preparation method includes the steps of firstly, preparing LiNi0.8Co0.1Mn0.1O2 through a wet chemistry method with soluble salt of nickel, cobalt and manganese and excessive lithium salt as raw materials; secondly, mixing LiNi0.8Co0.1Mn0.1O2 with zirconium nitrate, and preparing the LiNi0.8Co0.1Mn0.1O2 composite positive electrode material with the surface wrapped by Li2ZrO3 through a rheological phase method. The LiNi0.8Co0.1Mn0.1O2 composite positive electrode material with the surface wrapped by Li2ZrO3 has the advantages of being excellent in circulation performance, good in rate capability, simple and convenient in preparation process, low in cost, and the like, and is beneficial for being applied and popularized on a large scale as the positive electrode material of lithium ion batteries.
Owner:GUANGDONG TEAMGIANT NEW ENERGY TECH CORP
Nickel-based catalyst for hydrogen production by ethanol steam reforming and preparation method thereof
InactiveCN101444737AHigh mechanical strengthHigh catalytic activityHydrogenMetal/metal-oxides/metal-hydroxide catalystsSteam reformingNanowire
The invention discloses a nickel-based catalyst for hydrogen production by ethanol steam reforming and a preparation method thereof. The catalyst is made by taking nanoporous silicon dioxide aerogel as a catalyst carrier, a metal elementary substance nickel nanowire as an active component, and nano-particles of MgO or CaO or ZrO2 or TiO2 or CeO2 or compound nano-particles thereof as an adjuvant. The preparation method comprises the following steps: preparing a sol from silanolate, an alcohol solvent, nickel nitrate or magnesium nitrate or calcium nitrate or zirconium nitrate or cerous nitrate or complex nitrate thereof and an acidic catalyst at certain proportions; forming a wet gel complex, and then performing supercritical fluid drying. The catalyst has strong catalytic activity and selectivity for the hydrogen production by the ethanol steam reforming, has higher hydrogen yield and stronger CO2 selectivity at a lower temperature, and limits the selectivity of byproducts CH4 and CO at a lower level. Meanwhile, the preparation method has simple process, low cost and certain mechanical strength.
Owner:HUNAN SHANGYIFENG NEW MATERIAL TECH CO LTD
Load type nano-au catalyst and the preparing method
InactiveCN101036887AEasy to manufactureLow costPreparation by oxidation reactionsCatalyst carriersCyclohexanoneKetone
The present invention discloses a supported gold nano-particles catalyst and the preparation method of the same, which comprises Au, Ce, Zr, Al and Si; wherein Au is the main active component and has a mass percent of 0.1~4.0%; Ce and Zr are the assistant active component and has a mass percent of 3~57%, wherein the mol ratio of Ce to Zr is 1~10; Al and Si are the carrier and has a mass percent of 40~97%. The catalyst is prepared by a sequential immersion method, wherein the alumina or silicon oxide carrier is firstly impregnated by an aqueous liquid containing cerous nitrate and zirconium nitrate and then the gold component is impregnated. The present invention has the advantages of that: the preparation method is simple, the stability is excellent and the cost is low. For the reaction of preparing cyclohexanone and cyclohexanol by cyclohexane oxygenation, the catalyst of the present invention can obtain high ketone alcohol selectivity even under the condition of a high conversion, besides the peroxide content in the product is very low. For example, when the conversion of cyclohexane is 12.8%, the ketone alcohol selectivity is up to 92.6% and the peroxide content is only 0.7%.
Owner:ZHEJIANG UNIV
Metal oxide nanometer material for treating wastewater containing dyes or heavy metal ions, preparation method and application thereof
InactiveCN101591044ARaising the temperature can increase the active pointIncrease active pointOther chemical processesWater/sewage treatment by sorptionHigh pressureZinc nitrate
The invention relates to a metal oxide nanometer material for treating wastewater containing dyes or heavy metal ions, a preparation method and application thereof. Compositions of the metal oxide nanometer material is one or a mixture of more than two of CaO, ZrO2, SiO, ZnO, TiO2, MgO, Fe2O3 and NiO. The preparation method comprises the following steps: dissolving one or a mixture of more than two of calcium nitrate containing crystallization water, zirconium nitrate, ethyl orthosilicate, zinc nitrate, butyl titanate, magnesium nitrate, ferric nitrate and nickel nitrate into methanol, ethanol or propanol, adding a phenylcarbinol or benzylcarbinol structure-directing agent into the obtained solution, moving the mixture into a high-pressure kettle after the mixture is mixed evenly, heating the mixture to between 120 and 200 DEG C for 2 to 6h to ensure that nitrate is completely alcoholized under the protection of chlorine gas with the pressure of between 10 and 1.5*10 Pa, then heating the mixture to between 261 and 269 DEG C for 15h, drying the obtained product, and then performing high-temperature roasting at a temperature of between 300 and 500 DEG C to obtain the metal oxide nanometer material. The metal oxide nanometer material is added into the wastewater containing the dyes or the heavy metal ions to ensure that the metal oxide nanometer material and the wastewater are fully contacted to decolorize, absorb or degrade the wastewater containing the dyes.
Owner:SOUTH CENTRAL UNIVERSITY FOR NATIONALITIES
Method for preparing solid electrolyte by using lithium lanthanum zirconium oxide precursor coated powder
ActiveCN104124467ACalcination temperature is lowHigh sintering activitySecondary cellsElectrical batteryElectrochemistry
The invention discloses a method for preparing a solid electrolyte by using lithium lanthanum zirconium oxide precursor coated powder. The method specifically comprises the steps of dissolving a certain amount of lanthanum nitrate and zirconium nitrate into water, adding a precipitator, namely ammonium carbonate, controlling the pH value to ensure that La<3+> and Zr<4+> ions are simultaneously precipitated, and filtering and washing the precipitate; weighing a certain amount of lithium oxalate, dissolving lithium oxalate into water, adding the precipitate into the lithium oxalate solution, stirring, evaporating, crystallizing, and separating out lithium oxalate crystal on the surface of the precipitate to form precursor powder with a coated structure. The prepared powder has the advantages of uniform mixing, fine grains, high purity and the like; through the formed specific coated structure, the calcination temperature of the powder is low, the sintering time of the powder is short, and the room-temperature lithium ion electric conductivity of the sintered lithium lanthanum zirconium oxide is more than 2.2*10<-4>S / cm. According to the method, the process is simple, the cost is low, the preparation conditions are easy to control, and the prepared solid electrolyte is good in electrochemical stability and high in electric conductivity and can be used for preparing all-solid-state lithium ion batteries.
