Carbon dioxide-synthesized methanol catalyst and preparation method thereof
A technology for synthesizing methanol and carbon dioxide, which is applied in the direction of catalyst activation/preparation, hydroxyl compound preparation, organic compound preparation, etc. It can solve the problems of low selectivity, short life, poor thermal stability, etc., and achieve high conversion rate and long service life Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
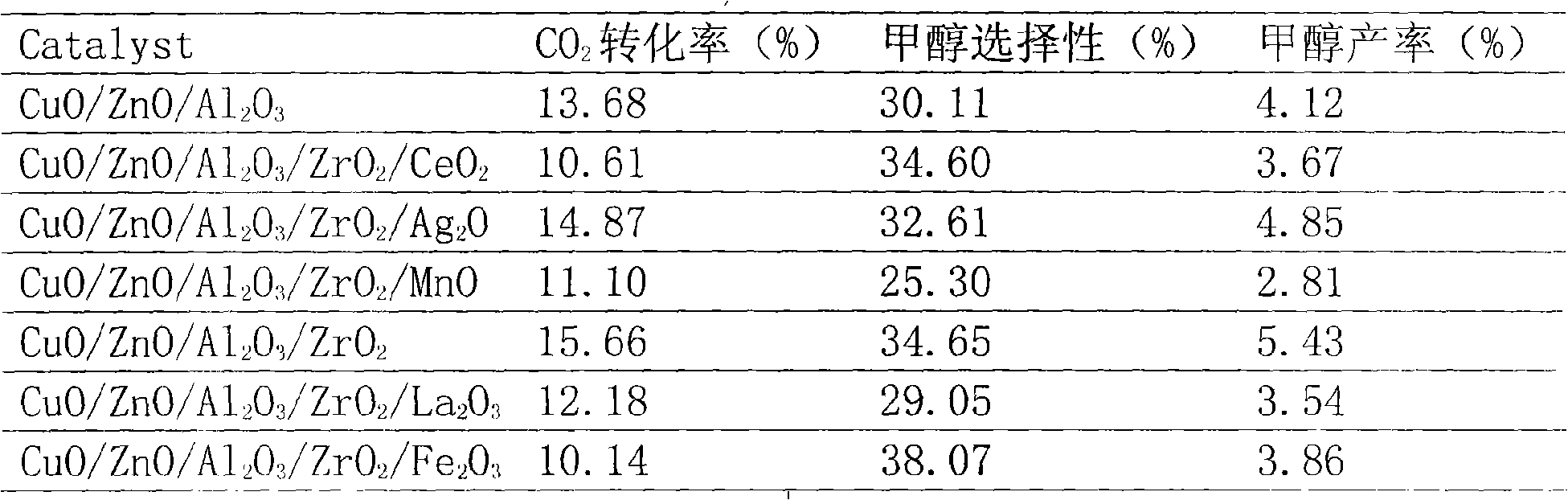
[0019] Weigh 13.59gCu(NO 3 ) 2 ·3H 2 O, 21.10gZn(NO 3 ) 2 ·6H 2 O, dissolved in 250ml deionized water, the solution is recorded as A solution, and Na 2 CO 3 26.52g was dissolved in 500ml deionized water, the solution was recorded as B solution, and 3.7g Al(NO 3 ) 3 9H 2 O, 1.1gZr(NO 3 ) 4 ·5H 2 O was dissolved in 50ml of deionized water, and the solution was recorded as solution C; take 100ml of solution B and solution C under stirring conditions, and co-precipitate in parallel at 65°C to obtain the precipitation solution (1), and mix solution A and solution B under stirring conditions , 65 ° C and flow into the precipitation solution (1) to co-precipitate until the complete precipitation of liquid A and titration is completed. During the precipitation process, the pH is controlled at 7-8. The precipitation is stirred for 20 minutes, then left to stand, aged at room temperature for 1 hour, and suction filtered. Wash with deionized water until the effluent from the ...
Embodiment 2
[0021] Weigh 13.59gCu(NO 3 ) 2 ·3H 2 O, 21.10gZn(NO 3 ) 2 ·6H 2 O, 0.28gMn(CH 3 COO) 2 ·5H 2 O was dissolved in 250ml deionized water, the solution was recorded as A solution, and Na was weighed 2 CO 3 26.52g was dissolved in 500ml deionized water, the solution was recorded as B solution, and 3.7g Al(NO 3 ) 3 9H 2 O, 1.1gZr(NO 3 ) 4 ·5H 2 O was dissolved in 50ml of deionized water, and the solution was recorded as solution C; take 100ml of solution B and solution C under stirring conditions, and co-precipitate in parallel at 65°C to obtain the precipitation solution (1), and mix solution A and solution B under stirring conditions , 65 ° C and flow into the precipitation solution (1) to co-precipitate, until the A solution is completely precipitated and titrated, the pH is controlled at 7 to 8 during the precipitation process, the precipitate is stirred for 20 minutes, then left to stand, aged at room temperature for 1 hour, and filtered. Wash with deionized wate...
Embodiment 3
[0023] Weigh 13.59gCu(NO 3 ) 2 ·3H 2 O, 21.10gZn(NO 3 ) 2 ·6H 2 O, 0.11gAgNO 3 Dissolve in 250ml deionized water, and record the solution as A solution; weigh Na 2 CO 3 26.52g was dissolved in 500ml deionized water, the solution was recorded as B solution, and 3.7g Al(NO 3 ) 3 9H 2 O, 1.1gZr(NO 3 ) 4 ·5H 2 O was dissolved in 50ml of deionized water, and the solution was recorded as solution C. Take 100ml of solution B and solution C under stirring conditions, and co-precipitate in parallel at 65°C to obtain the precipitation solution (1). Combine solution A and solution B under stirring conditions , 65 ° C and flow into the precipitation solution (1) to co-precipitate, until the A solution is completely precipitated and titrated, the pH is controlled at 7 to 8 during the precipitation process, the precipitate is stirred for 20 minutes, then stood still, aged at room temperature for 1 hour, and filtered. Wash with deionized water until the effluent from the lower e...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com