Iron base catalyst for fischer-tropsch synthesis and preparation method thereof
An iron-based catalyst and Fischer-Tropsch synthesis technology, applied in chemical instruments and methods, physical/chemical process catalysts, metal/metal oxide/metal hydroxide catalysts, etc., can solve the problem of difficulty in greatly increasing the solid content of slurry, etc. problems, to achieve the effect of increasing the effective specific surface area, good activity and selectivity, and high activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
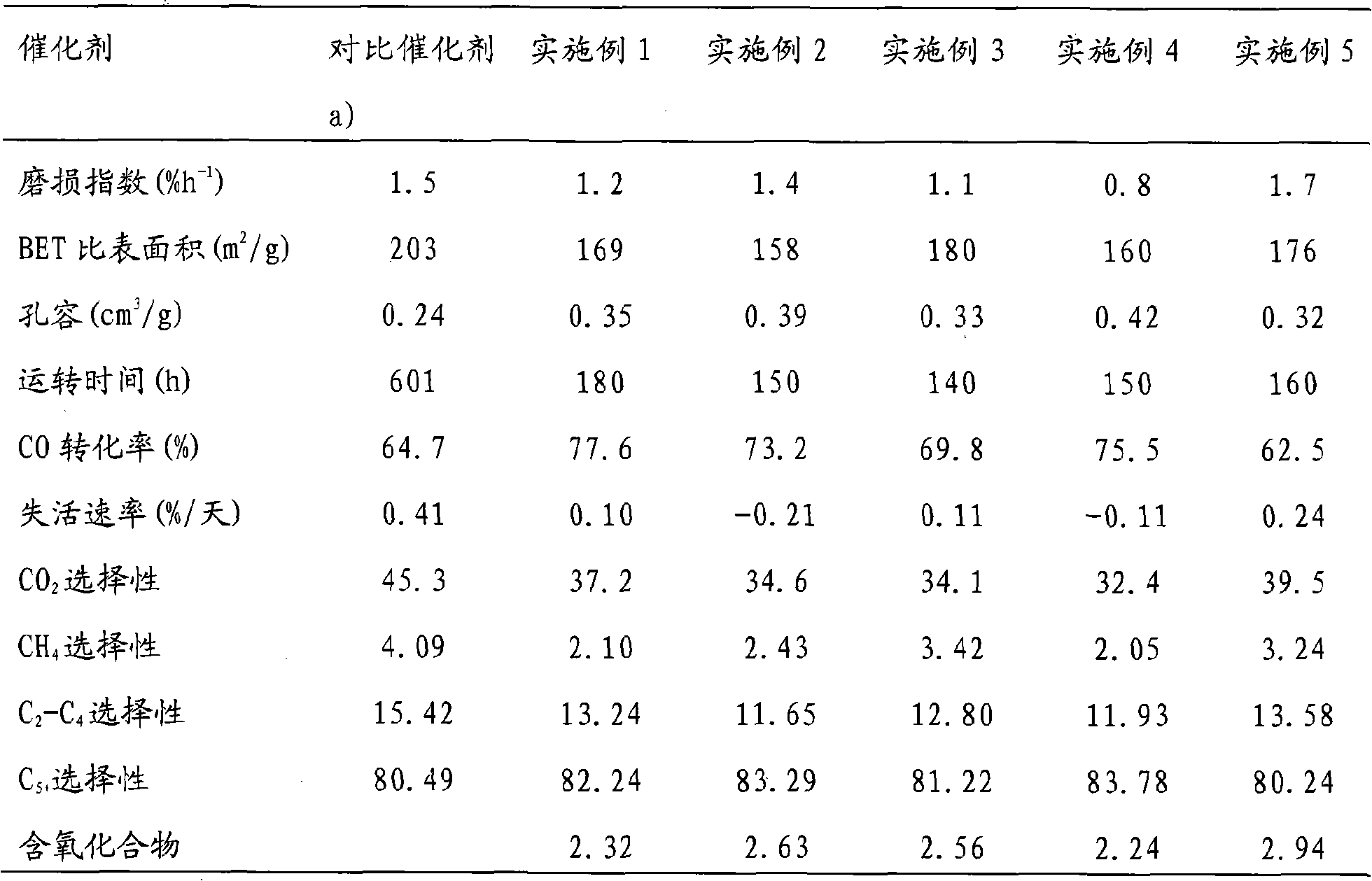
[0033] Weigh 4.5Kg Fe(NO 3 ) 3 9H 2 O, 0.15Kg Zr(NO 3 ) 4 ·5H 2 O, add 15L deionized water and stir to dissolve. It is the K of 20wt% that adding 0.2Kg mass concentration is 20wt% in this solution that is stirring again. 2 SiO 3 aqueous solution, stir well. Weigh 3.0Kg (NH 4 ) 2 CO 3 , add 15L deionized water and stir to dissolve. Heating the mixed solution of nitrate and potassium silicate to 80°C by means of jacket steam heating, and then co-flowing with the heated ammonium carbonate solution to produce precipitation in a vigorously stirred tank, and adjusting the salt and alkali salt delivery pumps respectively Control the precipitation temperature in the reaction tank: 80°C, pH: 7.5-8.0. After the precipitation, the precipitate was statically aged for 4 hours, vacuum filtered and washed repeatedly with deionized water. 0.25Kg containing 30wt% SiO 2 Add the silica sol in the precipitated filter cake, then add 0.1Kg 30wt% Cu(NO 3 ) 2 and 0.27 Kg of 30wt% KNO ...
Embodiment 2