Owner:WUHAN UNIV OF TECH
Ni-based catalyst adopting core-shell structure and used in DRM (dry reforming of methane) and preparation method
InactiveCN107552054AImprove anti-sinteringImprove anti-carbon performanceHydrogenMetal/metal-oxides/metal-hydroxide catalystsGas compositionReaction temperature
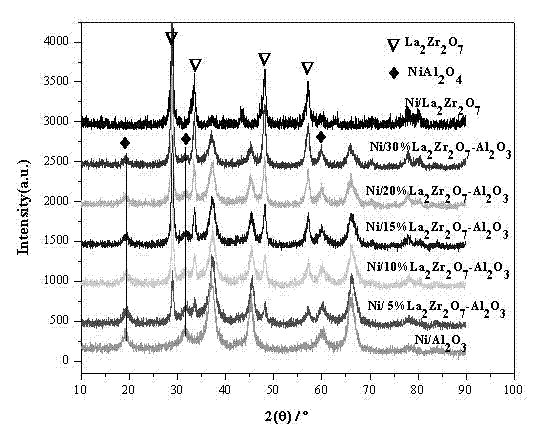
The invention discloses a Ni-based catalyst adopting a core-shell structure and used in DRM (dry reforming of methane) and a preparation method. A Ni-MOx@SiO2 (M is Zr, La and Ce) multi-core core-shell material is prepared with a reversed-phase microemulsion method with nickel nitrate, zirconium nitrate and the like as precursor salt. The additive amount of metal oxide in the catalyst is 1%-5% ofthe weight of the catalyst and the additive amount of nickel is 5%-10% of the weight of the catalyst. The sintering resistance and carbon deposit resistance of the catalyst are remarkably enhanced dueto addition of the metal oxide. The catalyst shows high activity, high stability and extremely high carbon deposit resistance and sintering resistance under the reaction conditions of normal pressure, reaction gas composition CH4:CO2 being 1.05:1, the air speed being 1,8000 ml.gcat<-1>.h<-1> and the reaction temperature being 800 DEG C. The catalyst has the advantages of being simple to prepare,free of secondary pollution to the environment, low in cost, high in catalytic efficiency and the like.
Owner:NANCHANG UNIV
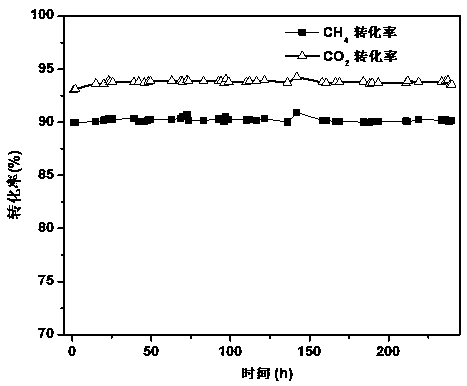
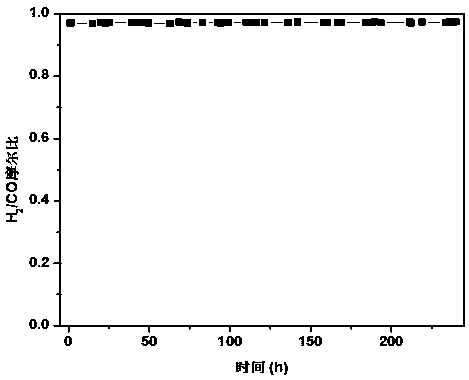
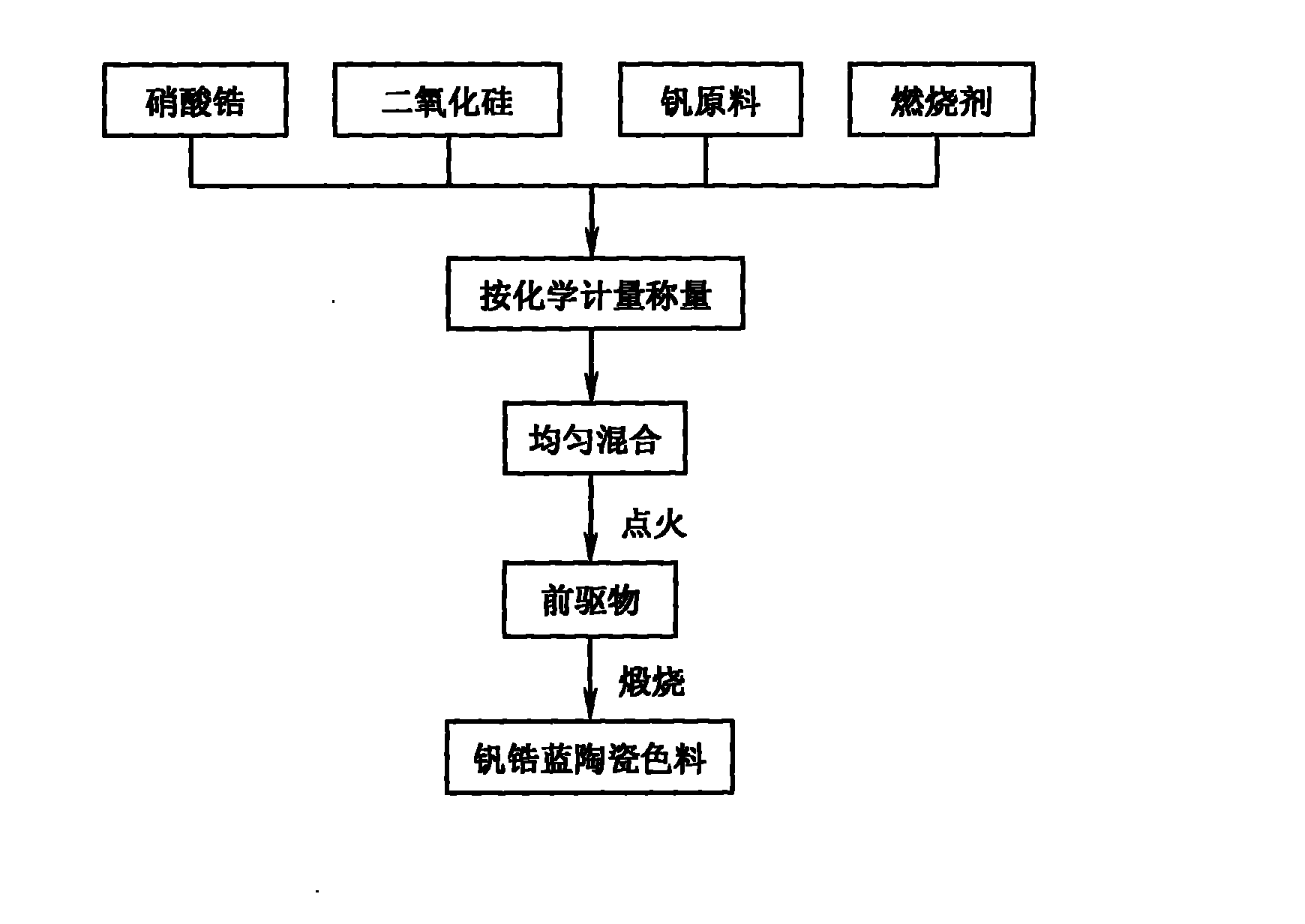
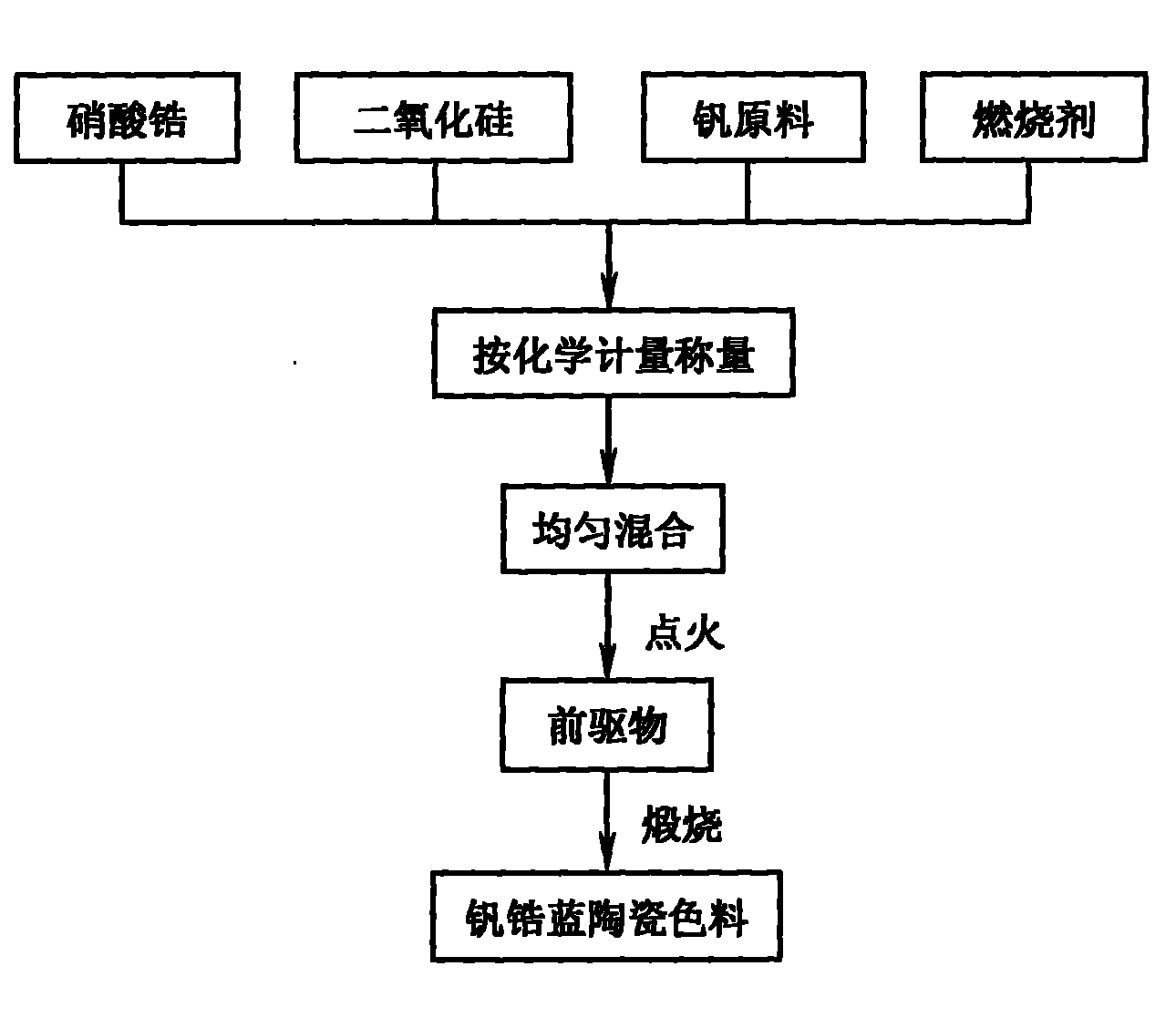
Method for preparing zircon-vanadium blue ceramic pigment
InactiveCN102050640ALow equipment requirementsSimple production processAmmonium metavanadateSilicon dioxide
The invention discloses a method for preparing a zircon-vanadium blue ceramic pigment, and belongs to the technical field of fine ceramics. In the method, zirconium nitrate and silicon dioxide are respectively taken as a vanadium raw material and a silicon raw material, ammonium acetate and the like are taken as organic incendiary agents, vanadium pentoxide, ammonium metavanadate and the like aretaken as zircon raw materials, sodium chloride, sodium fluoride and sodium fluosilicate are taken as a composite mineralizer, and the zircon-vanadium blue ceramic pigment is prepared by combustion synthesis and calcining technology. The method has the advantages that: the production process is simple, the equipment requirement is low, and the method is suitable for industrial production. The prepared zircon-vanadium blue ceramic pigment has narrow size distribution and stable color.
