Method for preparing solid electrolyte by using lithium lanthanum zirconium oxide precursor coated powder
A technology of solid electrolyte, lithium lanthanum zirconium oxide, applied in circuits, electrical components, secondary batteries, etc., can solve the problems of sintering temperature, powder not fine enough, long sintering time, etc. Lower calcination temperature and lower cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
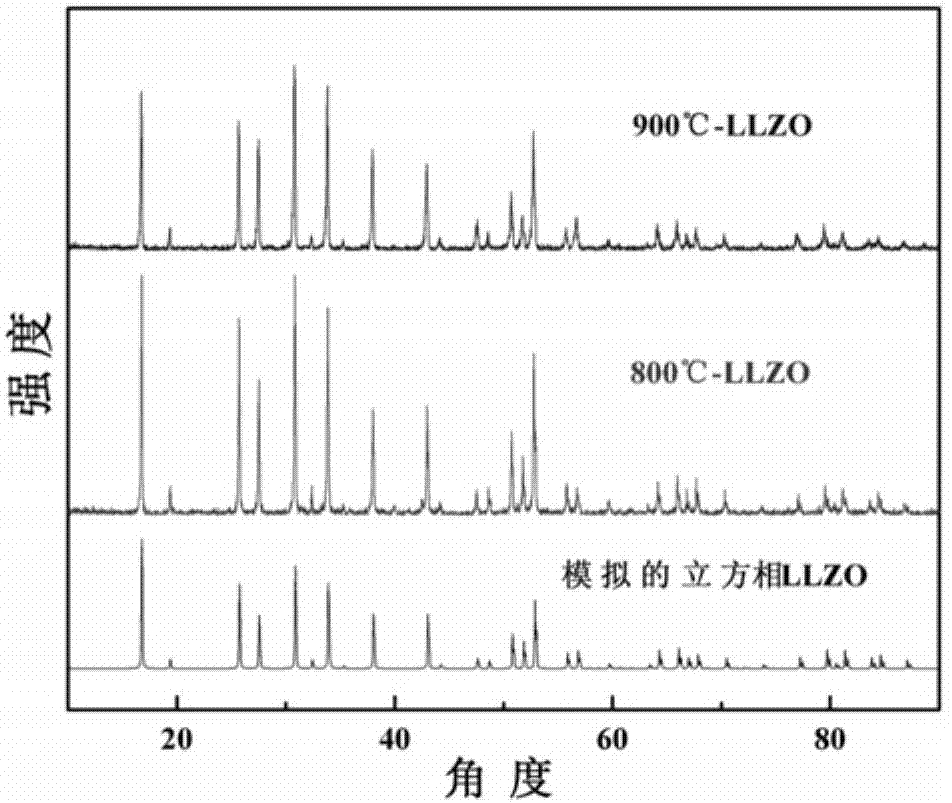
[0034] Weigh lanthanum nitrate (LaN3 o 9 ·6H 2 O) 2.6055g, zirconium nitrate (N 4 o 12 Zr·5H 2 O,) 1.722g is dissolved in 500ml water, and in the above solution, add precipitating agent ammonium carbonate ((NH 4 ) 2 CO 3 ) 0.9636g, adjust the pH of the solution with ammonia water, control the pH of the solution to be 7, make La 3+ 、Zr 4+ The ions were simultaneously precipitated, and the precipitate was subsequently filtered and washed three more times with deionized water. Weigh lithium oxalate (Li 2 C 2 o 4 ) 0.6725g was dissolved in 200ml of water, and the washed precipitate was added to the lithium oxalate solution, stirred and evaporated to crystallize in a water bath at 60°C until all the water was evaporated to obtain a precursor composite powder. Then it was calcined at 600°C for 8 hours, and the calcined powder was pressed into a tablet at 20 MPa, and then sintered at 600°C under normal pressure for 10 hours, and the obtained product was the solid electroly...
Embodiment 2
[0036] Weigh lanthanum nitrate (LaN 3 o 9 ·6H 2 O) 2.5835g, zirconium nitrate (N 4 o 12 Zr·5H 2 (O) 1.7075g is dissolved in 500ml water, adds precipitation agent ammonium carbonate ((NH 4 ) 2 CO 3 ) 1.4332g, adjust the pH of the solution with ammonia water, and control the pH of the solution to be 8, so that La 3+ 、Zr 4+ The ions were simultaneously precipitated, and the precipitate was subsequently filtered and washed three more times with deionized water. Weigh lithium oxalate (Li 2 C 2 o 4 ) 0.7090g was dissolved in 250ml of water, and the washed precipitate was added to the lithium oxalate solution, stirred and evaporated to crystallize in a water bath at 80°C until all the water was evaporated to obtain a precursor composite powder. Then it was calcined at 700°C for 6 hours, and the calcined powder was pressed into a tablet at 40 MPa, and then sintered at 700°C under normal pressure for 8 hours, and the obtained product was the solid electrolyte material of th...
Embodiment 3
[0038] Weigh lanthanum nitrate (LaN 3 o 9 ·6H 2 O) 2.5650g, zirconium nitrate (N 4 o 12 Zr·5H 2 (O) 1.6955g is dissolved in 600ml water, adds precipitation agent ammonium carbonate ((NH 4 ) 2 CO 3 ) 1.8974g, adjust the pH of the solution with ammonia water, and control the pH of the solution to be 9, so that La 3+ 、Zr 4+ The ions were simultaneously precipitated, and the precipitate was subsequently filtered and washed three more times with deionized water. Weigh lithium oxalate (Li 2 C 2 o 4 ) 0.7395g was dissolved in 250ml of water, and the washed precipitate was added to the lithium oxalate solution, stirred and evaporated to crystallize in a 90°C water bath until all the water was evaporated to obtain a precursor composite powder. Then calcined at 800°C for 4 hours, the calcined powder was sintered with electric field assistance, the sintering pressure was 20MPa, the heating rate was 300°C / min, the sintering temperature was 800°C, and the holding time was 5 min...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com