Method for solidifying actinium series nuclide by pyrochlore type rare earth zirconate
A zirconate and pyrochlore technology, applied in nuclear engineering, radioactive purification, etc., can solve the problems of long time, low efficiency, and cumbersome steps, and achieve the effect of high risk, high efficiency, and good safety
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
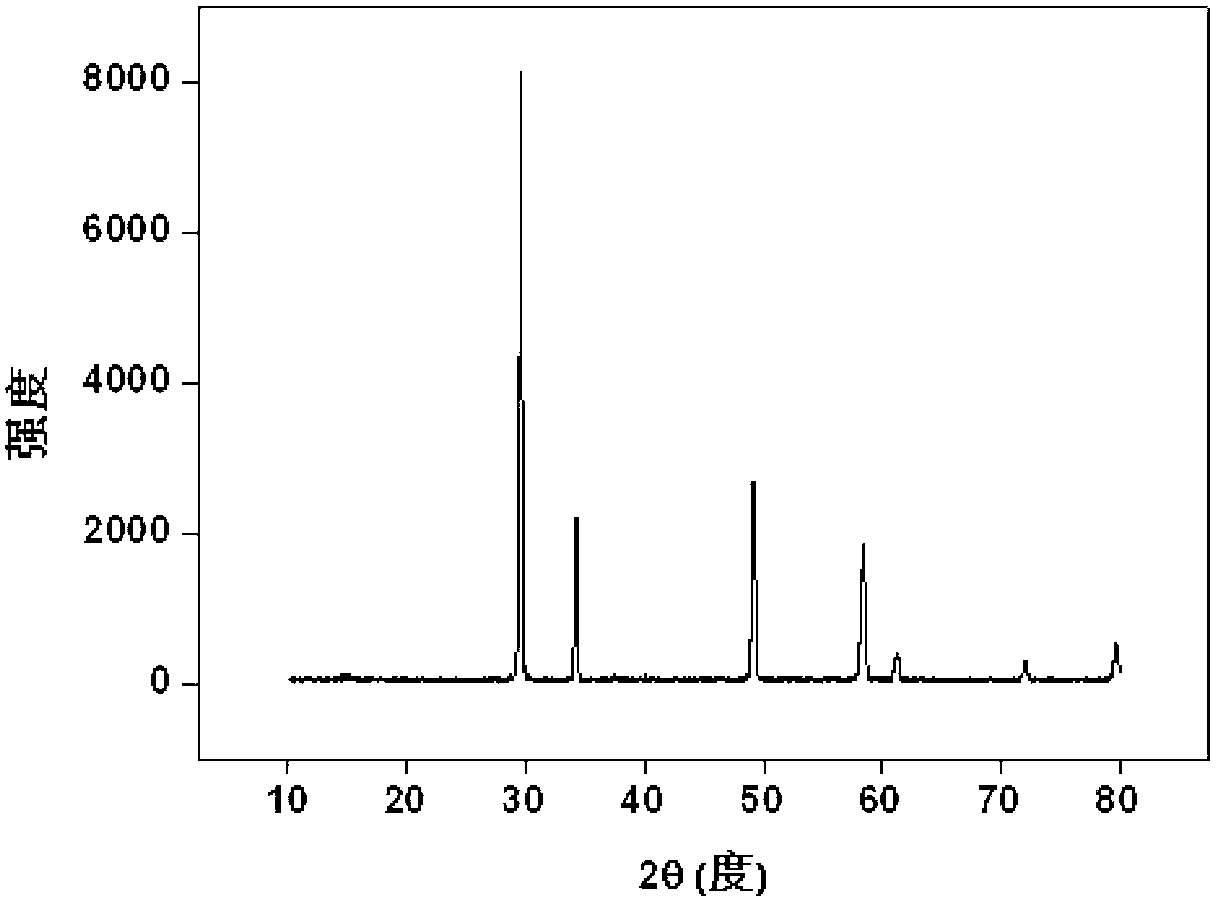
[0035] use Ce 4+ replace Pu 4+ Simulated curing of Pu (Note: Ce 4+ and Pu 4+ The ionic radii are very close and have similar reaction characteristics): Weigh 1.29g Zr(NO 3 ) 4 .5H 2 O (0.003mol), 0.87g Ce(NO 3 ) 3 .6H 2 O(0.002mol), 2.26g Gd(NO 3 ) 3 .6H 2 O (0.005mol) and 0.15gKF, fully ground, mixed evenly, transferred to the sintering furnace, from room temperature through 100min to 800 o C, kept for 6 hours; after it was naturally cooled, it was taken out, and the product was light yellow powder, which was put into 50ml water and soaked for 15min, filtered, washed, dried, and the product (Gd 2 Zr 0.6 Ce 0.4 o 7 ) powder X-ray diffraction pattern as figure 1 shown. The results show that the product is a single-phase pyrochlore structure, Ce 4+ It completely enters the crystal lattice of pyrochlore, that is, it is solidified into the crystal lattice by rare earth zirconate, and the inclusion capacity is 40%.