Owner:佛山市华南精细陶瓷技术研究开发中心
Preparation method of zirconic acid lanthanum ceramic fiber
ActiveCN103553596ASmall diameterEvenly distributedInorganic material artificial filamentsFiberFilamentation
The invention relates to a preparation method of a zirconic acid lanthanum ceramic fiber. The method comprises the following steps: preparing a gel fiber through a colloidal sol centrifugal fiber forming technology by using zirconium nitrate as a zirconium source, lanthanum nitrate as a lanthanum source, citric acid as a chelating agent, and water as a solvent, and calcining the gel fiber to form the zirconic acid lanthanum ceramic fiber. A precursor prepared by the invention has stable colloidal sol property and is good in filamentation property with no need of adding a polymer spinning additive. The prepared zirconic acid lanthanum ceramic fiber is good in flexibility, compact in microstructure, good in phase stability an low in heat conductivity coefficient and can be used for high-temperature thermal-insulation material, and the crystal form of the zirconic acid lanthanum ceramic fiber is pyrochlore form.
Owner:SHANDONG UNIV
Preparation method of zirconium carbide ceramic powder
A preparation method of zirconium carbide ceramic powder belongs to the preparation field of ceramic powder. By improving a raw material mixing method and enabling subparticles with smaller particle size to be directly contacted and reacted, reaction activity of precursors is improved, thereby helping to enable the precursors to be compounded to high-purity fine-grained ceramic powder at a low temperature. A zirconium source is zirconium nitrate or zirconium oxychloride, a carbon source is glucose, and additives are carbamide, ammonium nitrate and hydrogen nitrate. Molar ratio of the zirconium source to the carbon source (Zr: C) is 1: 5-18. Molar ratio of +5 valence of nitrogen and -3 valence of nitrogen is N +5: N-3 = 1: 0.1-10. The particle size of the zirconium source and the carbon source in the precursors is small, mixing is even, reaction activity is good, carbothermic reduction reaction temperature can be lowered, reaction rate can be improved, and nano-level zirconium carbide ceramic powder with good dispersibility can be prepared. In addition, a variety of raw material sources are available, price is low, production cost is low, and prepared zirconium carbide nano-powder is stable in property, simple in production process and capable of achieving mass production.
