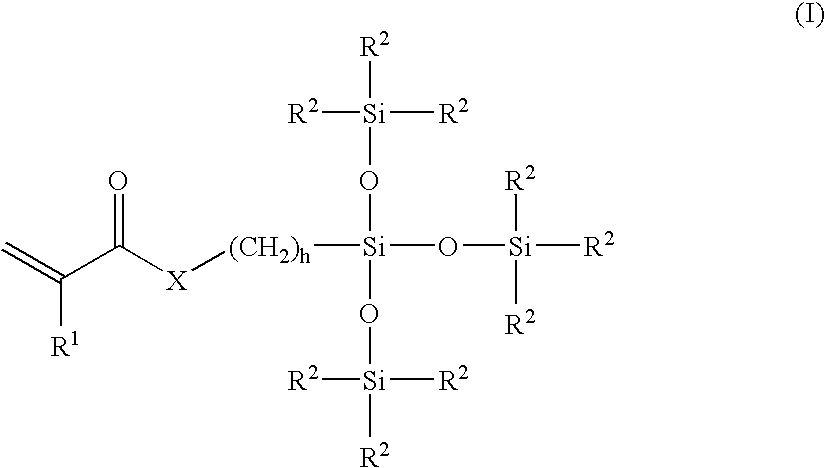
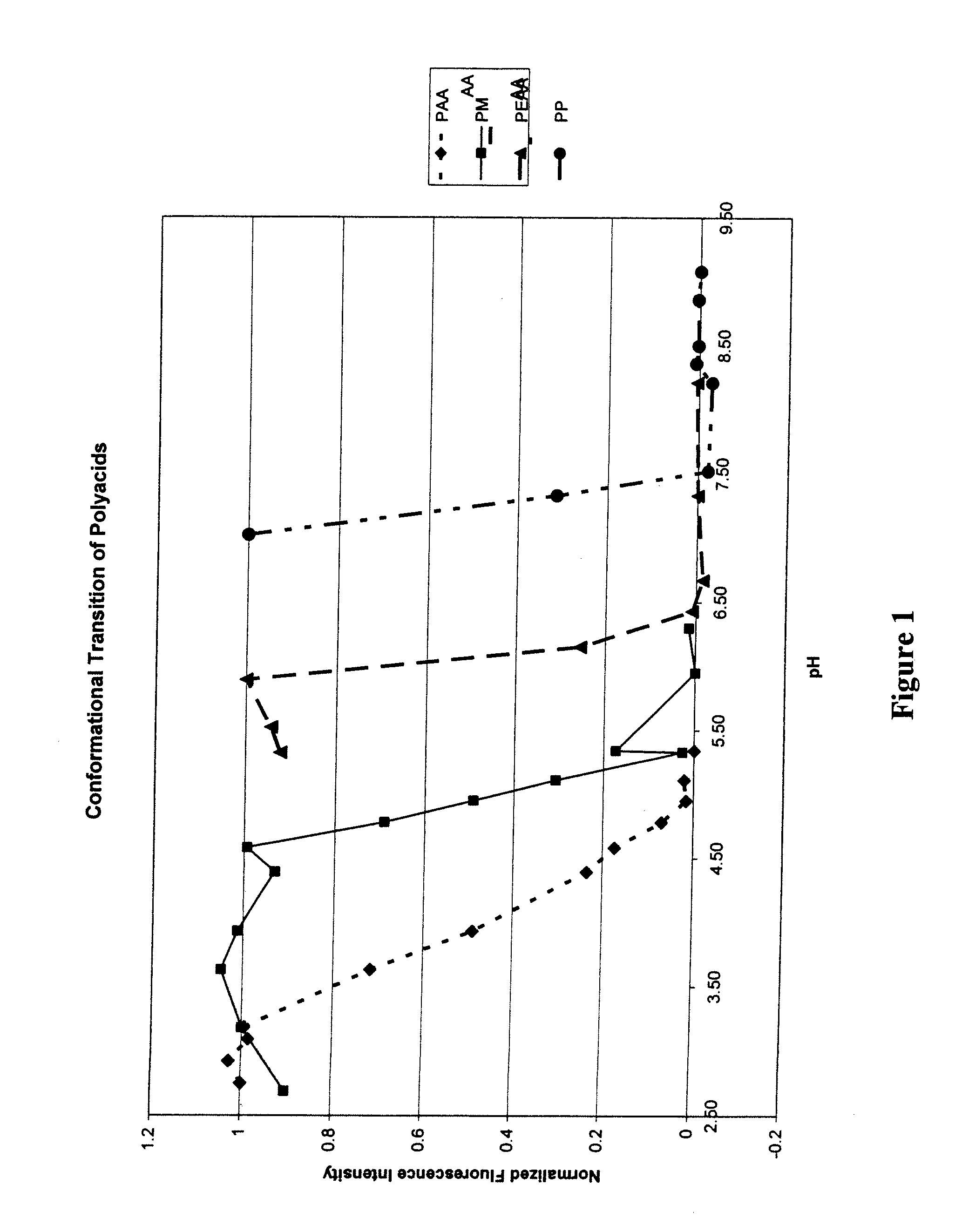
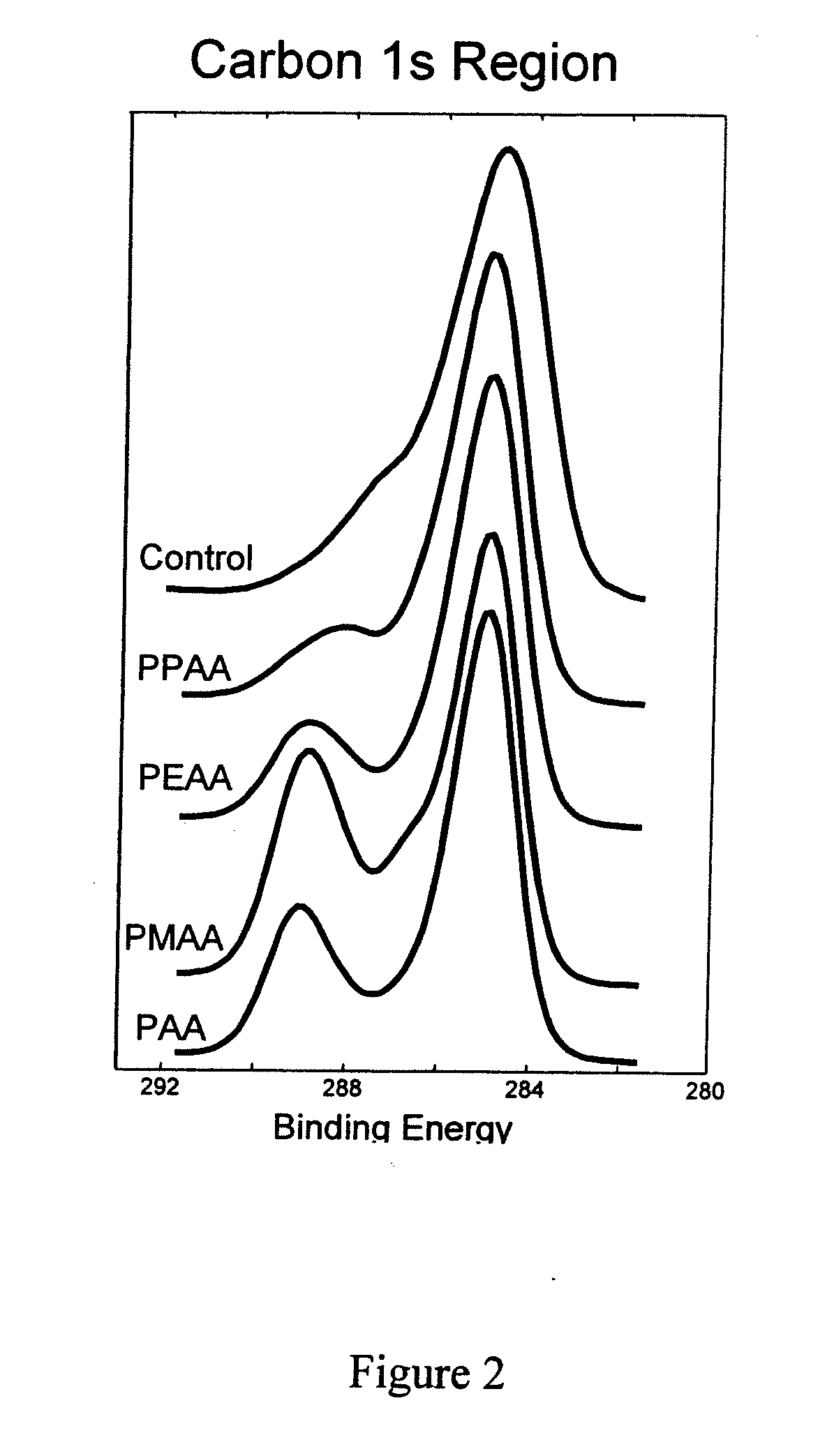
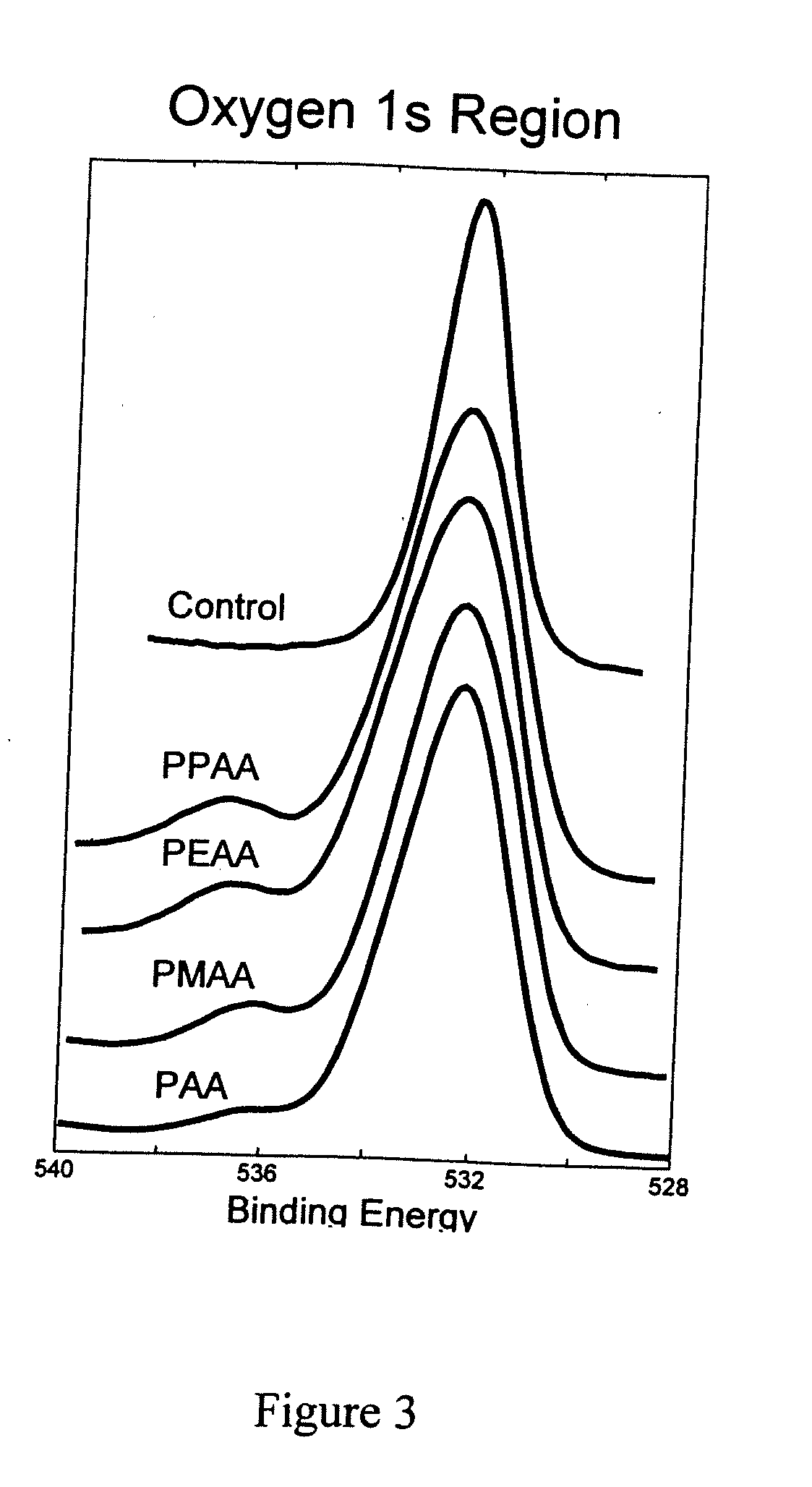
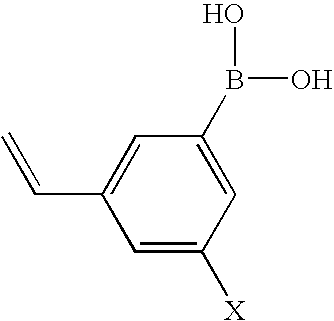
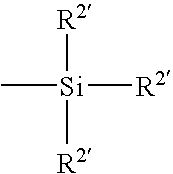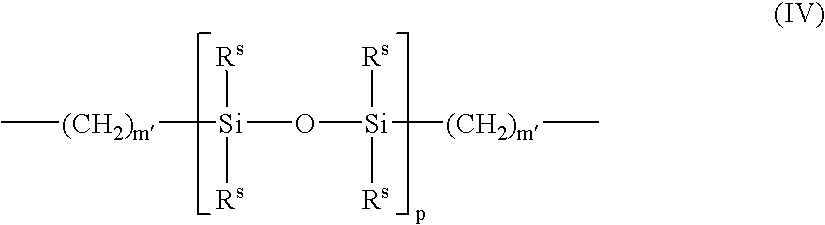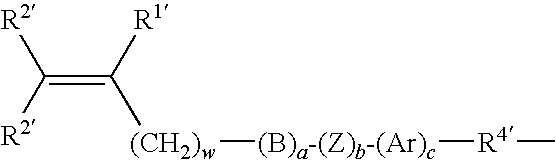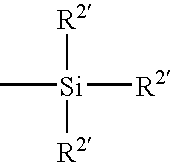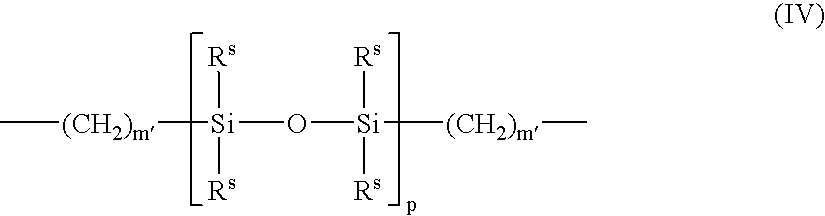Patents
Literature
36results about How to "Preserve sterility" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
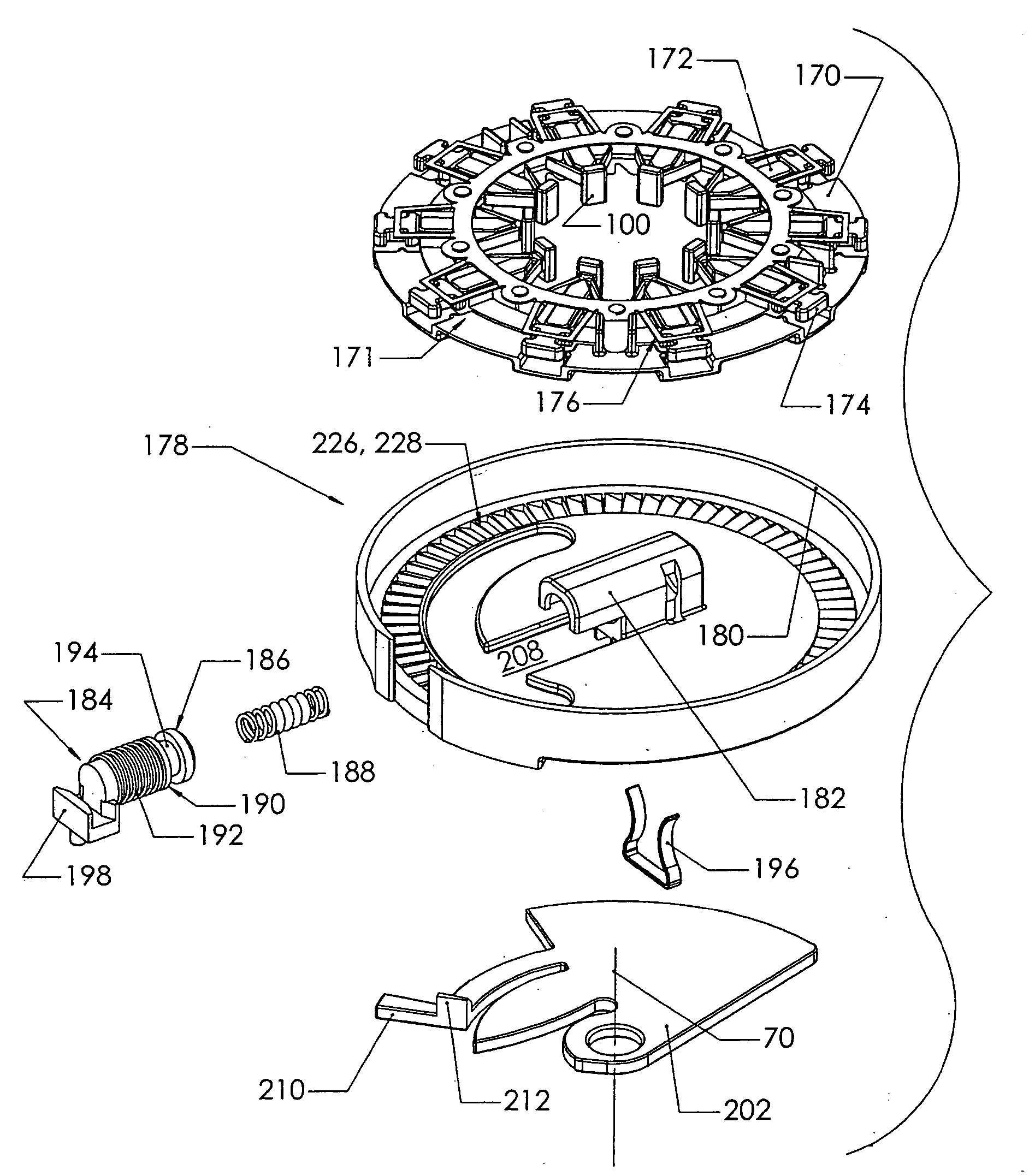
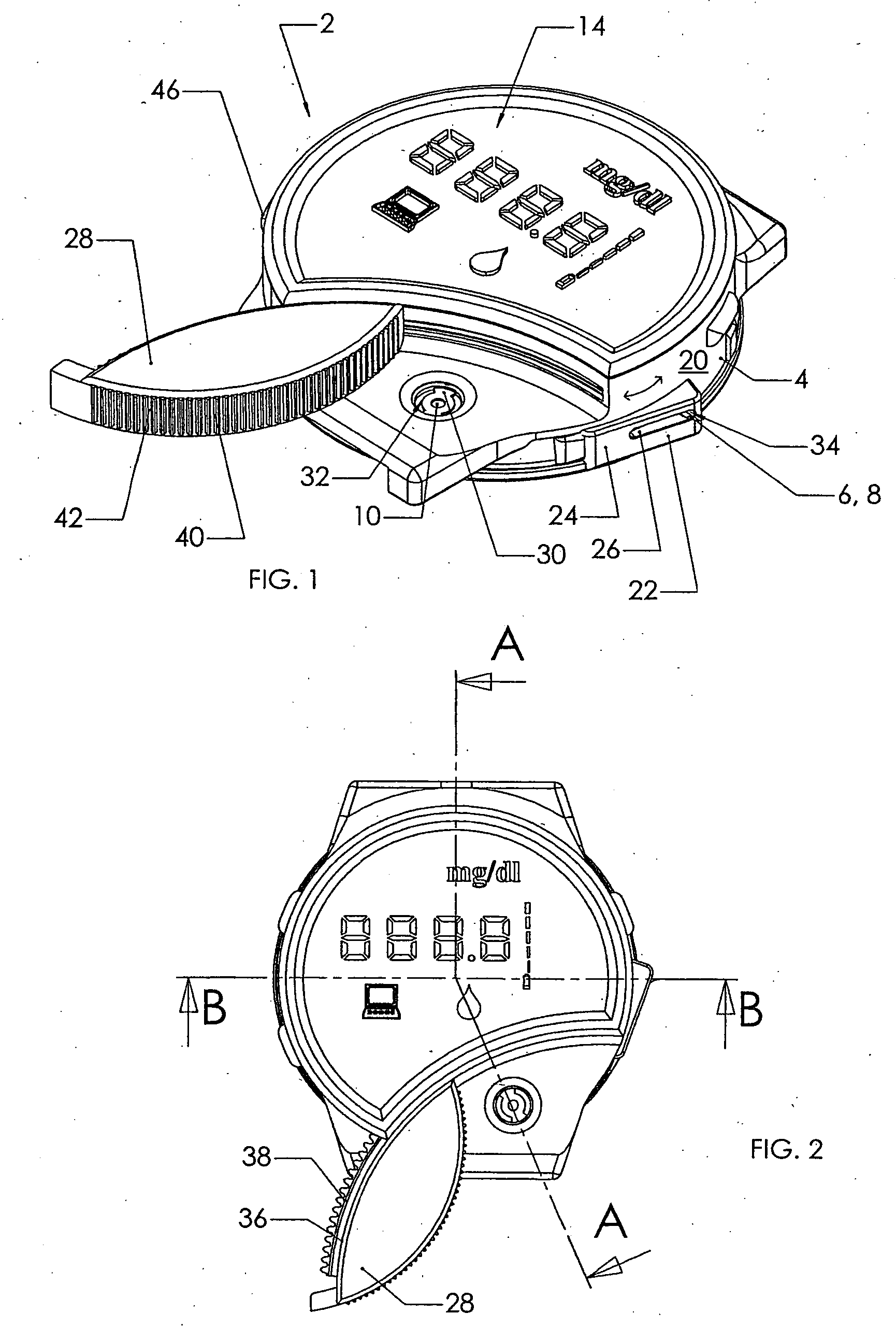
