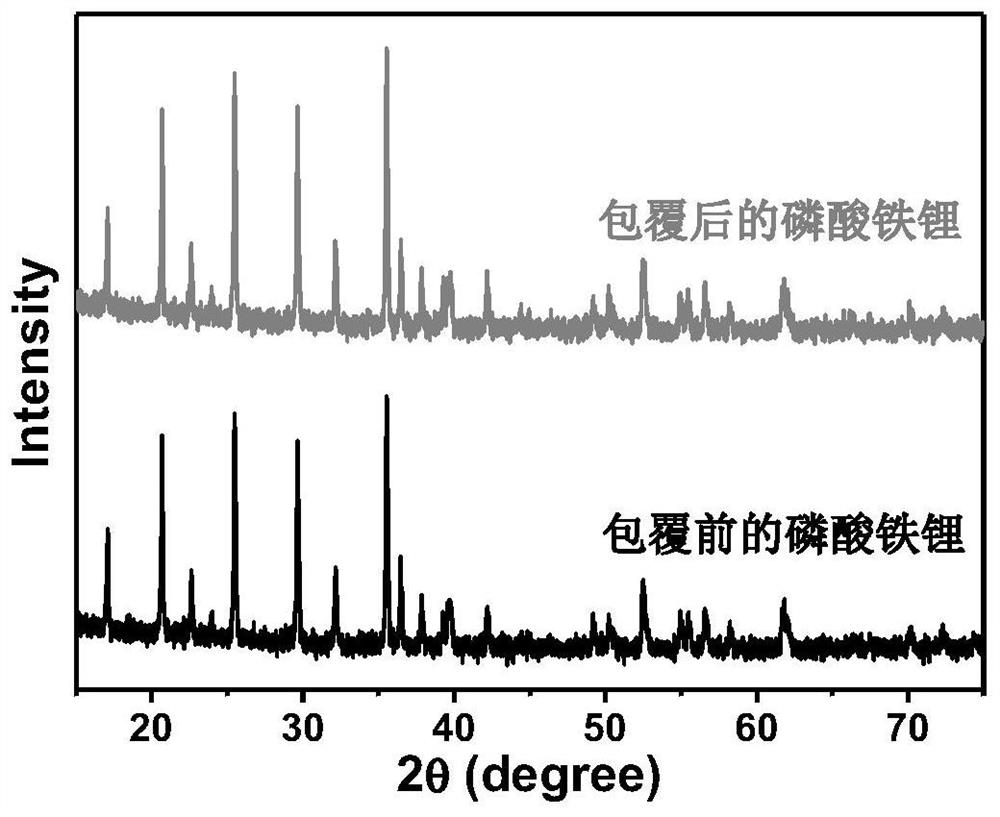
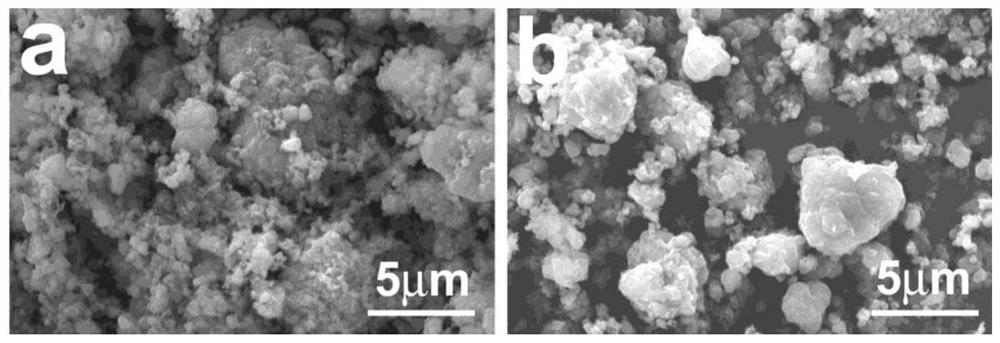
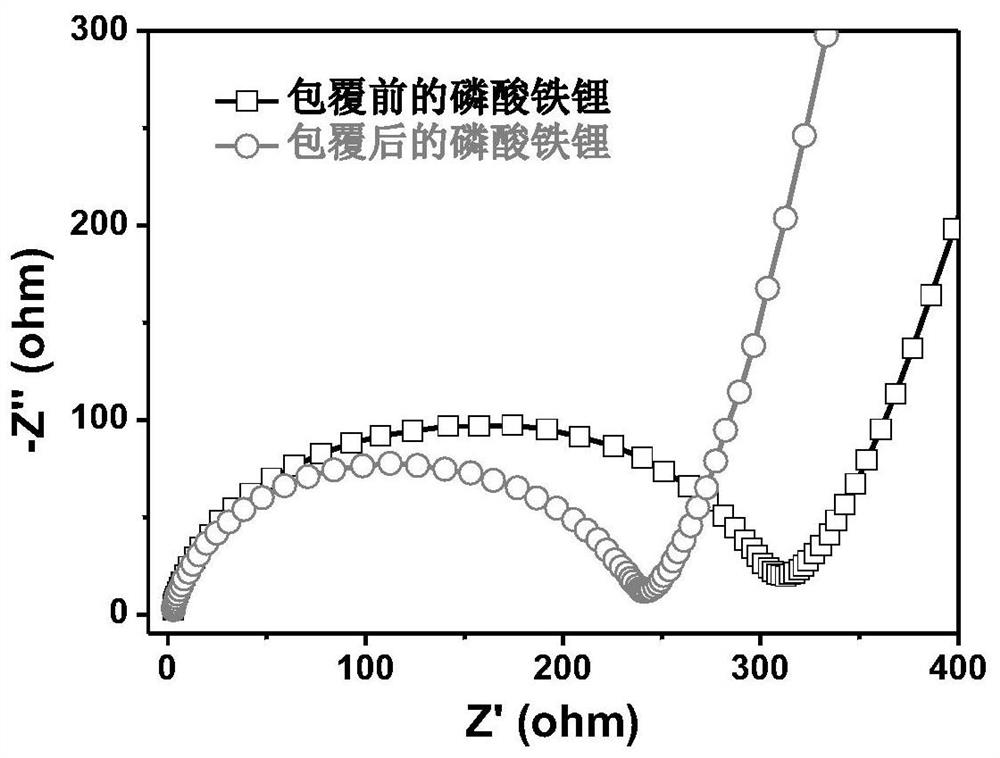
Method for compositely coating lithium battery positive electrode material with tungsten oxide and nitrogen-doped carbon
A battery cathode, nitrogen-doped carbon technology, applied in battery electrodes, positive electrodes, secondary batteries, etc., can solve the problems of inability to improve the conductivity of cathode materials, unfavorable industrial production and manufacturing, and achieve excellent electronic conductivity and electrochemical performance. Effects of improved stability, electronic conductivity and electrochemical stability, low energy consumption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Dissolve ammonium metatungstate in 19 mL of water, and then disperse 1.9 grams of lithium iron phosphate in the ammonium metatungstate solution; dissolve dopamine in 1 mL of deionized water, and add it to the solution containing lithium iron phosphate and ammonium metatungstate In the dispersion, a mixed reaction solution was prepared, wherein the amount of the ammonium metatungstate substance in the mixed reaction solution was 0.024mmol, and the amount of the dopamine substance was 1.26mmol;
[0029] The mixed reaction solution was stirred at 25°C for 24 hours, and the obtained product was centrifuged, washed, and dried to obtain a tungsten and carbon precursor co-coated lithium iron phosphate material;
[0030] The dry tungsten and carbon precursor co-coated lithium iron phosphate positive electrode material was calcined under argon protection and the calcination temperature was 500°C for 6 hours to obtain the tungsten oxide and nitrogen-doped carbon co-coated lithium ...
Embodiment 2