[0036] Refer to the American PCT (product cons...
Embodiment 2
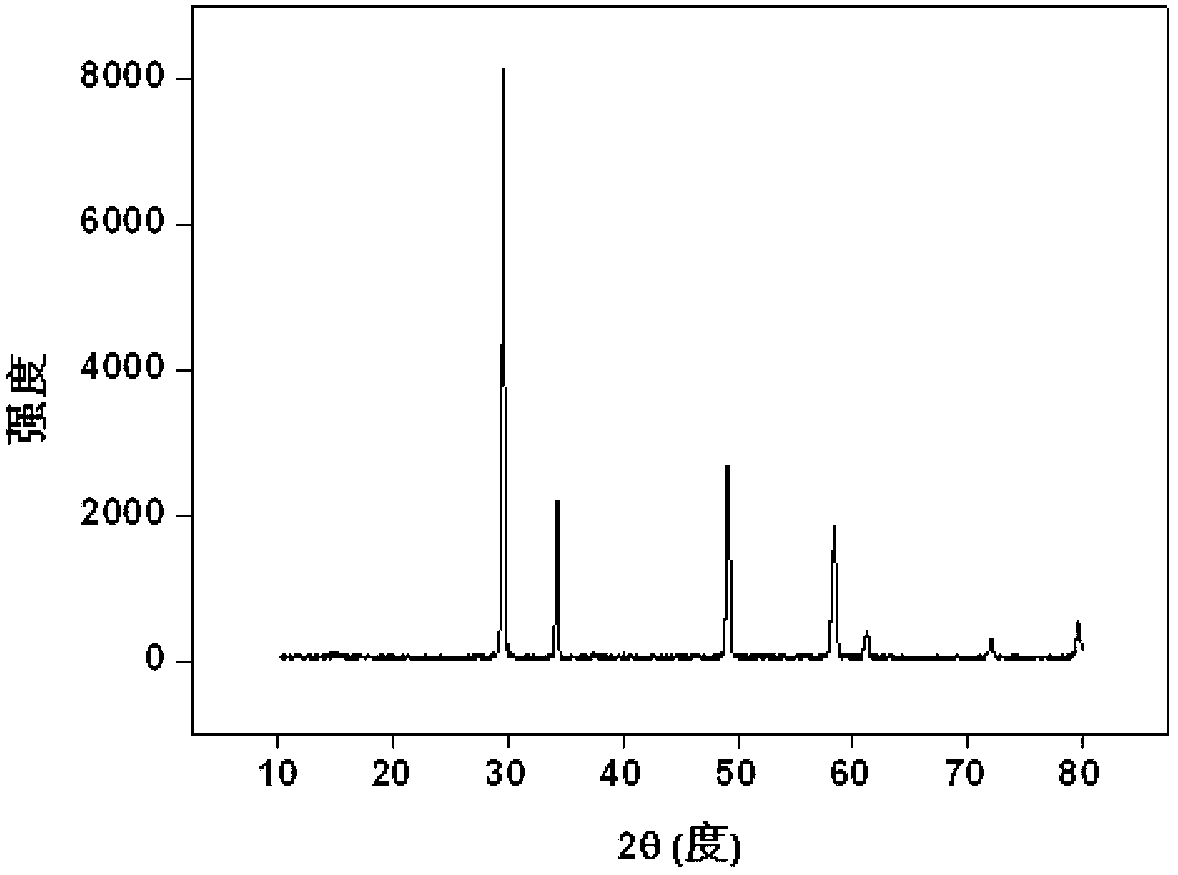
[0038] with Nd 3+ Substitute trivalent actinides for simulated solidification: Weigh 2.15g Zr(NO 3 ) 4 .5H 2 O (0.005mol), 0.63g Nd(NO 3 ) 3 .5H 2 O(0.0015mol), 1.59g Dy(NO 3 ) 3 .6H 2 O (0.0035mol) and 0.2gKCl, fully ground, mixed evenly, transferred to the sintering furnace, from room temperature to 1000 after 120min o C, kept for 10 hours; after it was naturally cooled, it was taken out, and the product was a light pink powder, which was put into 50ml water and soaked for 15min, filtered, washed, dried, and the product (Dy 1.4 Nd 0.6 Zr 2 o 7 ) powder X-ray diffraction pattern as figure 2 shown. The results show that the product is a single-phase pyrochlore structure, Nd 3+ It completely enters the crystal lattice of pyrochlore, that is, it is solidified into the crystal lattice by rare earth zirconate, and the inclusion capacity is 30%.