Owner:UNIV OF SCI & TECH BEIJING
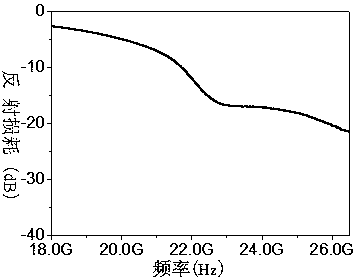
Zirconium-doped barium ferrite wave-absorbing material and preparation method thereof
ActiveCN104030667AApplication frequency band wideningMatching Thickness ReductionOther chemical processesBarium nitrateElectromagnetic shielding
The invention discloses a zirconium-doped barium ferrite wave-absorbing material having a chemical formula of BaFe12-xZrxO19, wherein x is 0.3-0.5, zirconium-doped barium ferrite is a polycrystalline powder, and Fe<3+> and Fe<2+> exist in the barium ferrite simultaneously. A preparation method comprises the preparation steps: mixing barium nitrate, iron nitrate and zirconium nitrate, adding deionized water, and dissolving into a nitrate solution; placing EDTA in deionized water, and dissolving into an EDTA solution; adding the nitrate solution into the EDTA solution, heating, drying, and thus obtaining a dry gel; and sintering the dry gel to obtain a zirconium-doped barium ferrite powder, then grinding, and thus obtaining the zirconium-doped barium ferrite wave-absorbing material. The wave-absorbing material has the characteristics of thin matching thickness and wide wave-absorbing frequency band, can be used for a wave-absorbing coating layer, and can have wide applications in the electromagnetic shielding and stealth fields.
Owner:ZHEJIANG UNIV
Method for solidifying actinium series nuclide by pyrochlore type rare earth zirconate
ActiveCN102779561AEasy disposalImprove efficiencyRadioactive decontaminationWaste processingDeep geological repository
The invention discloses a method for solidifying actinium series nuclide by pyrochlore type rare earth zirconate and belongs to the technical field of radioactive nuclear waste processing. The method includes adopting rare earth nitrate, zirconium nitrate or zirconium oxynitrate, actinium series nuclide raw materials and a little fluxing agent as raw materials, grinding and mixing the raw materials and directly placing the raw materials in a sintering furnace to conduct sintering under certain temperature to obtain a pyrochlore type rare earth zirconate solidified body containing actinium series nuclide. Therefore, radioactive nuclide can be solidified in lattices of the rare earth zirconate to facilitate deep geology processing. The whole solidification process does not require complex high-energy-consumption dangerous steps of repeated grinding, piece pressing, long-time high-temperature sintering, sol gel preprocessing, piece pressing, long-time high-temperature sintering and the like. The method is energy-saving, high in efficiency and good in safety and reduces consumption.
Owner:TSINGHUA UNIV
Iron base catalyst for fischer-tropsch synthesis and preparation method thereof
ActiveCN101293206AImprove wear resistanceIncrease the areaHydrocarbon from carbon oxidesMetal/metal-oxides/metal-hydroxide catalystsPotassium nitratePotassium silicate
The present invention relates to a fetto-synthesized iron catalyst, which is characterized in that the catalyst comprises the components with the following weight ratio Fe: Cu: K: Zr: SiO2=100: (0.01-10): (0.01-10): (0.01-15): (0.1-28); wherein, Fe, Cu, K and Zr exist in the form of oxide. The present invention also relates to a preparation method of the catalyst, which includes that the mixed water solution of ferric nitrate, zirconium nitrate and potassium silicate is deposited by salvolatile, the sediment is filtered and washed, and the filter mass is mixed with silica sol, cupric nitrate and potassium nitrate for drying and calcining. More than 90 percent of grains of the catalyst provided by the present invention are micro-sphere, which are an even micro-sphere with smooth surface and has the characteristics of high anti-abrasive performance, high stability and activity, good product selectivity, etc. In addition, the catalyst can reduce the preparation cost and energy consumption of the catalyst and is suitable for mass industrial production.
Owner:CHNA ENERGY INVESTMENT CORP LTD +2
Metal surface treatment agent as well as preparation method thereof
ActiveCN103540917AImprove corrosion resistanceImprove coating adhesionMetallic material coating processesTitanium fluorideSilanes
The invention relates to a metal surface treatment agent as well as a preparation method thereof. The metal surface treatment agent consists of titanium fluoride, zirconium nitrate, zirconium fluoride, amino silane, 1,2-di(triethoxy silyl) ethane and di(triethoxy silyl) hexane. The titanium fluoride, the zirconium nitrate and the zirconium fluoride account for 110ppm-140ppm or 550ppm-650ppm of the total amount of the treatment agent in terms of metal elements, wherein a ratio of metal element atoms of the titanium fluoride to metal element atoms of the zirconium nitrate and the zirconium fluoride is 1:1-1:3; a ratio of metal element atoms of the zirconium nitrate to the metal element atoms of the zirconium fluoride is 2:5; a molar ratio of the amino silane to the 1,2-di(triethoxy silyl) ethane and the di(triethoxy silyl) hexane is 17:3-9:1; a molar ratio of the 1,2-di(triethoxy silyl) ethane to the di(triethoxy silyl) hexane is 1:1.
Owner:宁波英科特精工机械股份有限公司
Esterification catalyst for synthesizing ethylene/propylene glycol ether carboxylate, and preparation method thereof
InactiveCN101722027AShort processLess investmentMolecular sieve catalystsOrganic compound preparationHigh energyCerium
The invention provides an esterification method for synthesizing ethylene / propylene glycol ether carboxylate. A catalyst used in the method consists of an active substance and a carrier, wherein the active substance is tin salt (such as tin tetrachloride), titanium salt (such as titanium sulfate, titanium tetrachloride or titanium nitrate), zirconium salt (such as zirconium sulfate, zirconium tetrachloride or zirconium nitrate) or cerium salt (such as cerous sulfate, cerium tetrachloride or cerous nitrate); the carrier is silica gel, hydrogen-type ZSM-5, activated clay or diatomite; the content of the active substance in percentage by mass is 10 to 30 percent; and the content of the carrier in percentage by mass is 70 to 90 percent. The method has the advantages of inhibiting the occurrence of side reaction during production, improving the selectivity of target products, shortening production cycle, reducing investment in production equipment and avoiding the problems of production equipment corrosion, environmental pollution, high energy consumption and the like.
Owner:SHENYANG POLYTECHNIC UNIV
Composite cerium-zirconium-barium oxides and preparing method thereof
The invention provides a kind of super tiny cerium-zirconium-barium compound oxide and the manufacturing method. The molecular formula is CexZr1-xBayOz, the mol proportion of cerium, zirconium and barium is: Ce=X(X=0.1í½1) : Xr=1-X: Ba=Y(Y=0.1í½0.5), it uses metal nitrate such as cerium nitrate, zirconium nitrate, barium nitrate as materials, through polyglycol high molecular surface decorating agent, uses butyl alcohol azeotropy distillation. The baked temperature is 600 deg.C í½700 deg.C, the time is 3í½4 hours.