[0038]Dissolve ammonium paratungstate in 19 mL of water, then disperse 1.9 grams of lithium iron phosphate in the ammonium paratungstate solution; dissolve dopamine in 1 mL of deionized water, and add it to the dispersion containing lithium iron phosphate and ammonium paratungstate to prepare a mixed reaction solution , wherein, the amount of the ammonium paratungstate substance in the mixed reaction solution is 0.01mmol, and the amount of the dopamine substance is 0.44mmol;
[0039] The mixed reaction solution was stirred at 5°C for 5 hours, and the obtained product was centrifuged, washed, and dried to obtain a tungsten and carbon precursor co-coated lithium iron phosphate material;
[0040] The dry tungsten and carbon precursor co-coated lithium iron phosphate cathode material was calcined under argon protection and the calcination temperature was 300°C for 7 hours to obtain the tungsten oxide and nitrogen-doped carbon co-coated lithium iron phosphate cathode material;
[0...
Embodiment 3
[0043] Dissolve sodium phosphotungstate in 19 mL of water, then disperse 1.9 g of lithium iron phosphate in the sodium phosphotungstate solution; dissolve dopamine in 1 mL of deionized water, and add it to the solution containing lithium iron phosphate and sodium phosphotungstate In the dispersion, a mixed reaction solution is obtained, wherein the amount of the ammonium paratungstate substance in the mixed reaction solution is 0.01mmol, and the amount of the dopamine substance is 0.55mmol;
[0044] The mixed reaction solution was stirred at 68°C for 30 hours, and the obtained product was centrifuged, washed, and dried to obtain a tungsten and carbon precursor co-coated lithium iron phosphate material;
[0045] The dry tungsten and carbon precursor co-coated lithium iron phosphate positive electrode material was calcined under argon protection and the calcination temperature was 420°C for 18 hours to obtain the tungsten oxide and nitrogen-doped carbon co-coated lithium iron pho...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com