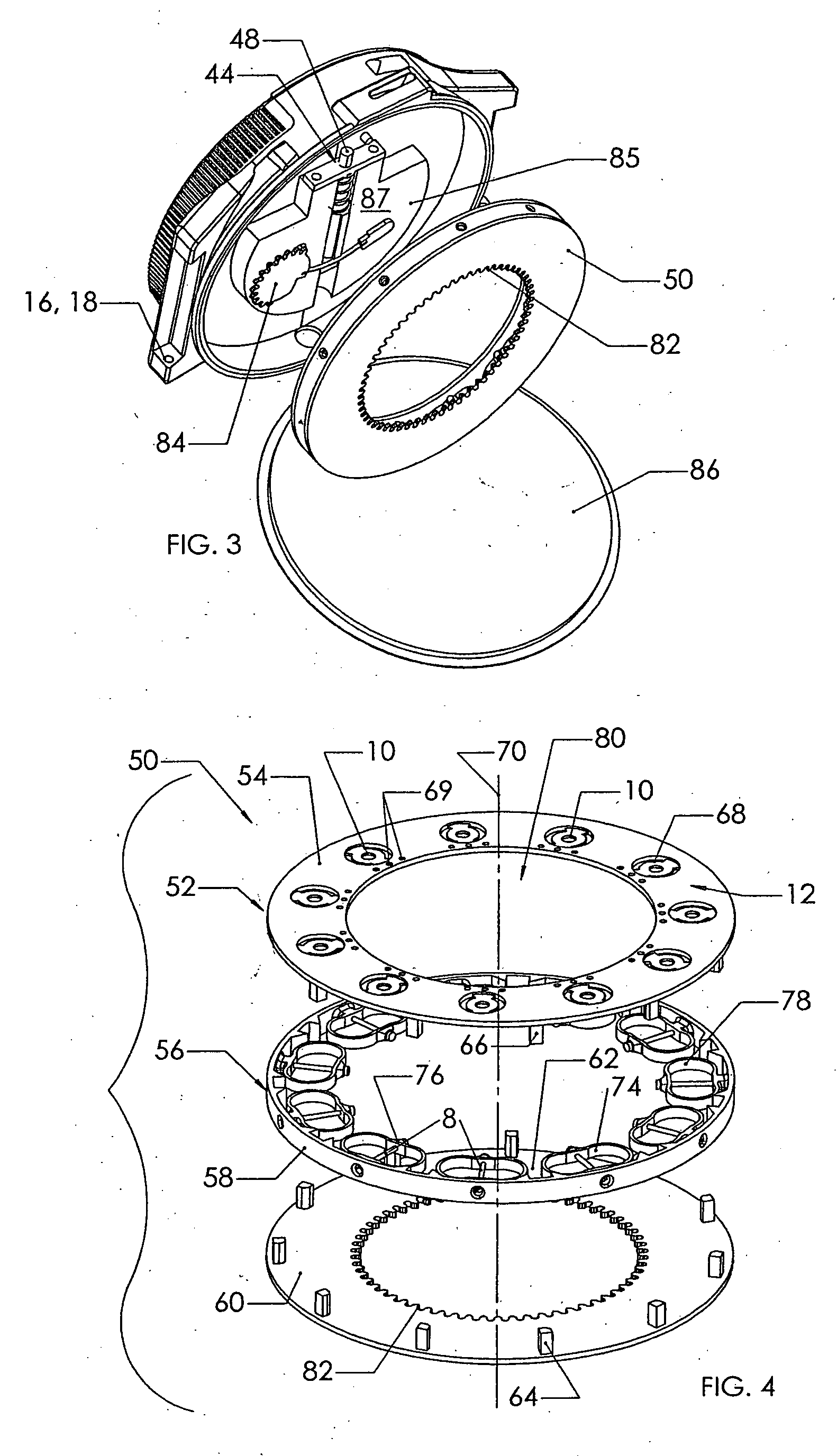
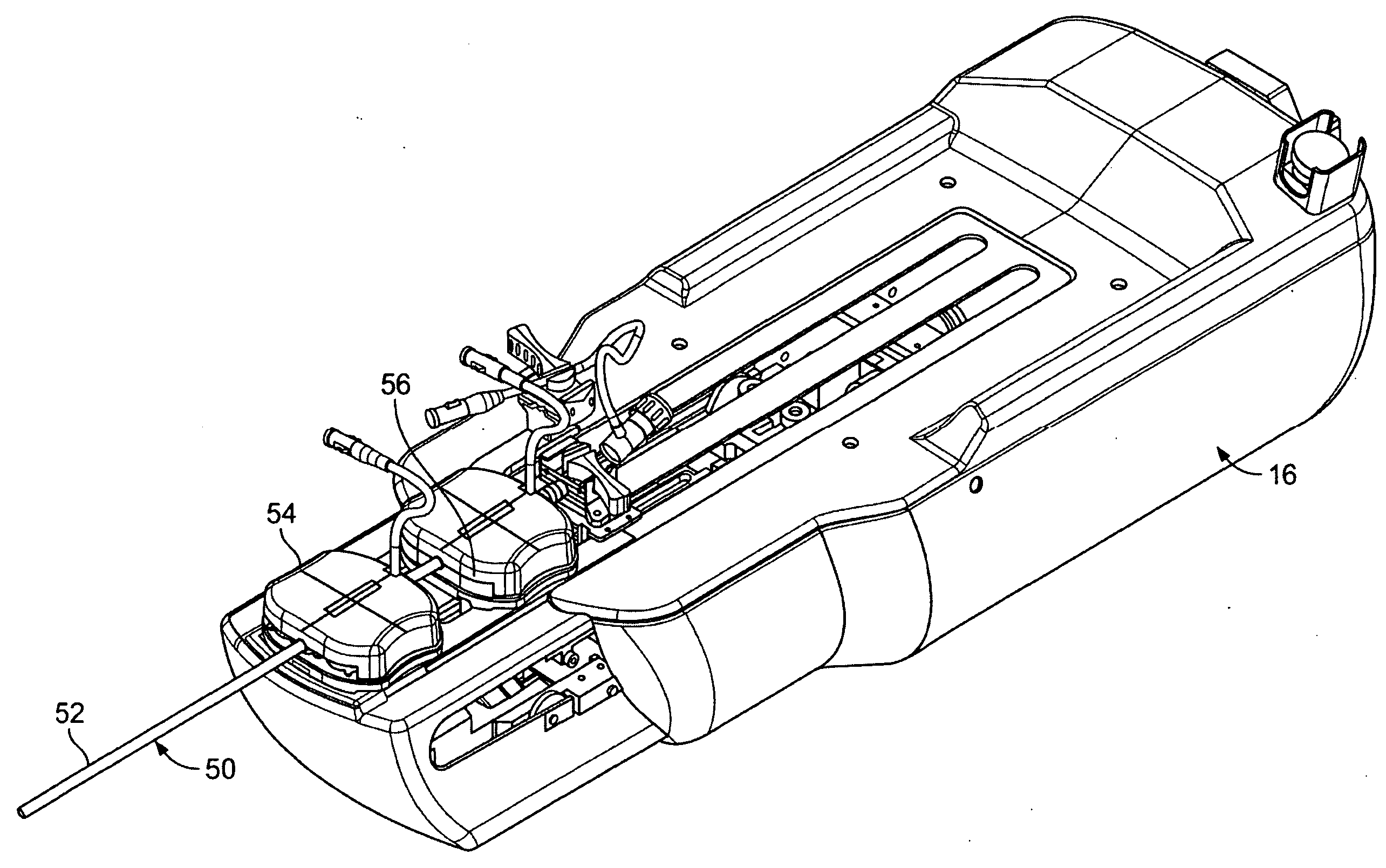
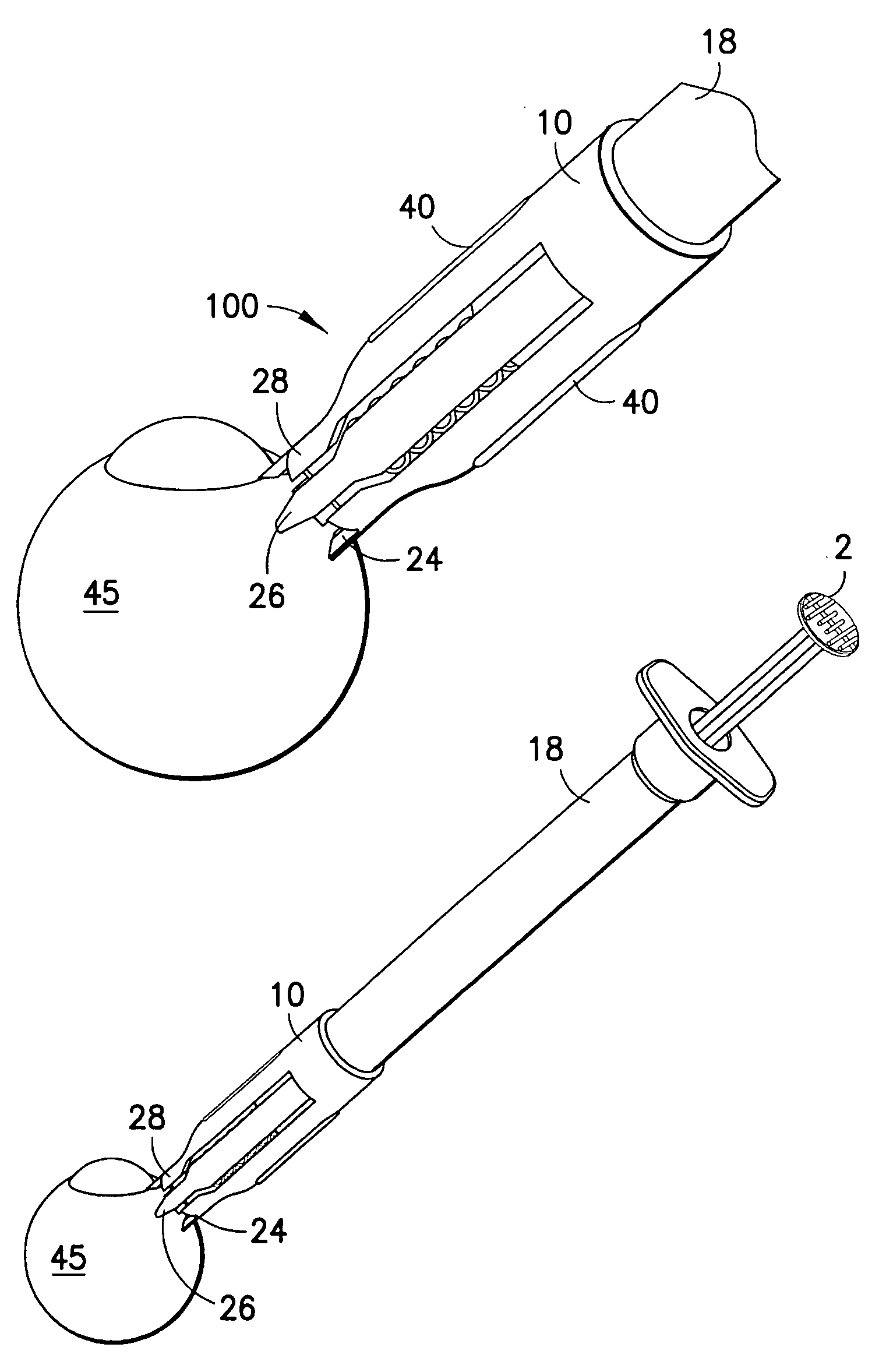
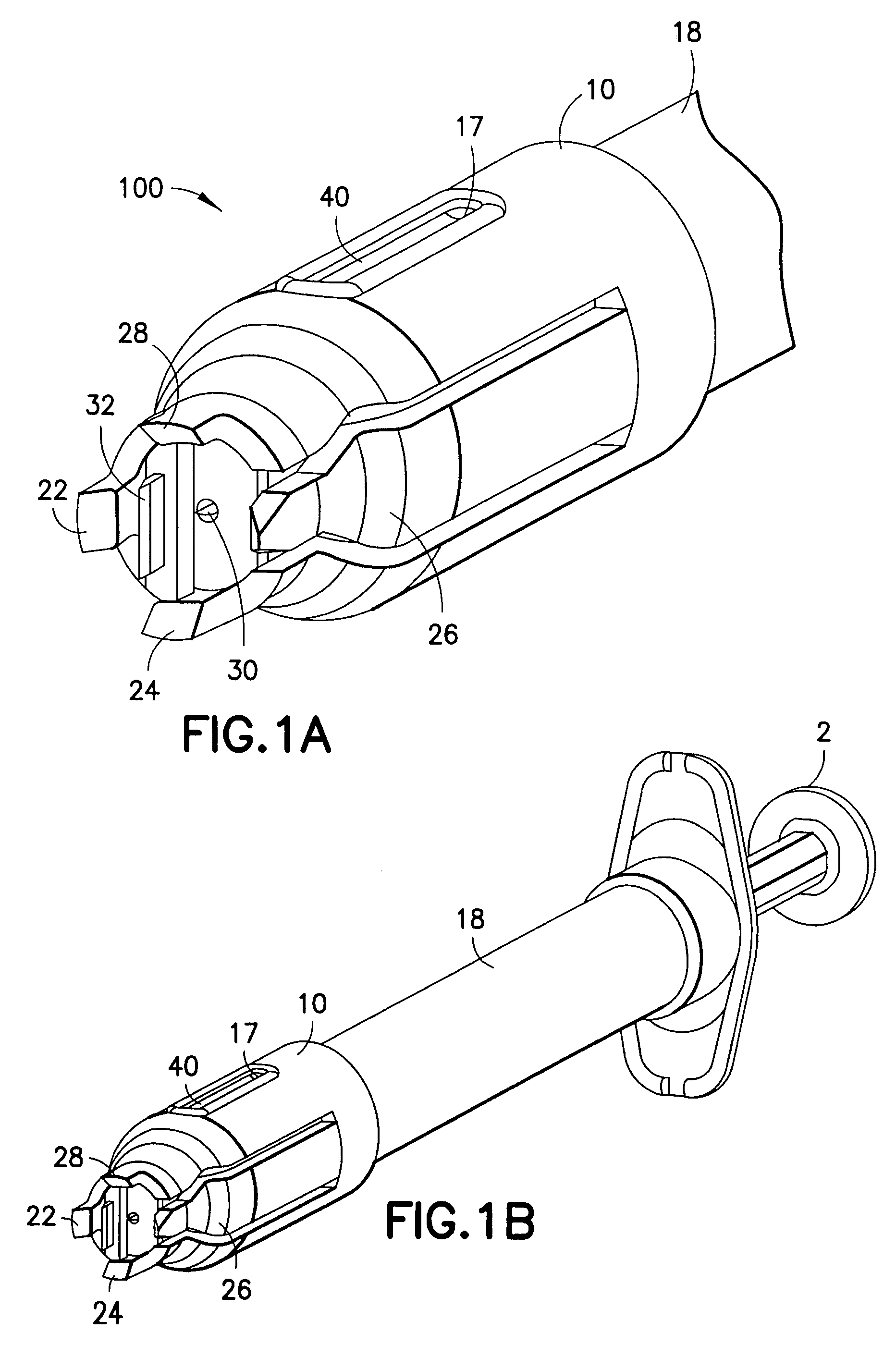
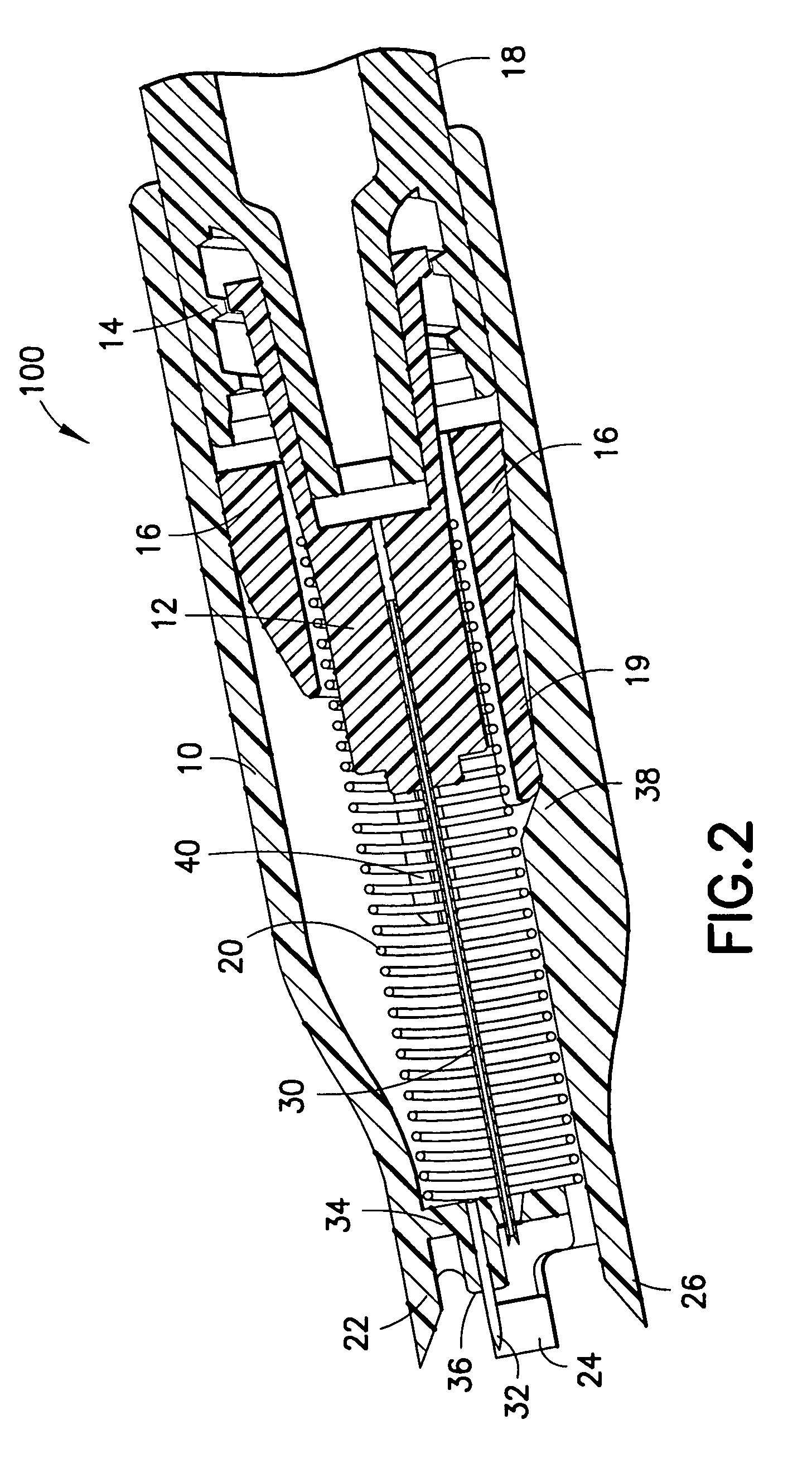
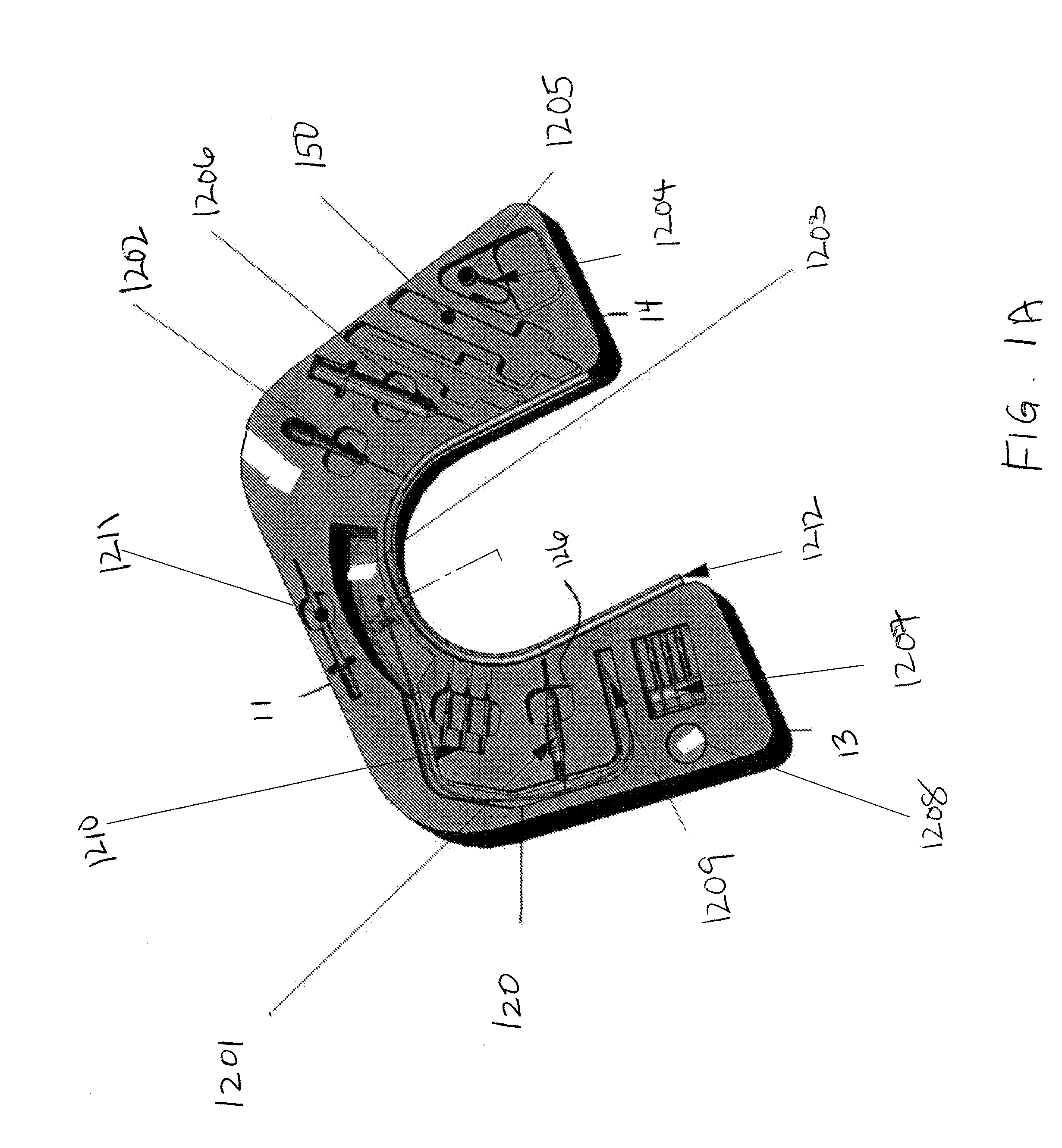
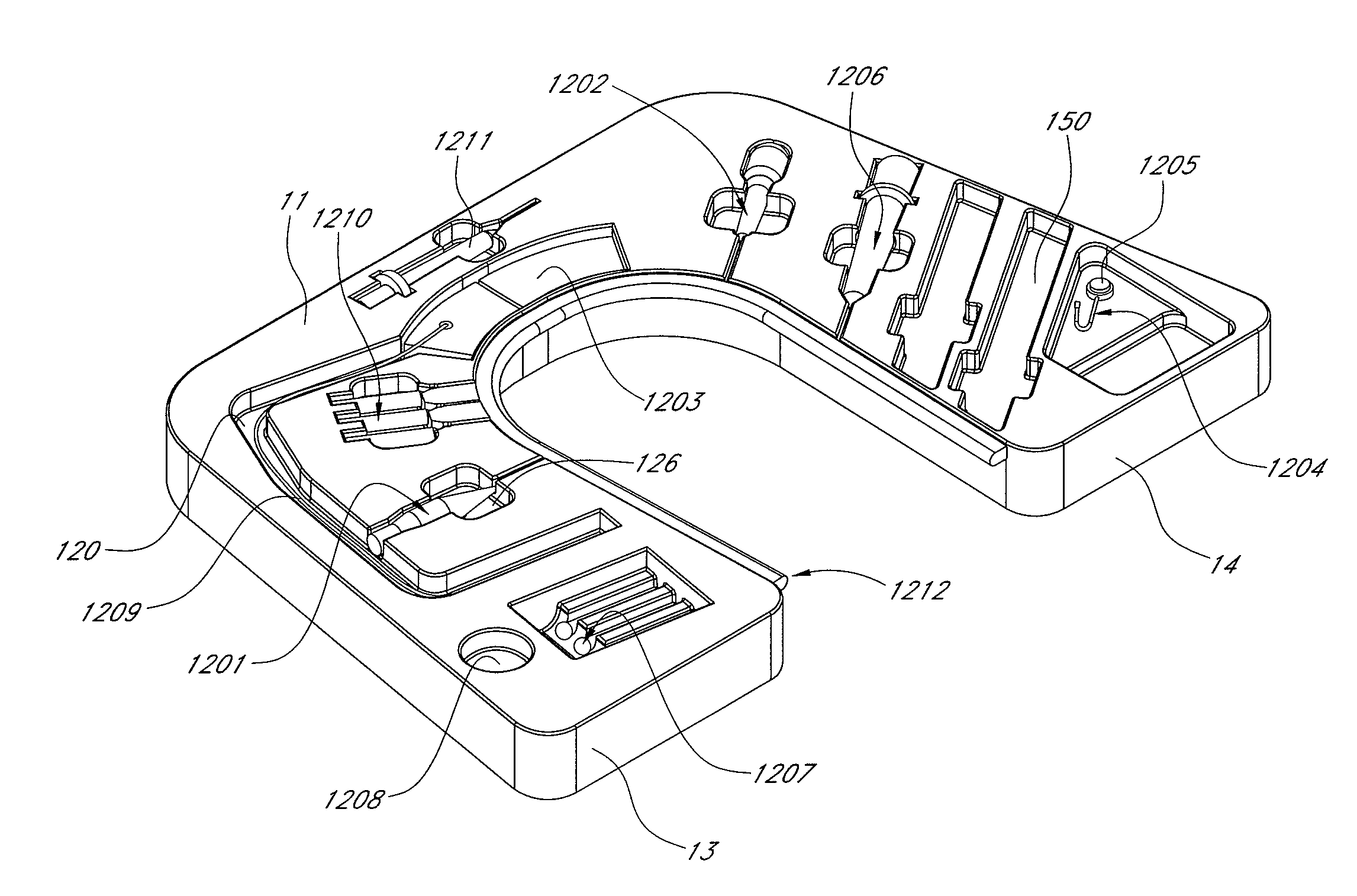
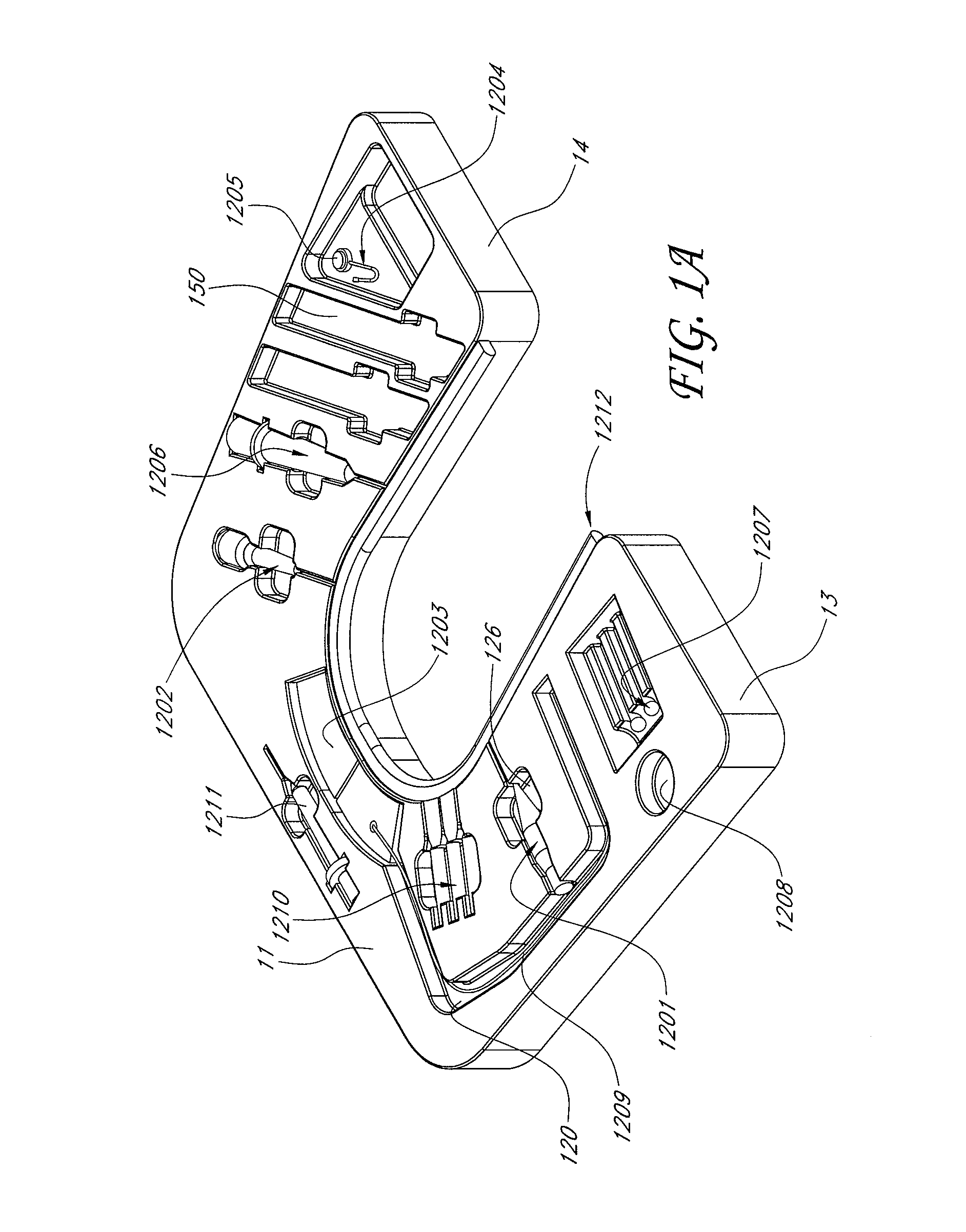
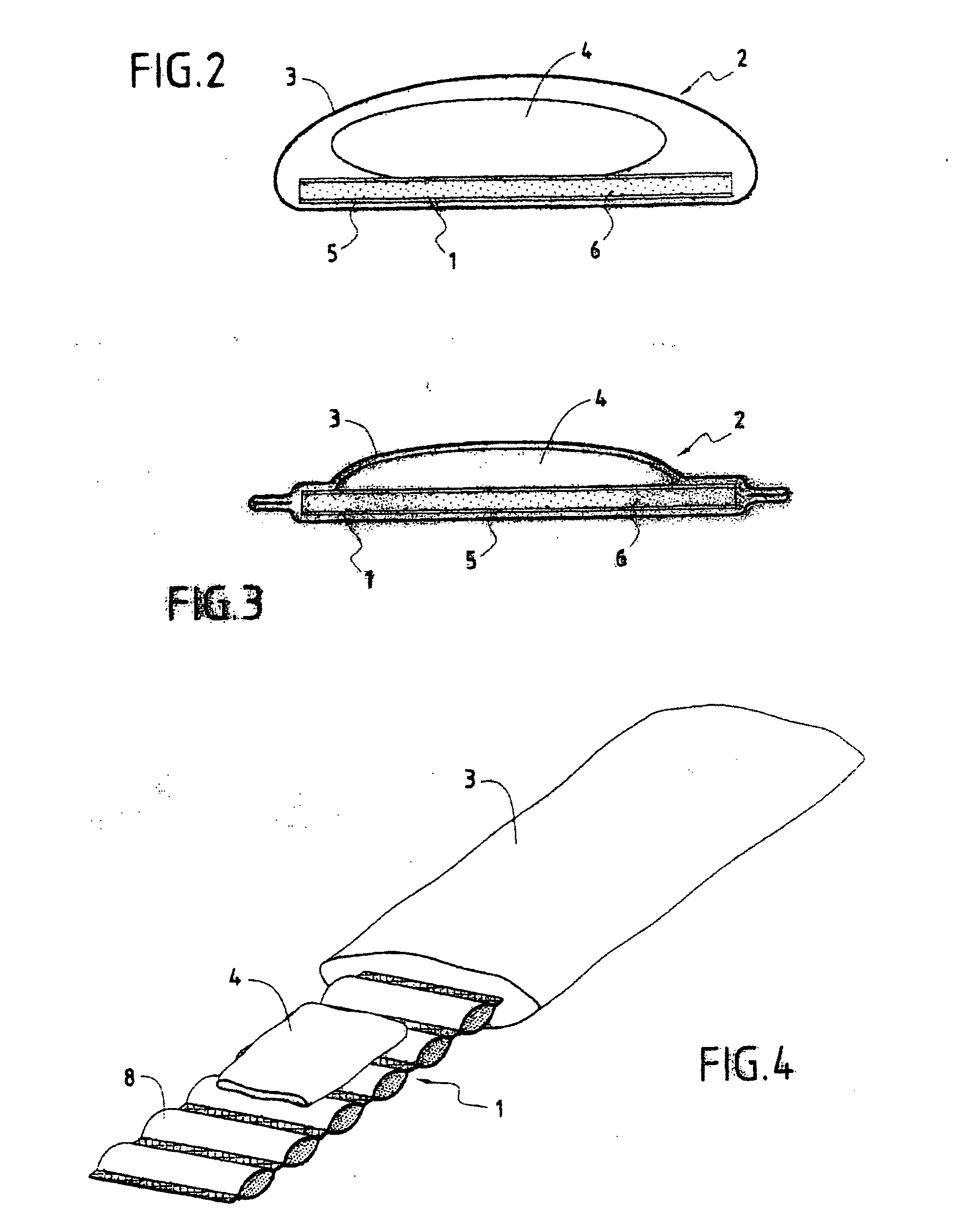
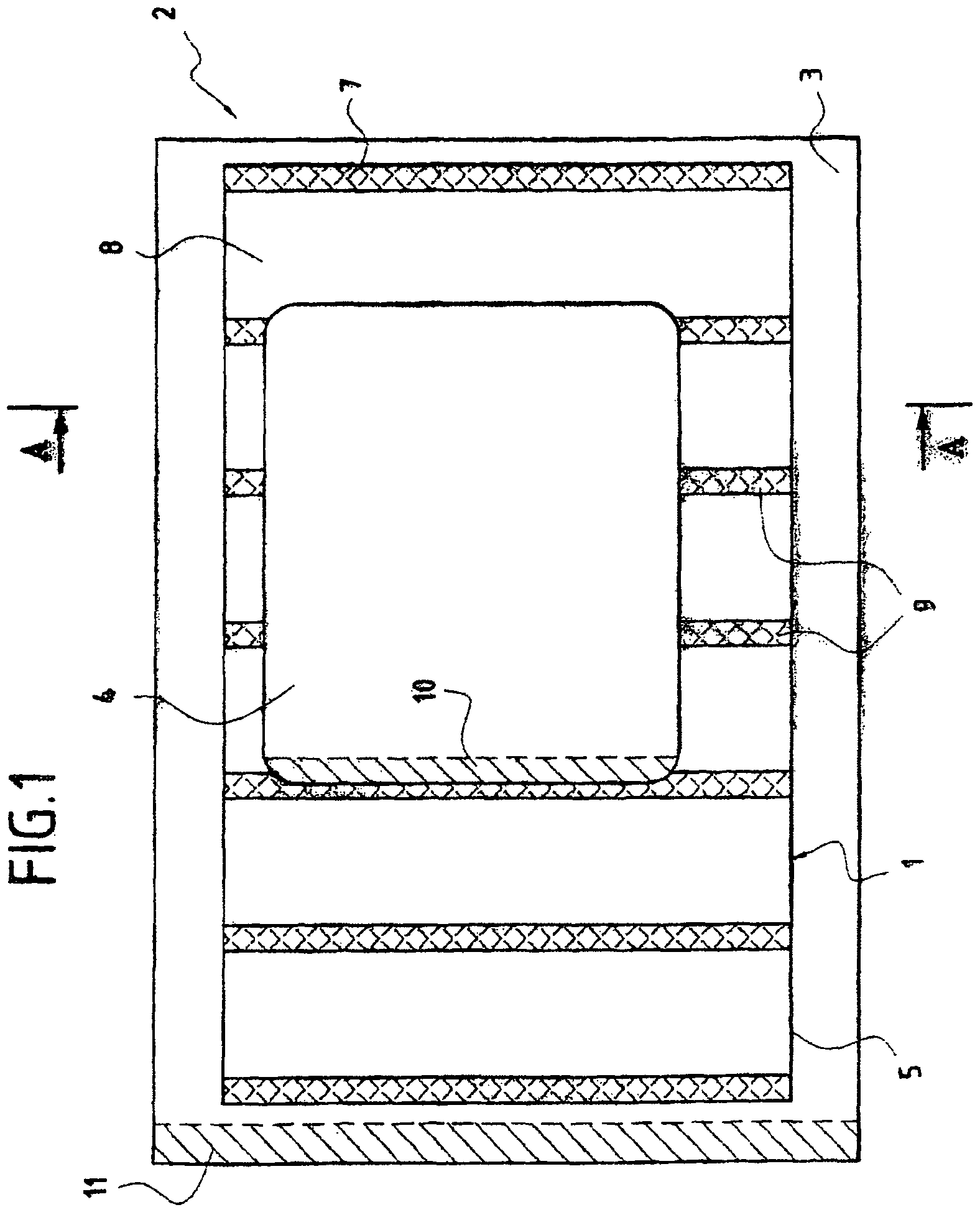
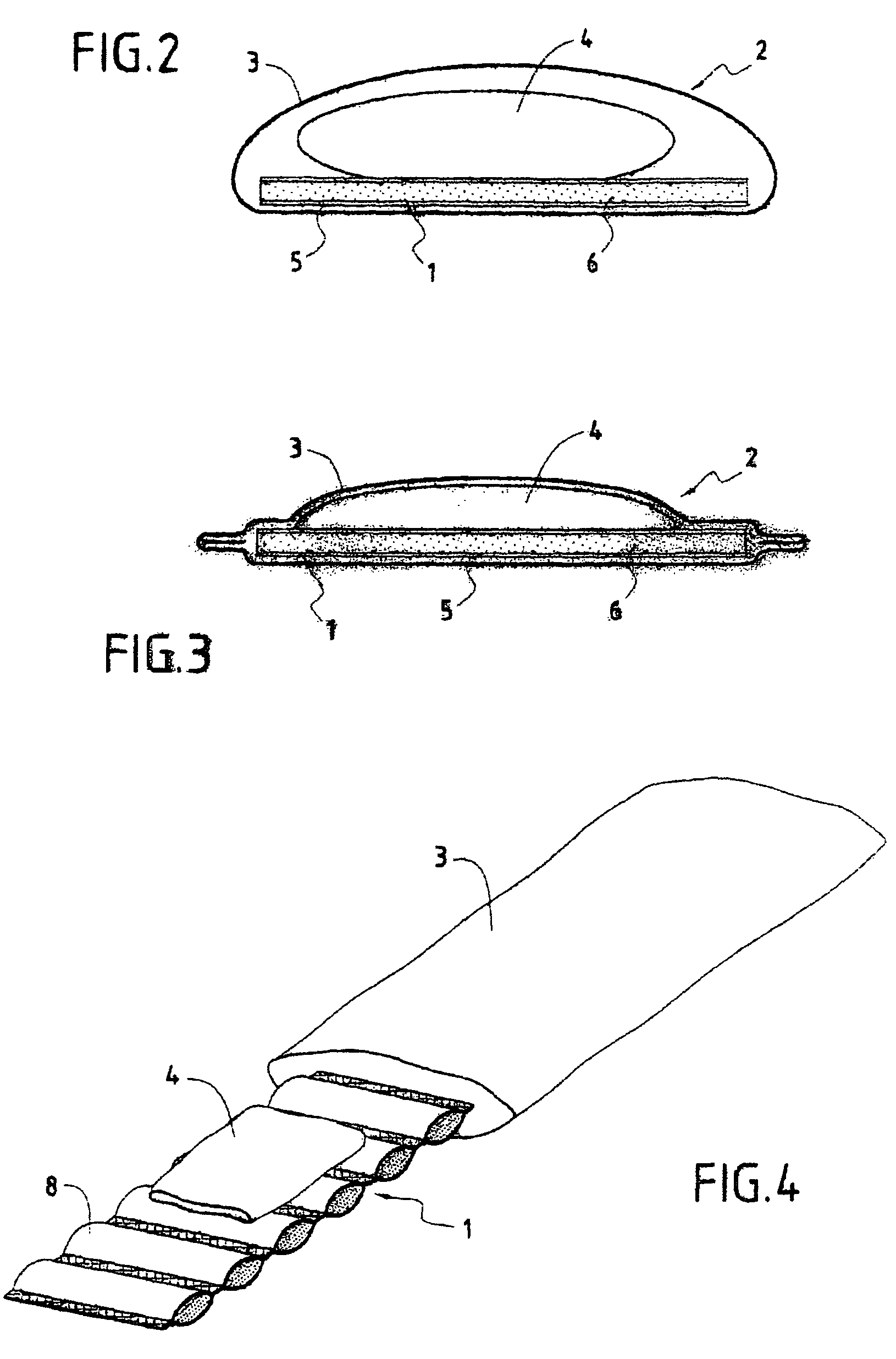
Blood sampling device
ActiveUS20040230216A1Preserve sterilityGood removal effectSurgical needlesCatheterDisplay deviceEngineering
A device for sampling and / or analyzing blood or other body fluid of a subject. A housing contains a plurality of lancets and optionally includes test elements to take up a sample of blood, an evaluation system and a display. A complete system that can be handled as a single device, for example in the form of a wristwatch, includes a multiplicity of test elements and lancets, which can be brought successively to a working position to perform multiple measurements. A cassette or carrier includes multiple lancets and / or test elements, for insertion into the device.
Owner:FACET TECH LLC
Modular interfaces and drive actuation through barrier
ActiveUS20100175701A1Preserve sterilityDifferent flexibilityDiagnosticsPortable liftingRoboticsControl system
The invention relates generally to robotically controlled systems, such as medical robotic systems. In one variation, a robotic catheter system is configured with a sterile barrier capable for transmitting a rotary force from a drive system on one side of the barrier to surgical tool on the other side of the sterile barrier for performing minimally invasive diagnostic and therapeutic procedures. Modularized drive systems for robotics arc also disclosed herein.