Owner:ZHEJIANG UNIV
Preparation method and application method of CuZr(PO4)2*4H2O material
InactiveCN103072965AReduce pollutionThe synthesis process is simpleAdditivesPhosphorus compoundsO-Phosphoric AcidCopper nitrate
The invention discloses a preparation method and an application method of a CuZr(PO4)2*4H2O material, and belongs to the field of inorganic functional materials. The preparation method and the application method are characterized in that the CuZr(PO4)2*4H2O material is directly prepared by a hydrothermal synthesis method, and further applied to the field of lubrication. The preparation method comprises the steps that one of a zirconium oxychloride solution and a zirconium nitrate solution with a molar concentration of 1.0-4.0mol / L is mixed with one of a copper chloride solution, a copper acetate solution and a copper nitrate solution with a molar concentration of 1.0-4.0mol / L on the condition that a molar ratio of Cu to Zr is 0.8-3.0, and stirred uniformly; then a pH value of a system is adjusted to be 0.5-5 with 85% of phosphoric acid solution by mass percent; stirring is conducted for 10-20min; blue gel is obtained, sealed in a polytetrafluoroethylene lining reaction kettle, and crystallized in a drying oven at 100-220 DEG C for 12-200h; a product is washed to be neutral with distilled water by a centrifuge, and dried at a room temperature; and the CuZr(PO4)2*4H2O material is obtained. The material is directly added to lubricant base oil to serve as a lubricant additive in an ultrasonic, heating and stirring, or three-roller grinding mode, and has good bearing capability, abrasion resistance and antifriction capability.
Owner:TAIYUAN UNIV OF TECH
Spray granulation preparation method for zirconium oxide toughened aluminum oxide powder
The invention discloses a spray granulation preparation method for zirconium oxide toughened aluminum oxide powder. The method comprises the following steps of: preparing nano zirconium nitrate and yttrium hydroxide mixed gel by adopting a co-precipitation method, adding nano aluminum oxide, mixing uniformly by using a wet ball milling method to form Zr(OH)4+Y(OH)3 / Al2O3 slurry, performing spray drying granulation and calcination on the slurry, and thus obtaining the zirconium oxide toughened aluminum oxide powder with fine zirconium oxide crystal, uniform distribution of the zirconium oxide crystal in the aluminum oxide, high sintering activity and good forming performance. The method for preparing the zirconium oxide toughened aluminum oxide granulated composite powder overcomes the defects of low uniformity, zirconium oxide agglomeration and the like caused by directly mixing and granulating zirconium oxide powder and aluminum oxide powder in the conventional method, powder making and granulation processes are integrated, the method is simple, and the zirconium oxide is dispersed uniformly and is low in granularity and high in sintering activity.
Owner:SOUTH CHINA UNIV OF TECH
Method for preparing aluminum oxide for catalyst
InactiveCN102658114AWell mixedFully packagedMetal/metal-oxides/metal-hydroxide catalystsCerium nitrateManufacturing technology
A method for preparing aluminum oxide for catalysts relates to the improvement of the preparation method of activated aluminum oxide used in catalysis field such as petrochemical engineering, industrial catalysis, automobile exhaust purification and the like. The method is characterized in that one or any two of lanthanum nitrate, zirconium nitrate and cerium nitrate is / are added in a reaction tank preparing pseudoboehmite and is / are used as modifying agents, and action is performed; slurry is processed through suction filtration, washing and drying after reaction and then modified pseudoboehmite powders are obtained; and the powders that are dried are roasted and then the aluminum oxide for the catalysts is obtained. The method is adopted to process the pseudoboehmite slurry through doping modification, is closely combined with the manufacturing process of the pseudoboehmite, effectively simplifies the manufacturing technology, lowers the manufacturing cost, achieves good surface modification effect, improves the heat stability of activated aluminum oxide powders, and improves the service performance of the activated aluminum oxide powders.
Owner:GUIZHOU BRANCH CHINA ALUMINUM IND
Carbon dioxide methanation catalyst (Ni/CexZr(1-x)O2) and preparation method thereof
InactiveCN105289616AEfficient hydrogenation reactionHigh catalytic activityMetal/metal-oxides/metal-hydroxide catalystsCerium nitratePtru catalyst
The invention discloses a carbon dioxide methanation catalyst (Ni / CexZr(1-x)O2) and a preparation method thereof. The preparation method comprises the following steps: (a) adding a certain amount of nickel nitrate, a certain amount of cerium nitrate and a certain amount of zirconium nitrate into 50ml of deionized water to prepare a mixed solution with the content of Ni being 10-40wt.% in the catalyst (Ni / CexZr(1-x)O2); (b) transferring the prepared mixed solution into a thermostat water bath pan at 80 DEG C, uniformly stirring, quickly pouring a 100ml of2 mol / Lsal volatile solution into the mixed solution, stirring, heating, drying at a constant temperature, and then acquiring the powder; (c) roasting the powder after being dried for 5h in a muffle furnace by increasing the room temperature to 500 DEG C at a heating rate of 2 DEG C / min, and then putting the powder after being roasted into a steel moldfor extruding and forming, breaking and sieving, thereby acquiring the grains with the grain sizes being 40-60 meshes; and(d) reducing for 2h at the temperature being 400 DEG C in a hydrogen atmosphere, thereby acquiring the Ni / CexZr(1-x)O2 catalyst. The catalyst prepared by adopting the preparation method has relatively high catalytic activity, selectivity and stability at 250-400 DEG C. The preparation method is simple, easy to operate and low in cost.
Owner:SHANGHAI UNIV
Stain resistant environment-friendly color-coated sheet
ActiveCN105002495AImprove corrosion resistanceImprove adhesionSuperimposed coating processEpoxyEnvironmental resistance
The invention provides a stain resistant environment-friendly color-coated sheet, comprising a substrate, a chemical passivation layer, a low fluorine base coat, a high fluorine surface coat and a back coat, wherein the substrate is a steel plate and the surface of the steel plate is coated with a zinc layer; the high fluorine surface coat comprises 3-5wt% of 1,4-butanediol, 1-2wt% of silicon dioxide, 1-3wt% of aluminium oxide and 90-95wt% of polyvinylidene fluoride; the low fluorine base coat comprises 15-25wt% of 4-hydroxybutyl vinyl ether, 10-20wt% of polyvinyl alcohol and 55-75wt% of polyvinylidene fluoride; the chemical passivation layer comprises 25-30wt% of molybdate, 45-50wt% of organic fluorine modified epoxide resin, 5-7wt% of zirconium nitrate, 10-15wt% of phosphoric acid and 5-15wt% of silicon dioxide. The color-coated sheet prepared by the invention is high in stain resistance and weather fastness, and the surface coat is excellent in adhesion force.
Owner:扬子江新型材料(苏州)有限公司
A preparing method of a catalyst for low-temperature denitrification
InactiveCN105521777AHigh activityHigh selectivityGas treatmentOrganic-compounds/hydrides/coordination-complexes catalystsOXALIC ACID DIHYDRATECellulose
The invention relates to a preparing method of a catalyst for low-temperature denitrification, and belongs to the field of flue gas denitrification. The denitrification efficiency of the catalyst for flue gas at 150-420 DEG C is 95% or above. Raw materials for preparing the catalyst comprise 55-91 parts of superfine titanium dioxide, 1-10 parts of lanthanum nitrate, 1-5 parts of polyoxyethylene, 1-10 parts of cerous nitrate, 1-10 parts of zirconium nitrate, 0.05-10 parts of ammonium metavanadate, 0.05-15 parts of ammonium heptamolybdate, 0.05-10 parts of ammonium paratungstate, 1-5 parts of carboxymethylcellulose, 10-30 parts of deionized water, 1-15 parts of monoetanolamine and 1-5 parts of oxalic acid. The catalyst is characterized by high selectivity, high activity and strong poison resistance.