[0039] Refer to the American PCT (product consistency test) method for powder soaking, the soaking agent is deionized w...
Embodiment 3
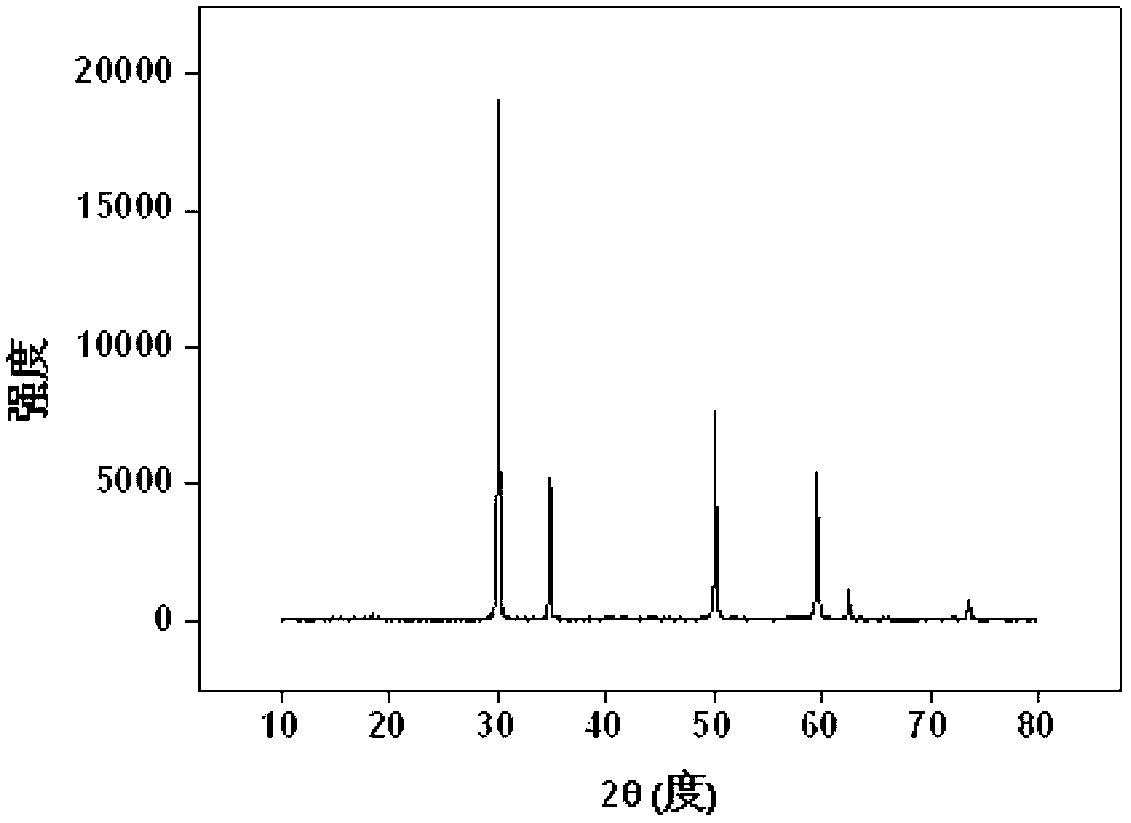
[0041] u 4+ Solidification: Weigh 1.72g Zr(NO 3 ) 4 .5H 2 O (0.004mol), 0.27g UO 2 (0.001mol), 1.91g Y(NO 3 ) 3 .6H 2 O (0.005mol) and 0.3gNaF, fully ground, mixed evenly, transferred to the sintering furnace, from room temperature through 120min to 1200 o C, kept for 10 hours; after it was naturally cooled, it was taken out, and the product was light brown powder, which was put into 50ml water and soaked for 15min, filtered, washed and dried, and the product (Y 2 Zr 1.6 u 0.4 o 7 ) powder X-ray diffraction pattern as image 3 shown. The results show that the product is a single-phase pyrochlore structure, U 4+ It completely enters the crystal lattice of pyrochlore, that is, it is solidified into the crystal lattice by rare earth zirconate, and the inclusion capacity is 20%.
[0042] Refer to the American PCT (product consistency test) method for powder soaking, the soaking agent is deionized water, 90±2 o C soaked for 7 days, the normalized leaching rate of U ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com