Owner:AURIS HEALTH INC
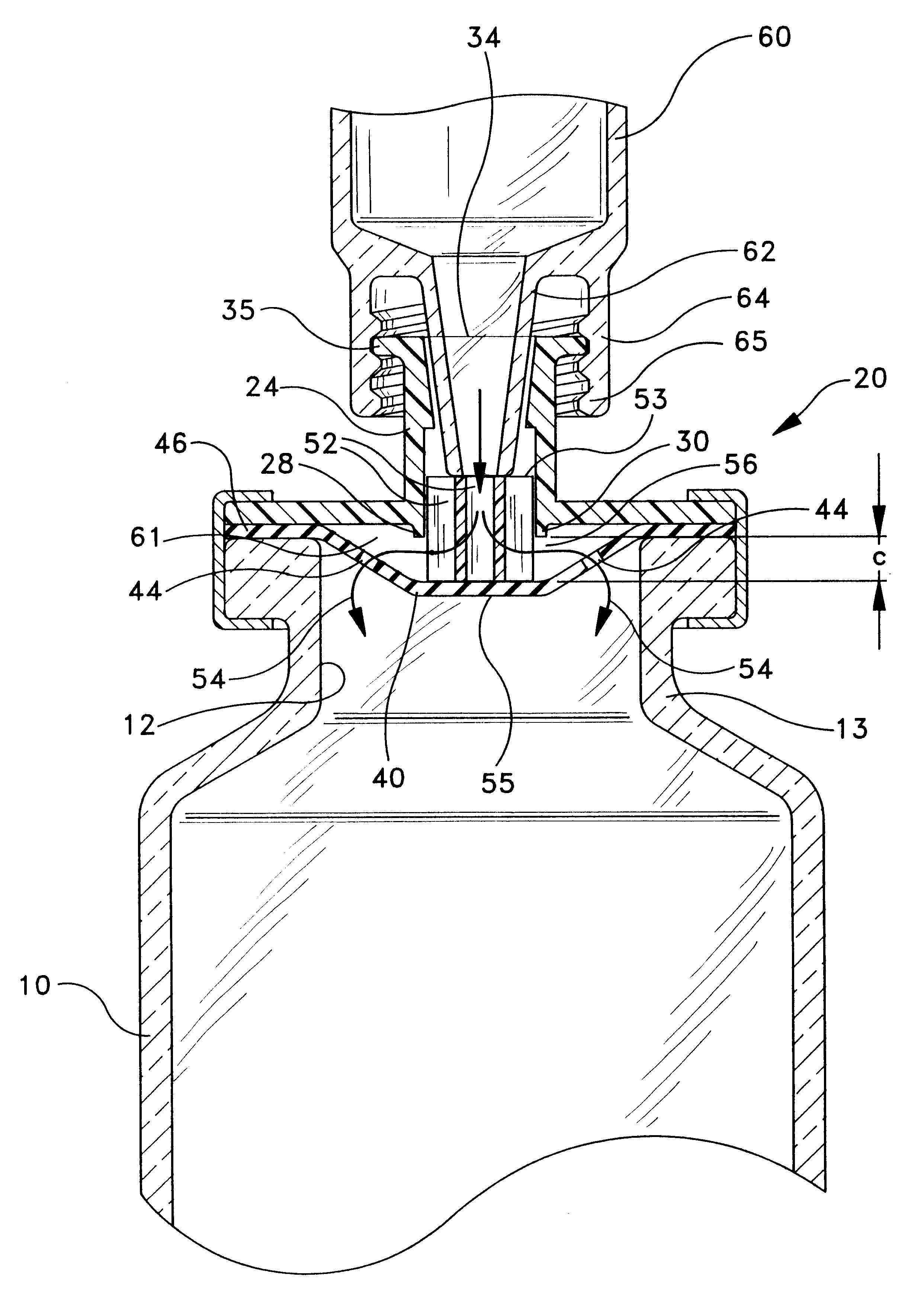
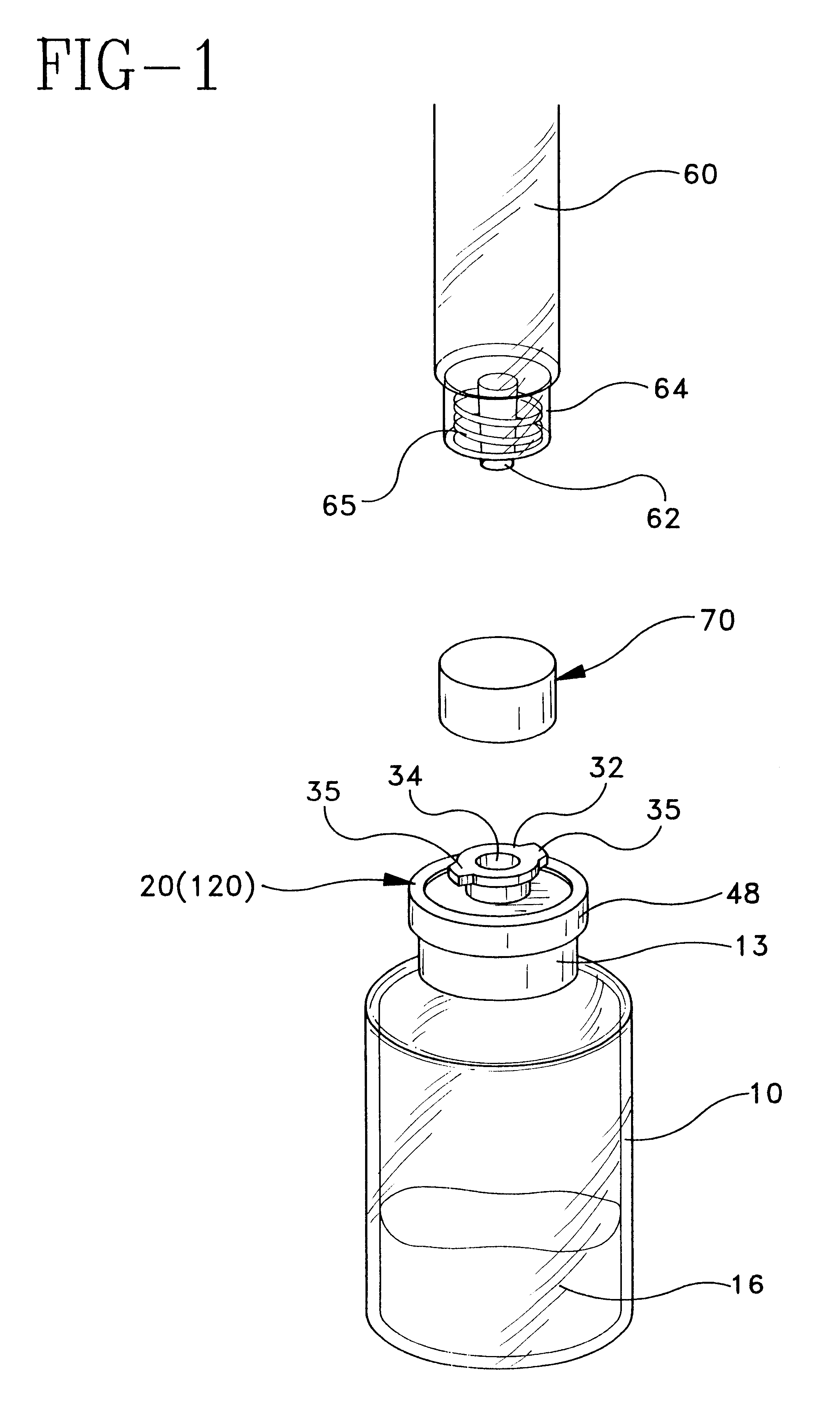
Resealable vial with connector assembly having a membrane and pusher
InactiveUS6168037B1Prevent inadvertent withdrawalPreserve sterilityCapsClosure using stoppersElastomerBottle
A resealable vial featuring a connector assembly having a membrane and a pusher for selectively opening or sealing the fluid passageway between the bottle and the connector end of a luer hub. The connector assembly includes a body disposed on said bottle, and means for communicating fluid such as a luer connector hub which may be separately provided with the body or formed integrally therewith. The luer connector hub features a connector end open for access by medical delivery instrument, and an opposed end which is disposed for fluid communication with a recess defined by the body. The body defines a recess having a fluid path with the open top of the bottle. A membrane, preferably formed from an elastomeric material, is secured across both the recess and the open top of the bottle, and may be retained between the top surface of the bottle and the body. The membrane preferably includes a central area sealing the recess from the open top of the bottle, with one or more fluid openings defined on a portion of the membrane outside of the central area. A pusher is located in the recess. A force exerted on the pusher deflects the membrane towards the interior of the vial, urging the membrane and fluid openings away from the body to open the fluid path between the bottle and the recess. The pusher may be structured to include one or more fluid pathways so as to facilitate fluid flow through the recess. A sealing rib may be provided around the portion of the periphery of the recess to enhance sealing contact between the central area of the membrane and the recess.
Owner:BECTON DICKINSON FRANCE
Sterile molded dispenser
ActiveUS9579484B2Keep sterilePreserve sterilitySurgical furnitureDispensing apparatusMedical deviceMaterial Perforation
Owner:MEDTRONIC VASCULAR INC
Intravitreal injection device and method
A coordinated cutting and spreading mechanism within a syringe dilator sub-assembly is applied to an eye surface during an intravitreal injection to provide an access window free of the conjunctival layer and through which an injection needle can be inserted. The system and method comprises a dilator sub-assembly including both the cutting and spreading mechanism and the intravitreal injection needle, for use with a conventional syringe. The dilator sub-assembly includes a number of projections to secure points of the surface of the conjunctival layer, a cutting member to incise the conjunctival layer, and at least one deflectable projection to move during the intravitreal injection, spreading the incision, and creating a window opening in the conjunctival layer through which the intravitreal injection needle then enters. Upon removal of the device from the injection site, the deflectable projection is released and the window opening in the conjunctival layer is closed.
Owner:BEAVER VISITEC INT US
Surgical Pack and Tray
ActiveUS20080272023A1Easy accessPreserve sterilitySurgical furnitureDispensing apparatusSurgical operationEngineering
A surgical pack includes a platform having a plurality of recesses configured to function as a surgical tray. A plurality of surgical instruments are contained in a corresponding recess of the platform. The recess may have the shape of the surgical instrument that it is designed to receive. The recess may also include safety mechanisms to protect the tips of certain instruments and to lower the risk of injury caused by them. A packaging or covering holds the platform and the plurality of surgical instruments in a substantially sterile condition.
Owner:DOHENY EYE INST
Surgical pack and tray
ActiveUS8177064B2Easy accessPreserve sterilitySurgical furnitureDispensing apparatusEngineeringSurgical pack
A surgical pack includes a platform having a plurality of recesses configured to function as a surgical tray. A plurality of surgical instruments are contained in a corresponding recess of the platform. The recess may have the shape of the surgical instrument that it is designed to receive. The recess may also include safety mechanisms to protect the tips of certain instruments and to lower the risk of injury caused by them. A packaging or covering holds the platform and the plurality of surgical instruments in a substantially sterile condition.
Owner:DOHENY EYE INST
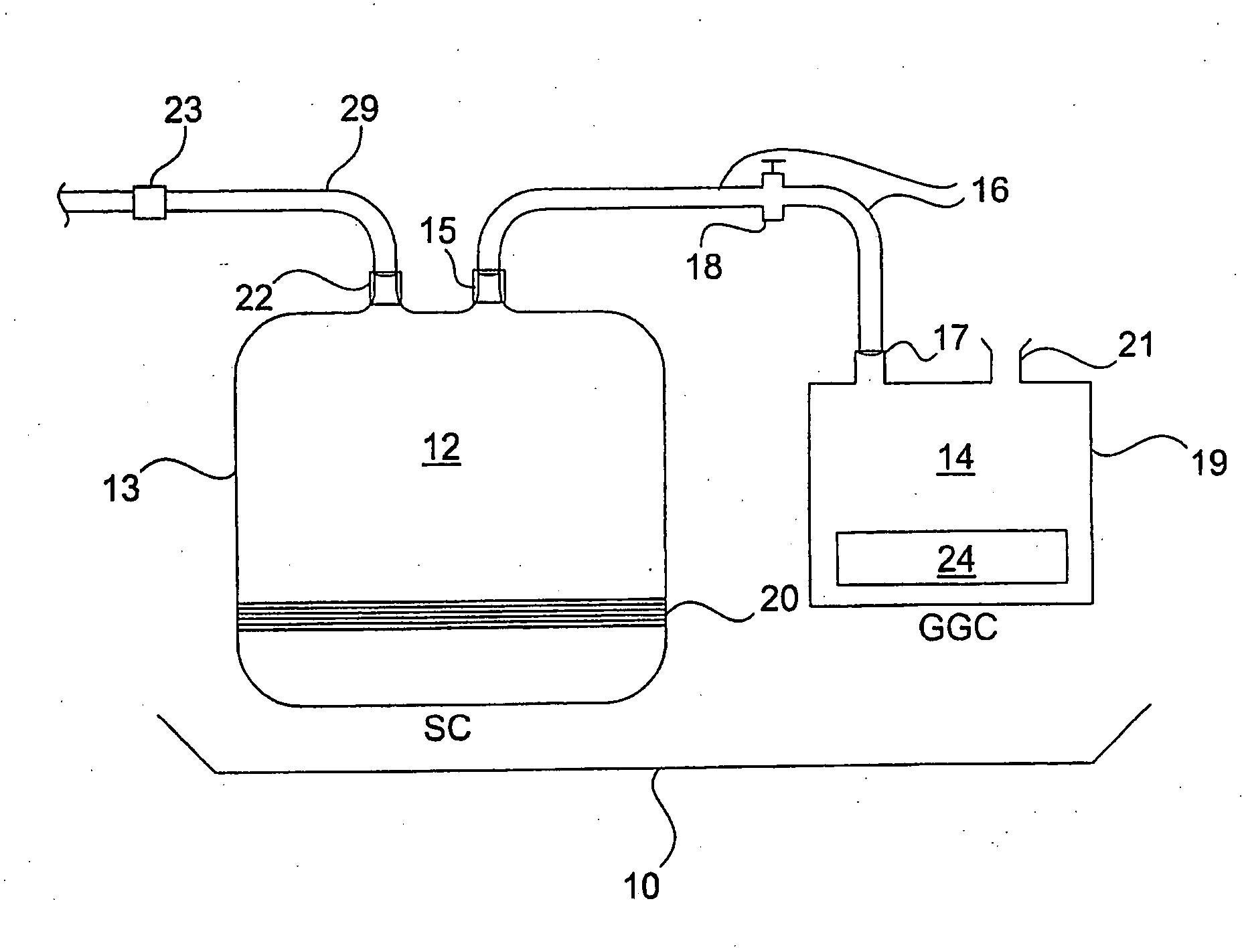
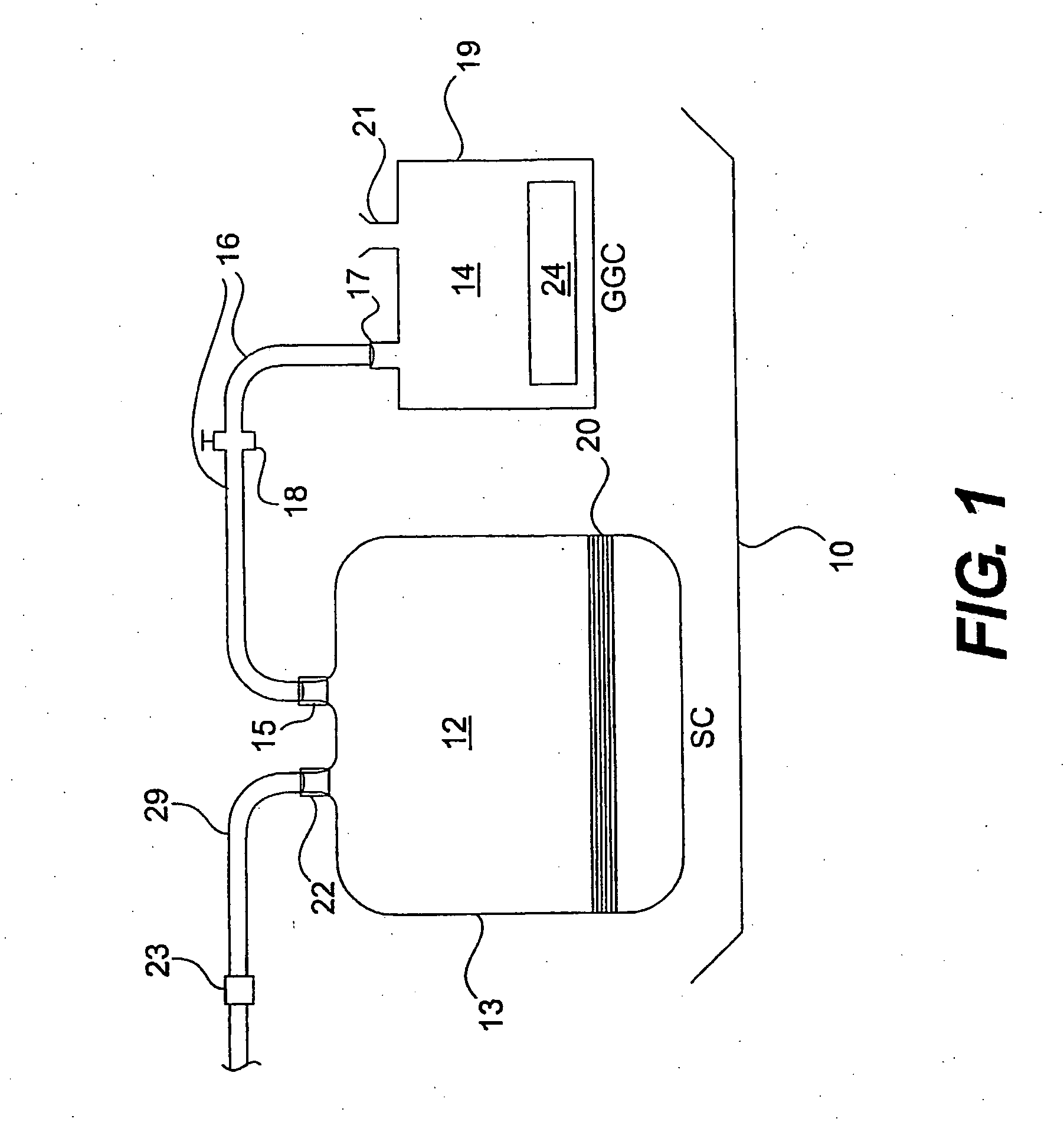
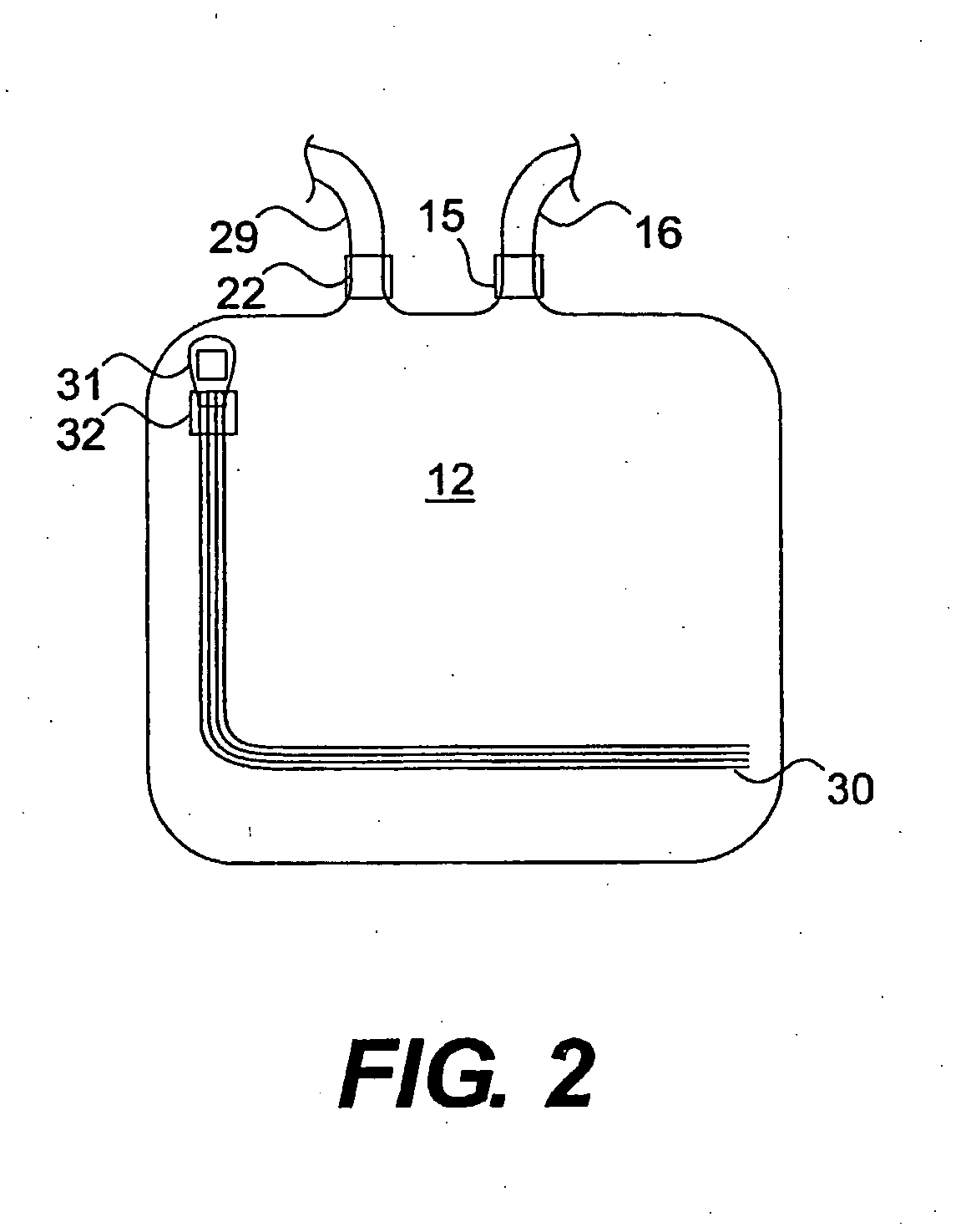
Sterilization System and Method
A system, device and method for sterilizing or decontaminating an object that includes a sealable sterilizing chamber (12) and a sterilant gas-generating composition (24) that preferably generates NO or a mixture of NO and NO2. The preferred sterilant gas-generating composition (24) includes a carbon-based diazeniumdiolate compound and a powdered acid.