Owner:ANHUI YUANCHEN ENVIRONMENTAL PROTECTION SCI & TECH
Preparation method of porous cerium-based composite oxide
ActiveCN106946282ALarge specific surface areaHigh yieldRare earth metal oxides/hydroxidesDispersed particle separationAir atmosphereCerium nitrate
A preparation method of a porous cerium-based composite oxide includes the following steps: (1) dissolving cerium nitrate, zirconium nitrate and an aluminum source in water to obtain a water solution and adding citric acid and uniformly mixing the solution, wherein the molar ratio of cerium nitrate, zirconium nitrate, aluminum source to citric acid is 1-5:0.5-3:0-8:2-10; (2) adding a surfactant and anhydrous ethanol to the solution, uniformly stirring the solution, and allowing the solution to stand for 8-12 h at 80-120 DEG C to obtain a foamed product; (3) placing the foamed product in an air atmosphere, increasing the temperature to 500-800 DEG C at the rate of 5-10 DEG C / min, and calcining the foamed product for 4-6 h to obtain the porous cerium-based composite oxide. There are two pore structures, mesopores and macropores, in the porous cerium-based composite oxide, so that the porous cerium-based composite oxide has high specific surface area and penetrating property of the pores. The preparation method employs easy-to-obtained raw materials, is low in cost and high in yield, is simple and controllable, is free of large-size special equipment and is easy to achieve in large-scale production.
Owner:INST OF RESOURCES UTILIZATION & RARE EARTH DEV GUANGDONG ACAD OF SCI
Process for separating zirconium and hafnium by solvent extracting method
ActiveCN106929695AFulfil requirementsImprove product qualityProcess efficiency improvementZirconium oxidesKeroseneHydrometallurgy