[0035] Weigh 4.5Kg Fe(NO 3 ) 3 9H 2 O and 0.075Kg Zr(NO 3 ) 4 ·5H 2 O, add 15L deionized water and stir to dissolve. It is the K of 20wt% that adding 0.2Kg mass concentration is 20wt% in this solution that is stirring again. 2 SiO 3 aqueous solution, stir well. Weigh 3.0Kg (NH 4 ) 2 CO 3 , add 15L deionized water and stir to dissolve. Heating the nitrate mixed solution to 80°C with the jacket water steam heating method, and then co-flowing with the heated ammonium carbonate and potassium silicate mixed solution to produce precipitation in a vigorously stirred tank, and transporting the salt and alkali salts respectively The precipitation temperature in the pump control reaction tank is: 80°C, and the pH is: 7.5-8.0. After the precipitation, the precipitate was statically aged for 4 hours, vacuum filtered and washed repeatedly with deionized water. 0.45Kg containing 30wt% SiO 2 Add the silica sol in the precipitated filter cake, then add 0.31Kg 30wt% Cu(NO 3 ) 2...
Embodiment 3
[0037] Weigh 4.5Kg Fe(NO 3 ) 3 9H 2 O, 0.15Kg Zr(NO 3 ) 4 ·5H 2 O, add 15L deionized water and stir to dissolve. It is the K of 20wt% that adding 0.2Kg mass concentration is 20wt% in this solution that is stirring again. 2 SiO 3 aqueous solution, stir well. Weigh 3.0Kg (NH 4 ) 2 CO 3 , add 15L deionized water and stir to dissolve. Heating the mixed solution of nitrate and potassium silicate to 80°C by means of jacket steam heating, and then co-flowing with the heated ammonium carbonate solution to produce precipitation in a vigorously stirred tank, and adjusting the salt and alkali salt delivery pumps respectively Control the precipitation temperature in the reaction tank: 80°C, pH: 7.5-8.0. After the precipitation, the precipitate was statically aged for 4 hours, vacuum filtered and washed repeatedly with deionized water. 0.37Kg containing 30wt% SiO 2 Add the silica sol in the precipitated filter cake, then add 0.18Kg 30wt% Cu(NO 3 ) 2 and 0.16Kg of 30wt% KNO ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com