Owner:NOXILIZER
Packaging solutions
InactiveUS20080110770A1Preserve sterilityIncrease lubricityPackage sterilisationOther accessoriesChemistryOsmolar Concentration
A packaging system for the storage of an ophthalmic device, such as a hydrogel contact lens, employs an aqueous packaging solution including a nonionic, nonpolymeric polyol and a nonionic polymeric conditioning agent. Preferably, the solution has an osmolality of at least about 200 mOsm / kg, a pH of about 6 to about 8 and is heat sterilized.
Owner:BAUSCH & LOMB INC
Surface Treatment of Biomedical Devices
InactiveUS20080143957A1Preserve sterilityImprove wettabilitySurgeryPharmaceutical containersChemistryRepeat unit
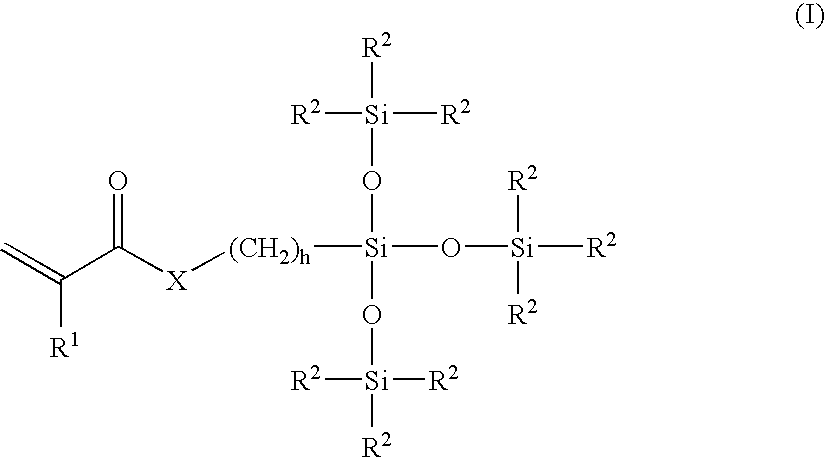
A surface modified biomedical device is provided comprising a biomedical device having a coating on at least a portion thereof, the coating comprising a polymer or copolymer having one or more repeating units of the formula:wherein R independently is a C2-C20 hydrocarbon radical and n is an integer of 2 to 5000.
Owner:BAUSCH & LOMB INC
Packaging Solutions
InactiveUS20100162663A1Preserve sterilityImprove the lubrication effectPackage sterilisationLavatory sanitoryEngineeringContact lens
Owner:BARCLAYS BANK PLC AS SUCCESSOR AGENT
Syringe and method of using
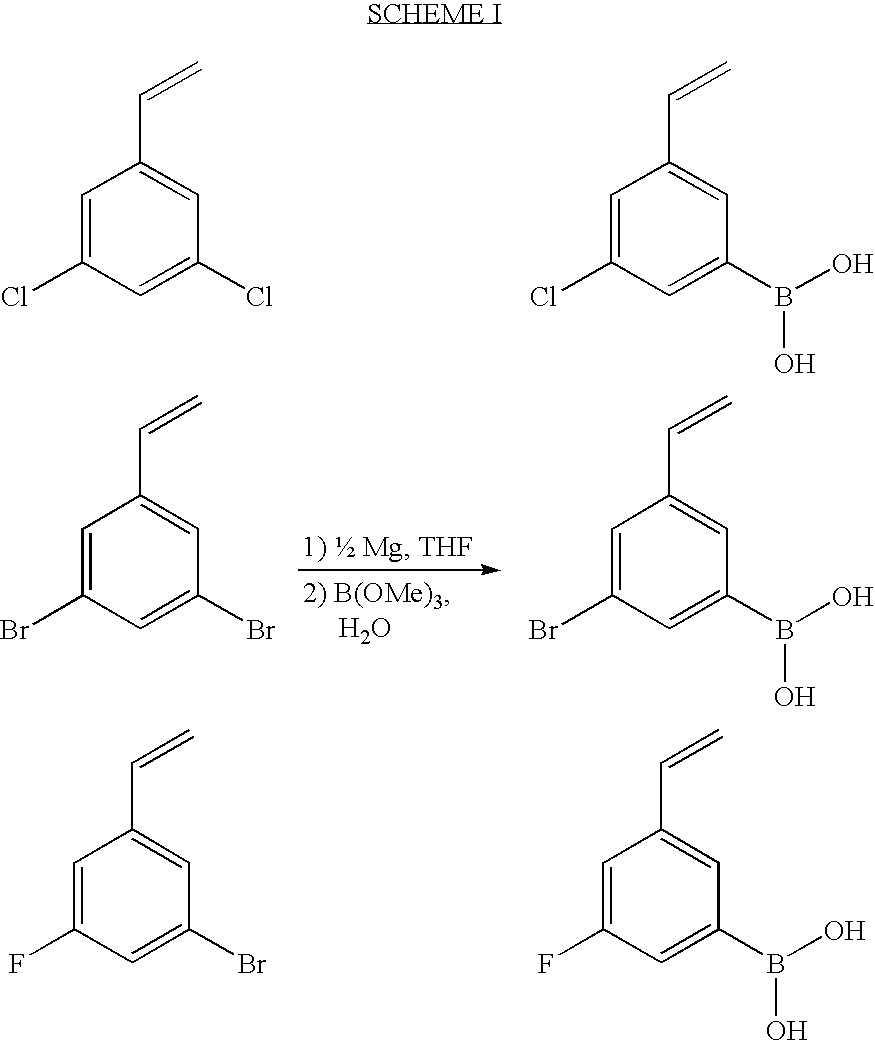
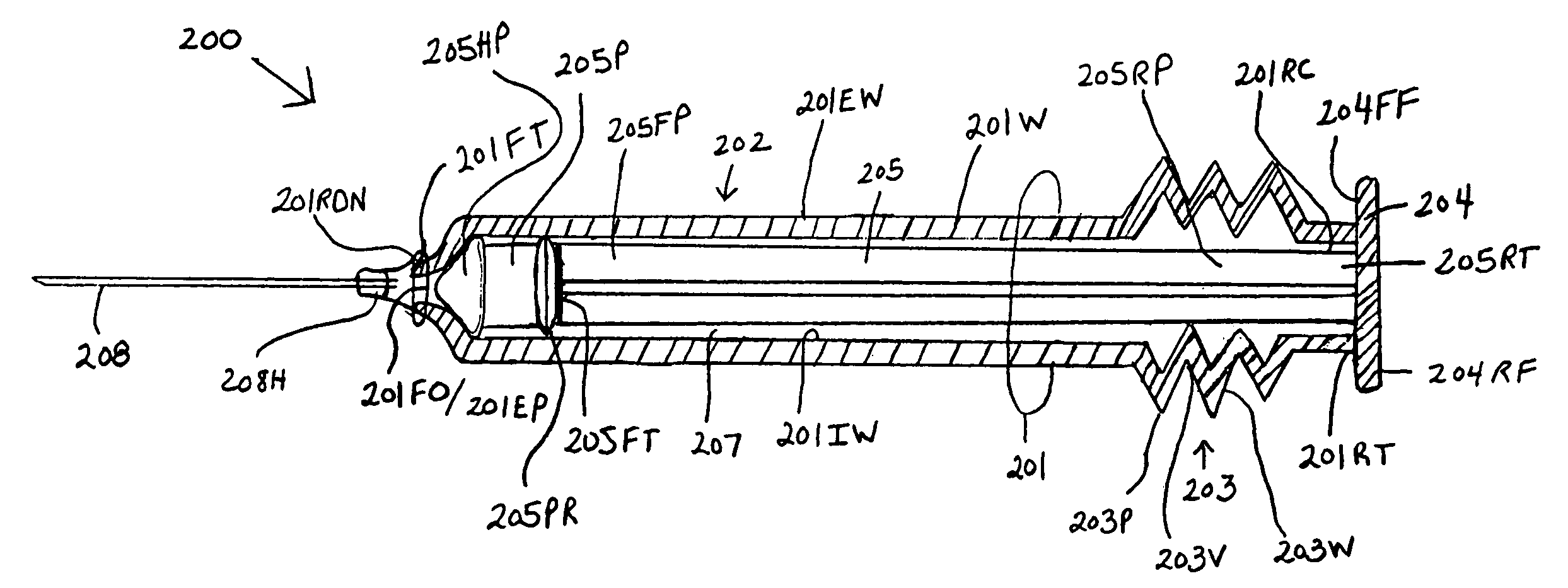
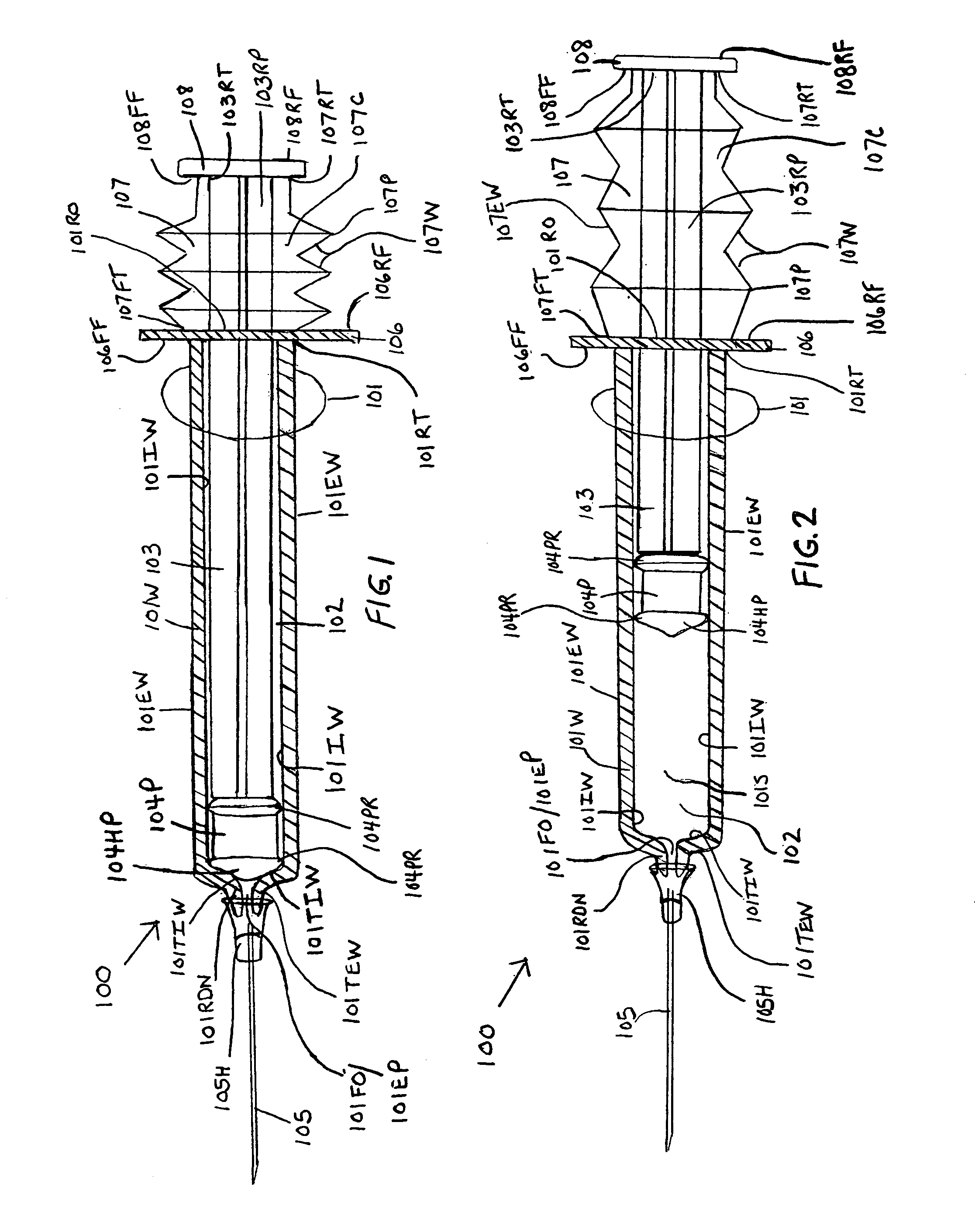
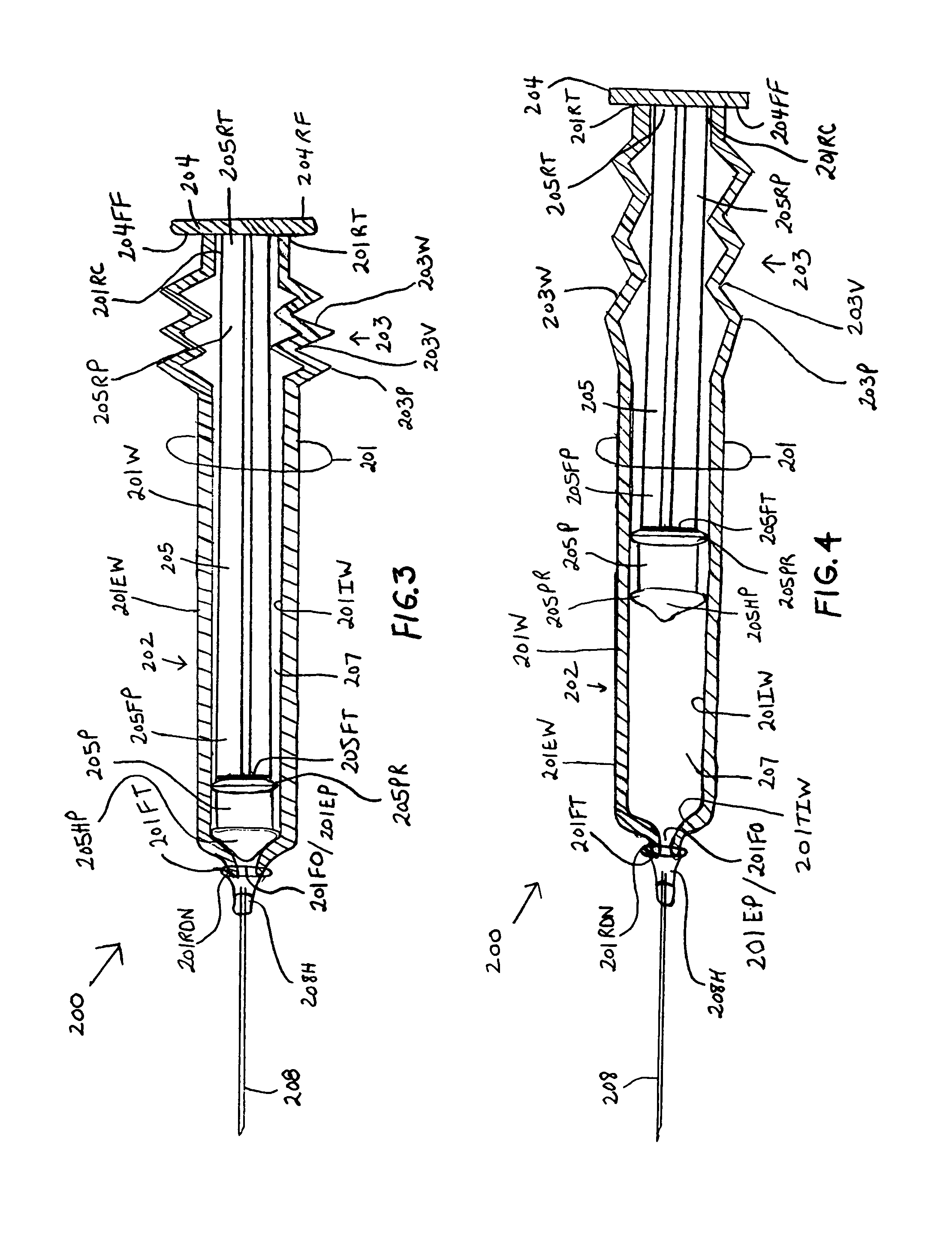
InactiveUS7175609B1Preserve sterilityInfusion syringesIntravenous devicesStraight segmentBiomedical engineering
Syringes and methods of using are described which protect the syringe barrel cavity from contaminants. A first syringe is formed with a corrugated sheath which encloses the plunger and space between the rearward end face surface of the syringe barrel handle member and the forward face of the plunger handle member. A second syringe is formed with a syringe barrel having a straight segment and a corrugated segment having the forward face of the plunger handle member molded to the rearward terminus of the corrugated segment of the syringe barrel. A third syringe is formed from mating syringe barrel and plunger member walls. The walls of the mating syringe barrel and plunger member are concentric and slide relative to each other while maintaining an enclosure around the plunger shaft. A fourth syringe is formed from inner and outer concentric syringe barrel walls mating with the walls of a plunger member. The mating walls are concentric and slide relative to each other while maintaining an enclosure around the plunger shaft. A fifth syringe is formed with an end cap contaminant shield having an extension wall that is mated with the rearward end opening of the syringe barrel cavity. Alternatively, the end cap contaminant shield can be provided with a flat design without the extending wall and is bonded or molded to the rearward end terminus of the syringe barrel. The end cap contaminant shield designs are provided with an opening defining the shape of the cross-section of the plunger shaft.