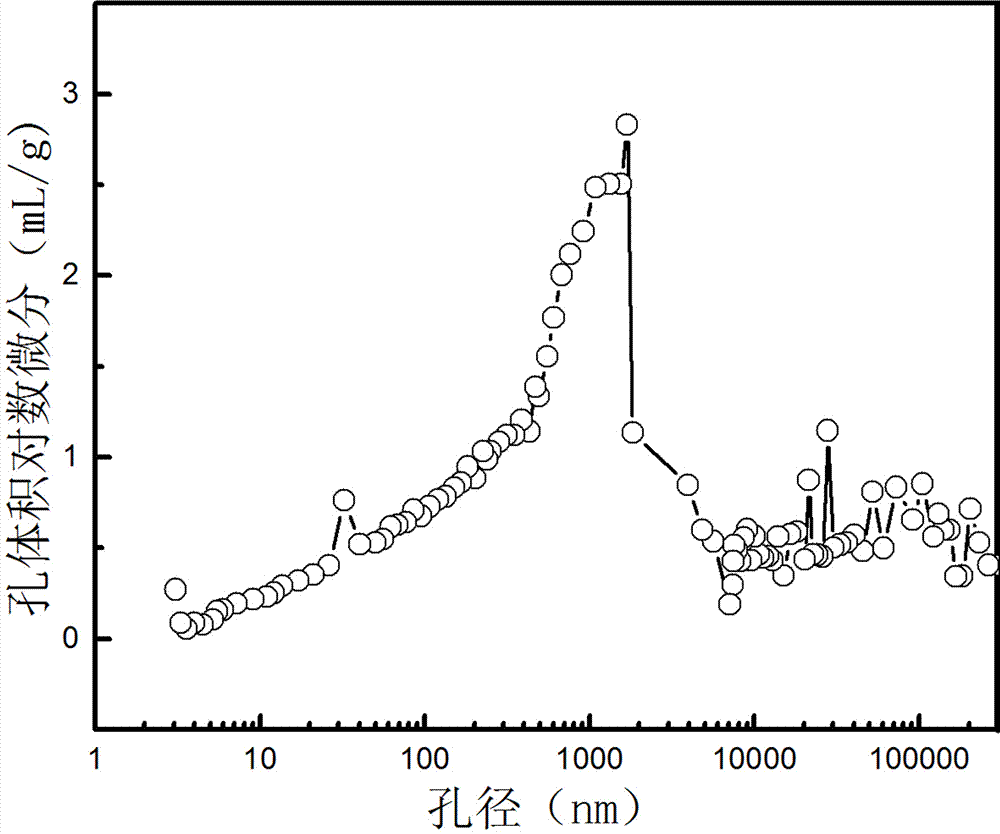
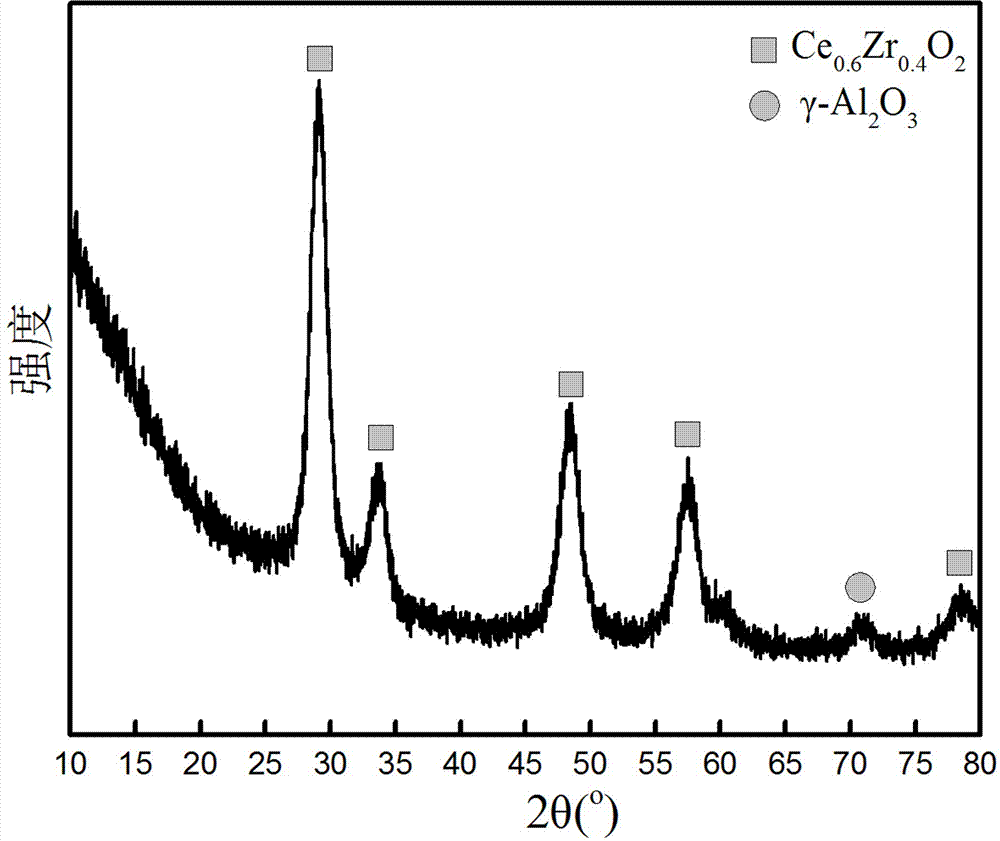
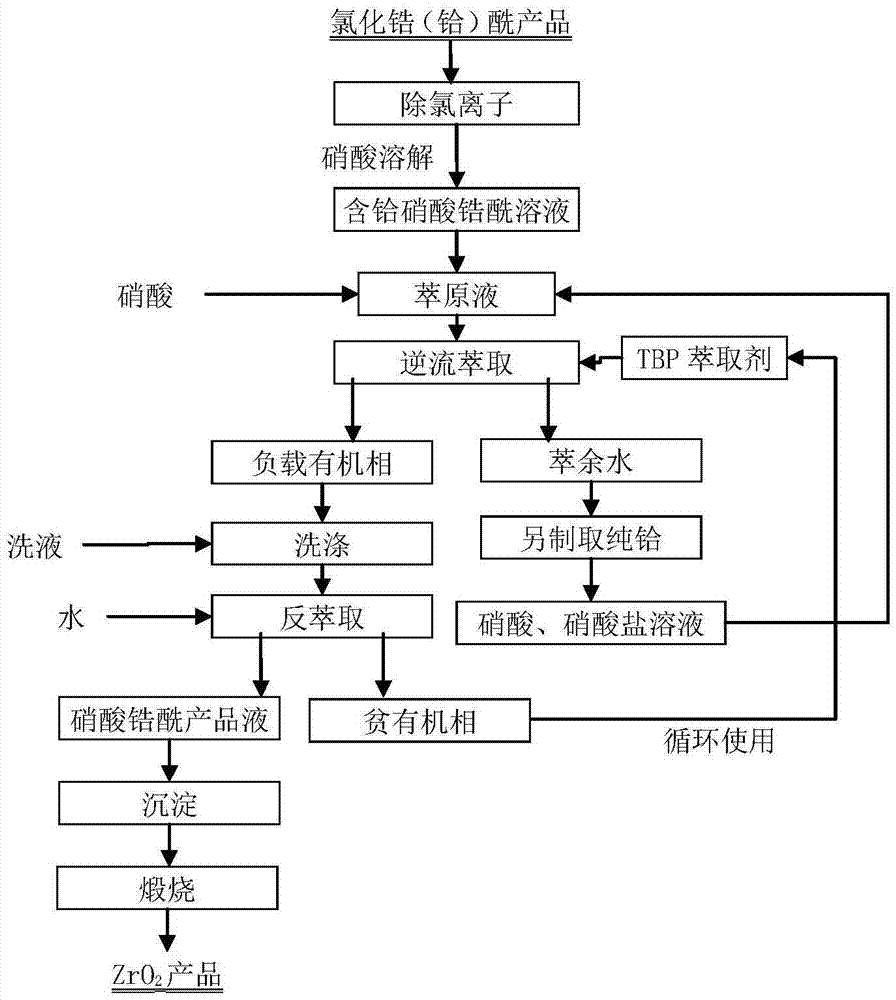
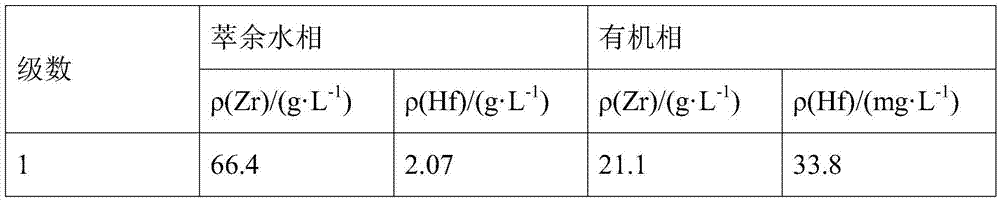
The invention belongs to the field of wet metallurgy and the technical field of zirconium and hafnium separation, and in particular, relates to a process for separating zirconium and hafnium by a solvent extracting method. Industrial products containing hafnium zirconium chloride acetyl are used as raw materials for such procedures as water solution, alkali sinking, washing and nitric acid dissolving to prepare zirconium (hafnium) nitrate acetyl solution; TBP kerosene solution of adding phase modifying agent octanol is used as an extracting agent; most zirconium and less hafnium are extracted into an organic phase through multistage counter-current extraction by using the characteristic of TBP priority extraction of zirconium; the loaded organic phase is washed by nitric acid solution with a certain concentration to further remove most hafnium in the organic phase; the acid-washed loaded organic phase is reversely extracted by water to obtain zirconium nitrate acetyl solution; the solution is precipitated in ammonia, dried and calcined to obtain zirconium dioxide powder, and the mass of the zirconium dioxide powder accords with the atomic energy-level zirconium dioxide standard; less zirconium and most hafnium only remained in residual water phase of zirconium after reverse extraction are extracted; the content of zirconium in hafnium meets the requirements on impurity zirconium by atomic energy-level hafnium; and atomic energy-level hafnium can be directly prepared by enrichment.