Owner:GRAY ROBIN SCOTT
Packaging Solutions
InactiveUS20090173643A1Preserve sterilityImprove the lubrication effectPackage sterilisationOther accessoriesPolyolAlcohol
Packaging systems for storing ophthalmic devices such as contact lenses and to methods for packaging such ophthalmic devices with solutions to improve the comfort of the lenses during wear are disclosed. A packaging system includes an ophthalmic device stored in an aqueous packaging solution comprising a copolymer which is the reaction product of one or more polymerizable polyhydric alcohols and one or more polymerizable fluorine-containing monomers.
Owner:LAI YU CHIN +1
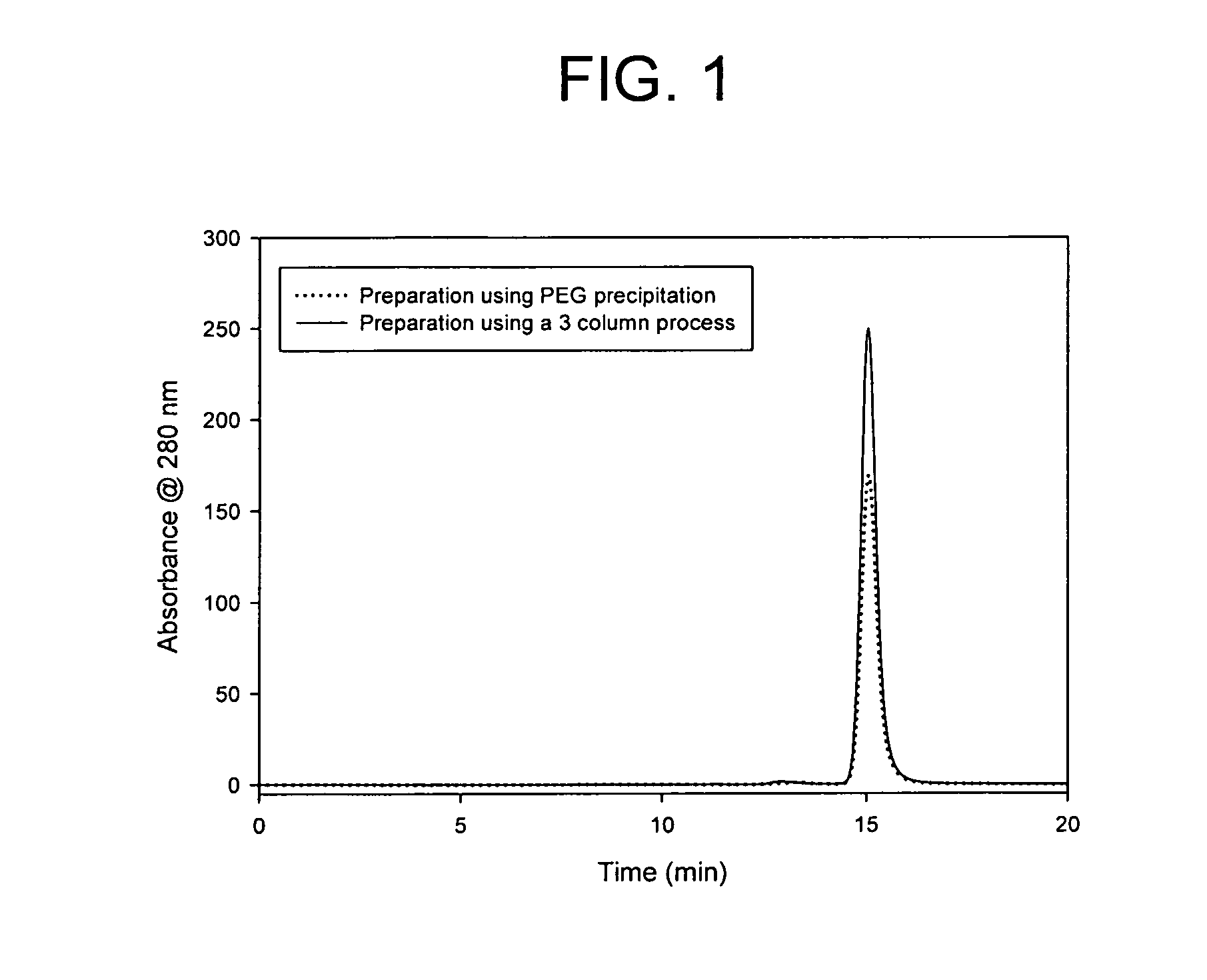
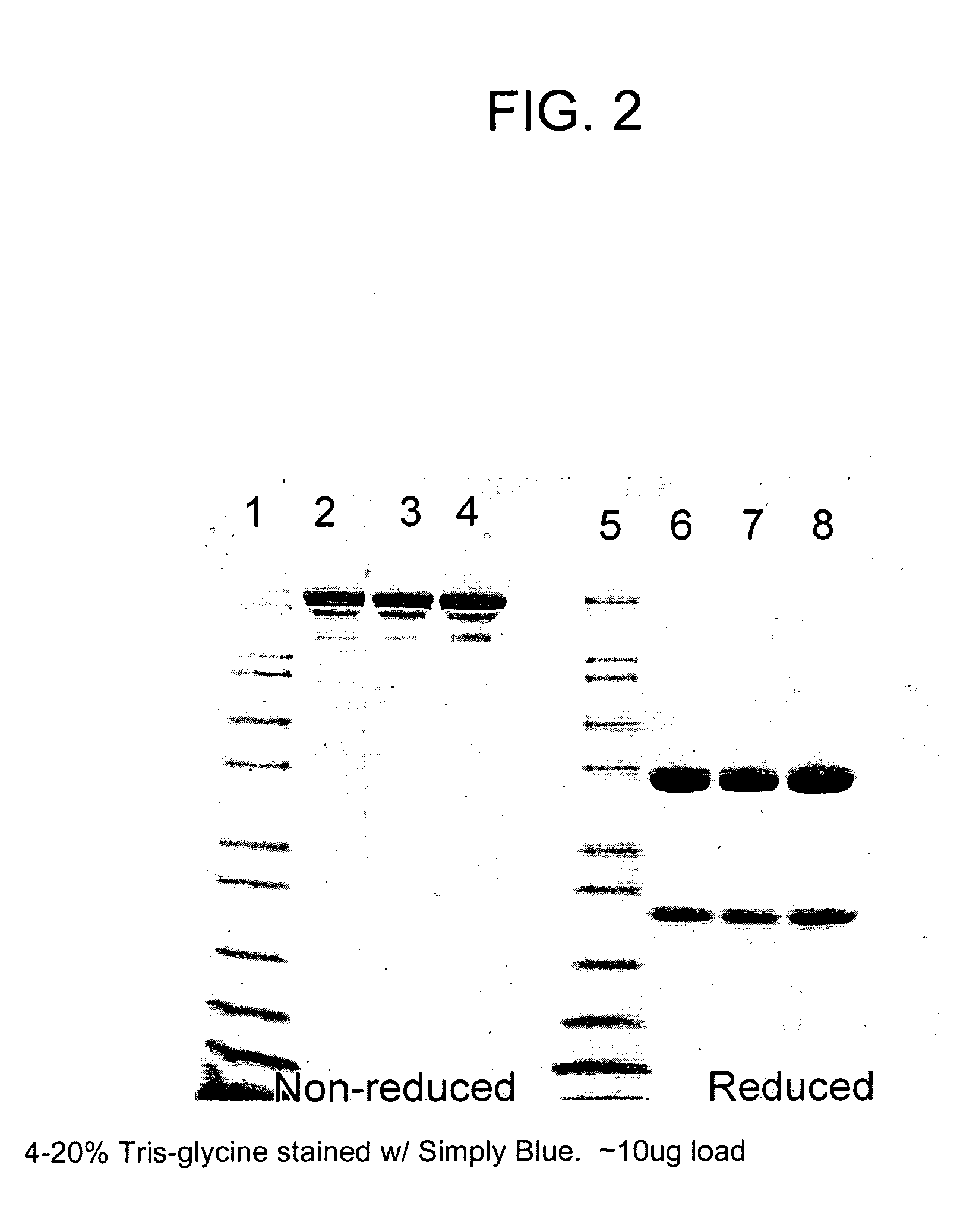
Method of isolating antibodies by precipitation
InactiveUS20080214795A1Preserve sterilityPeptide preparation methodsDepsipeptidesChromatographic separationAntibody Affinity Chromatography
Methods of isolating antibodies by precipitation are disclosed. Various precipitants that can be employed in the invention are also disclosed, with PEG being a preferred precipitant. In a representative embodiment of the invention, the pH of a solution comprising an antibody of interest is adjusted to ±0.5 pH unit of the pI of the antibody, a precipitant such as PEG is added and the antibody of interest is subsequently isolated from the resulting precipitate. The antibody can be further purified if desired or it can be resuspended in a buffer. The invention can be employed as an alternative to or in addition to chromatographic isolation methods, such as methods that employ affinity chromatography.
Owner:AMGEN INC
Compress with cooling effect in sterile pack
ActiveUS20050283212A1Preserve sterilitySafely completedDiagnosticsSurgeryAbsorption capacityCooling effect
The invention relates to a compress with a cooling effect, based on particles (6) of a polymer that has a high water absorption capacity, which comes enclosed with a reserve of water to activate it in an item (2) which comprises, inside a watertight outer sachet (3), on the one hand an inner pouch (4) filled with water and sealed in a watertight manner by a wall comprising a frangible region (10) and, on the other hand, the compress (1) produced in the form of a wrapper (5) at least partially permeable to water and containing the polymer particles (6) in the dry state. The sachet (3) is made of a material of a nature able to keep the pouch (4) and the compress (1) sterile.