Owner:BEIJING RESEARCH INSTITUTE OF CHEMICAL ENGINEERING AND METALLURGY
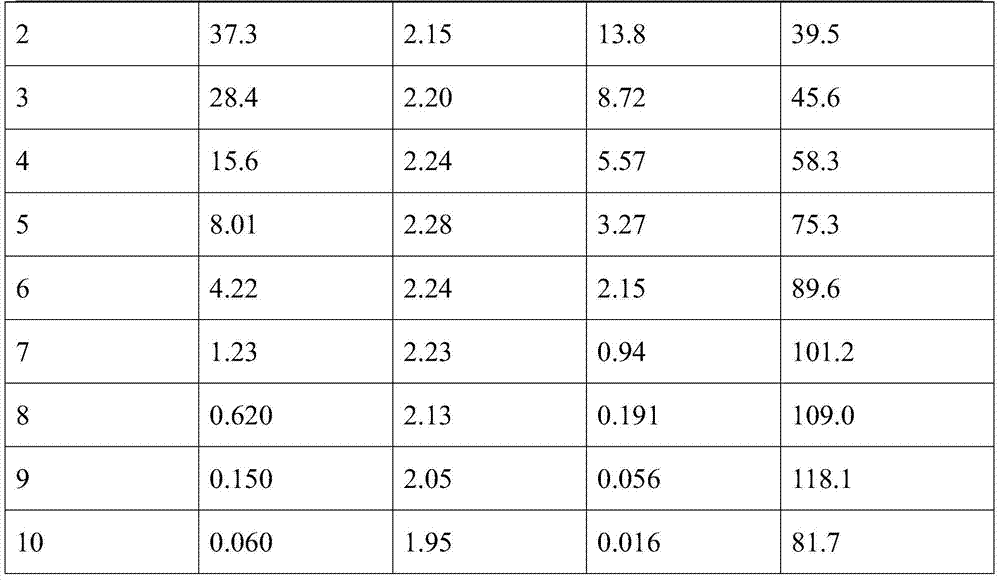
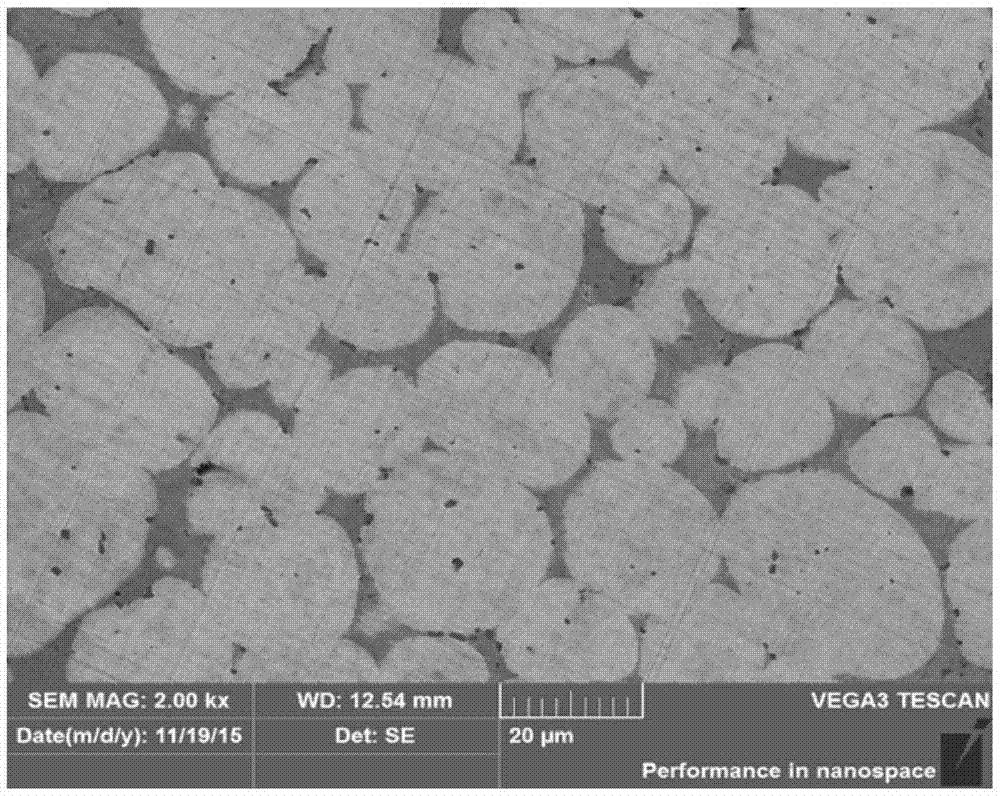
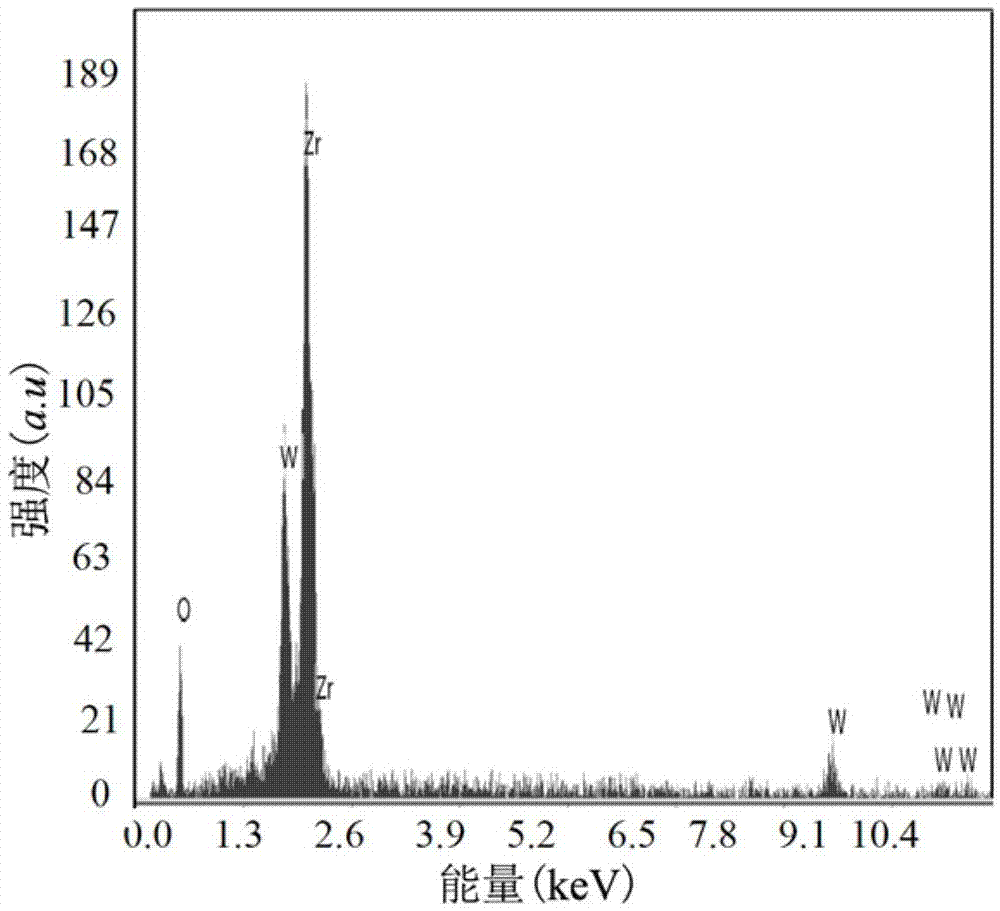
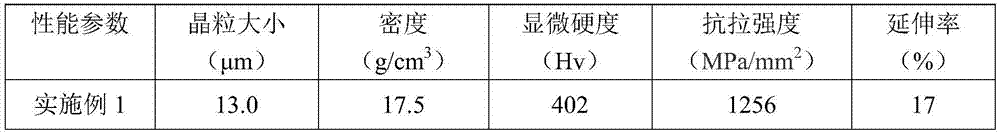
Missile heavy-gravity tungsten alloy and preparation method thereof
Owner:HENAN UNIV OF SCI & TECH
Cerium-zirconium base nitric oxide common temperature catalyst preparation method
InactiveCN103263925AIncreased oxidation absorption efficiencySimple processMetal/metal-oxides/metal-hydroxide catalystsCerium nitrateCatalytic oxidation
The invention provides a cerium-zirconium base nitric oxide common temperature catalyst preparation method, which is characterized by comprising: adopting active alumina powder as a carrier, carrying out cerium nitrate and zirconium nitrate impregnating loading, calcining, carrying out copper acetate and ammonia water mixed solution impregnating, and carrying out low temperature drying to obtain the catalyst so as to achieve common temperature catalysis oxidation on nitric oxide. Compared with the preparation method in the prior art, the preparation method of the present invention has characteristics of simple process, high catalysis efficiency and the like, wherein NO oxidation absorption efficiency is significantly increased.
Owner:SHANGHAI NAT ENG RES CENT FORNANOTECH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com