Owner:ALKANTIS
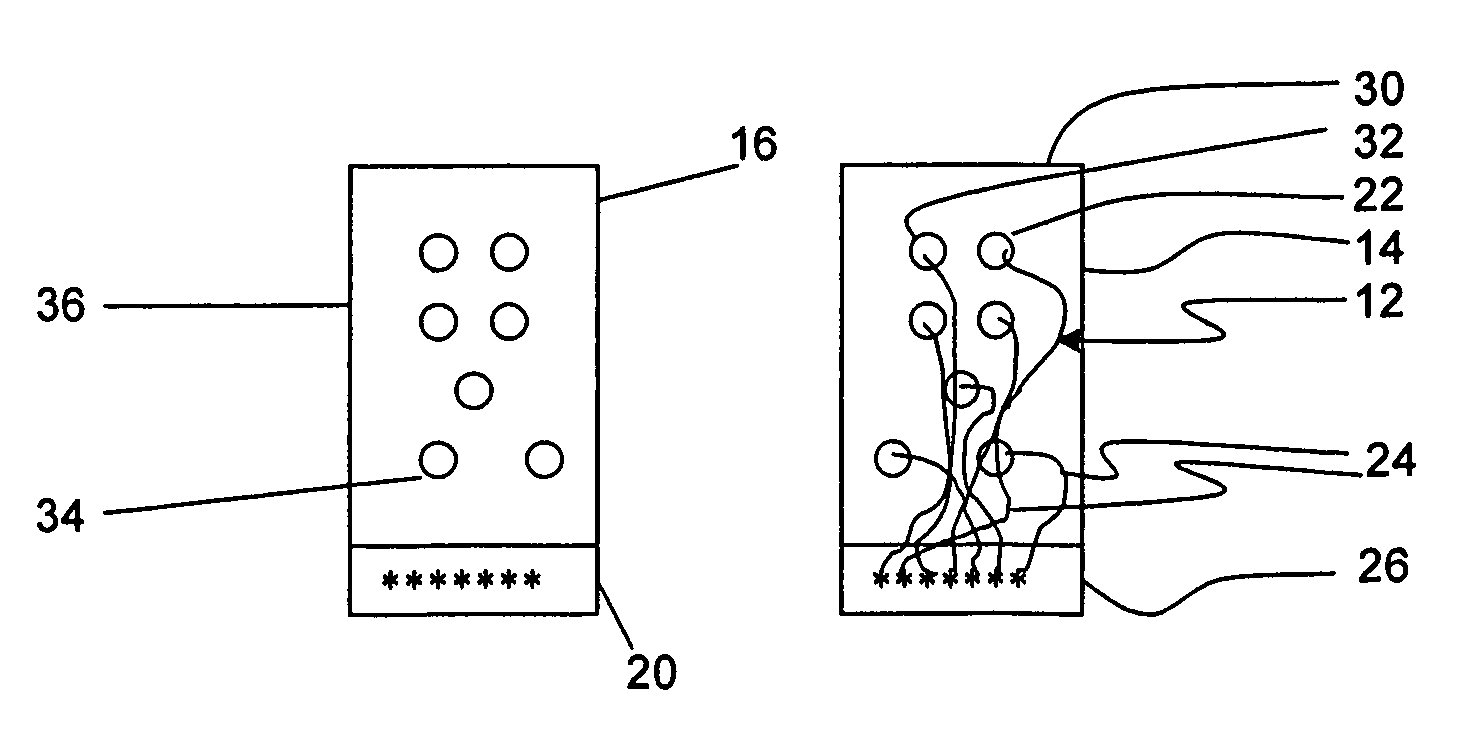
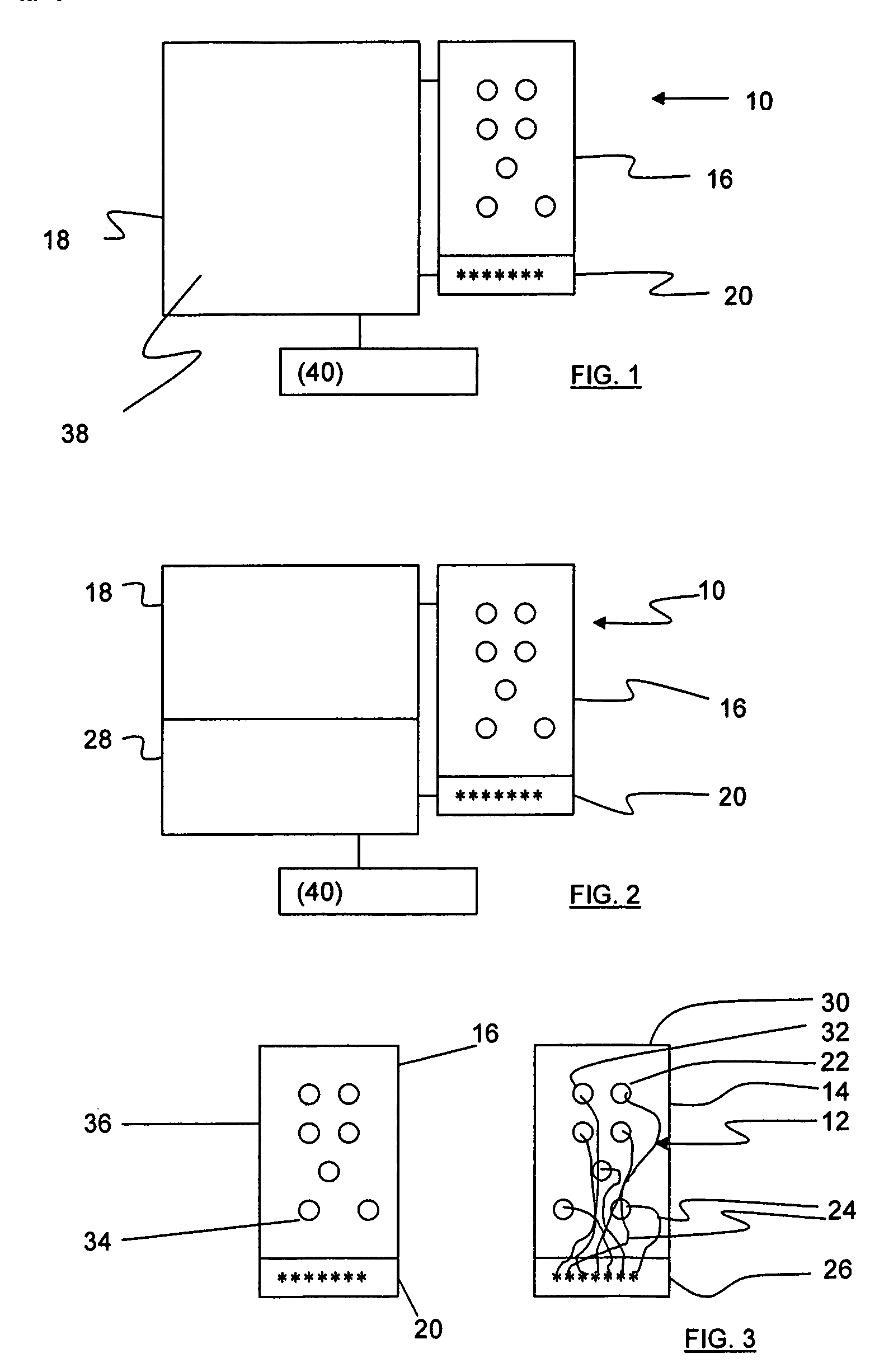
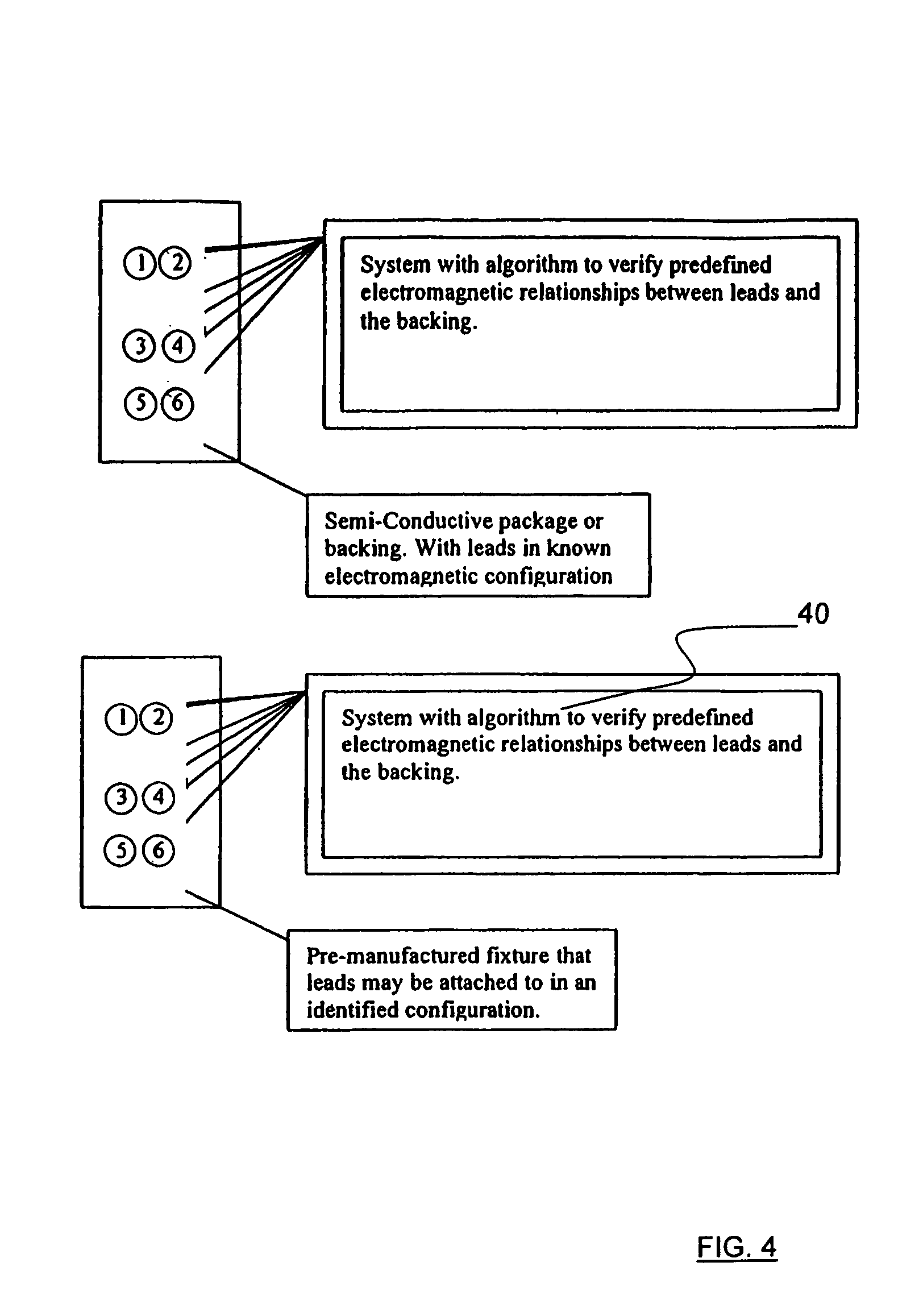
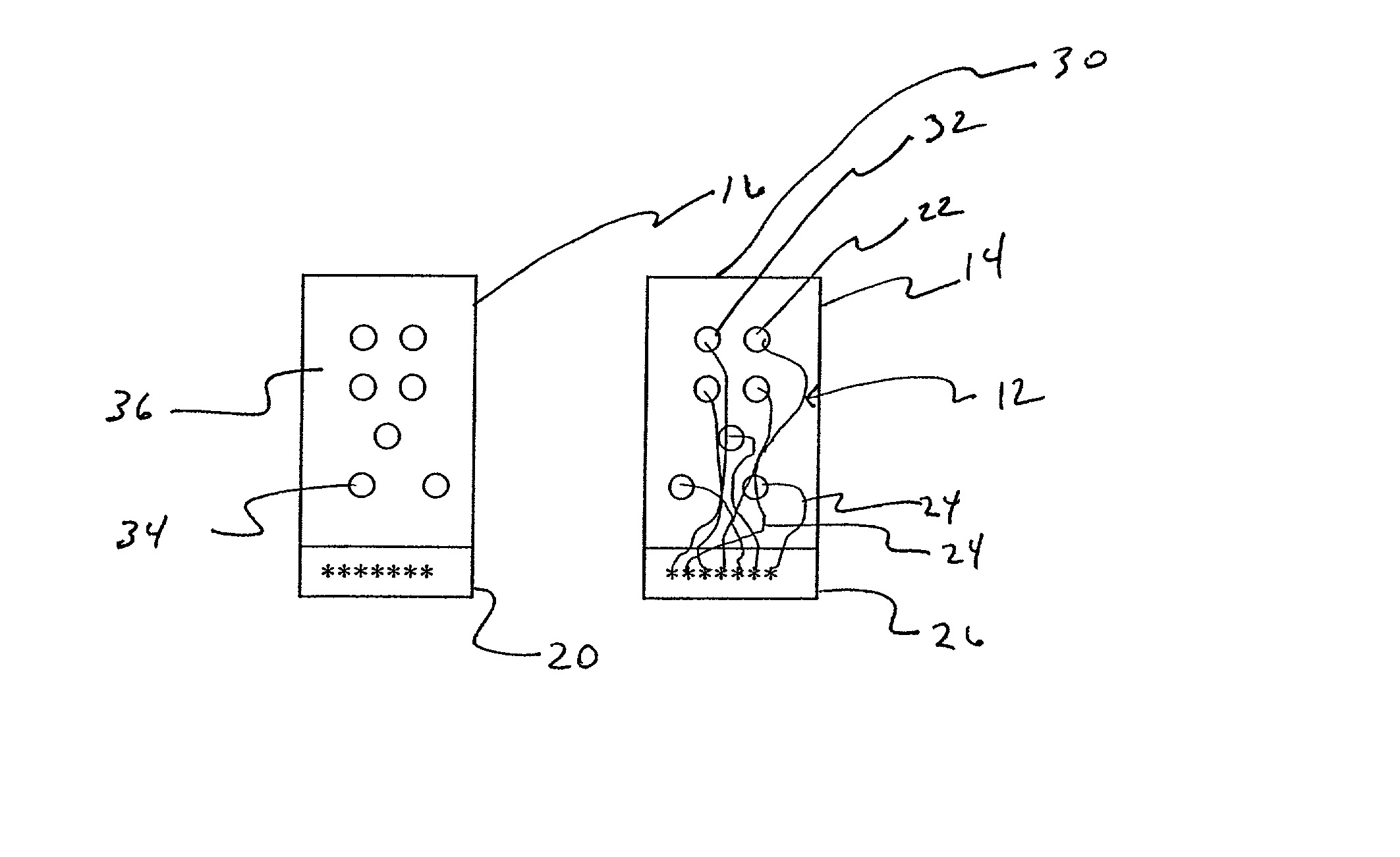
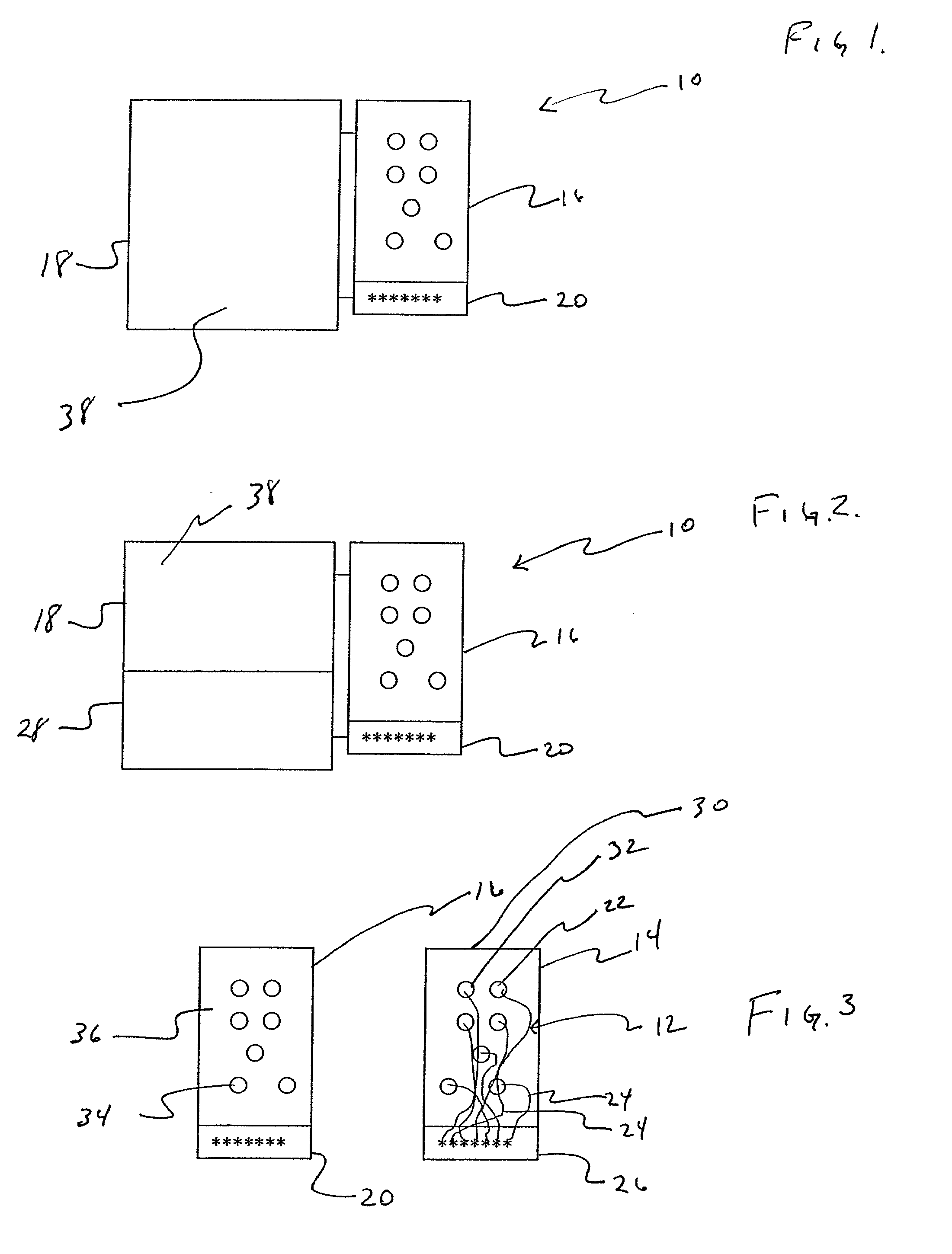
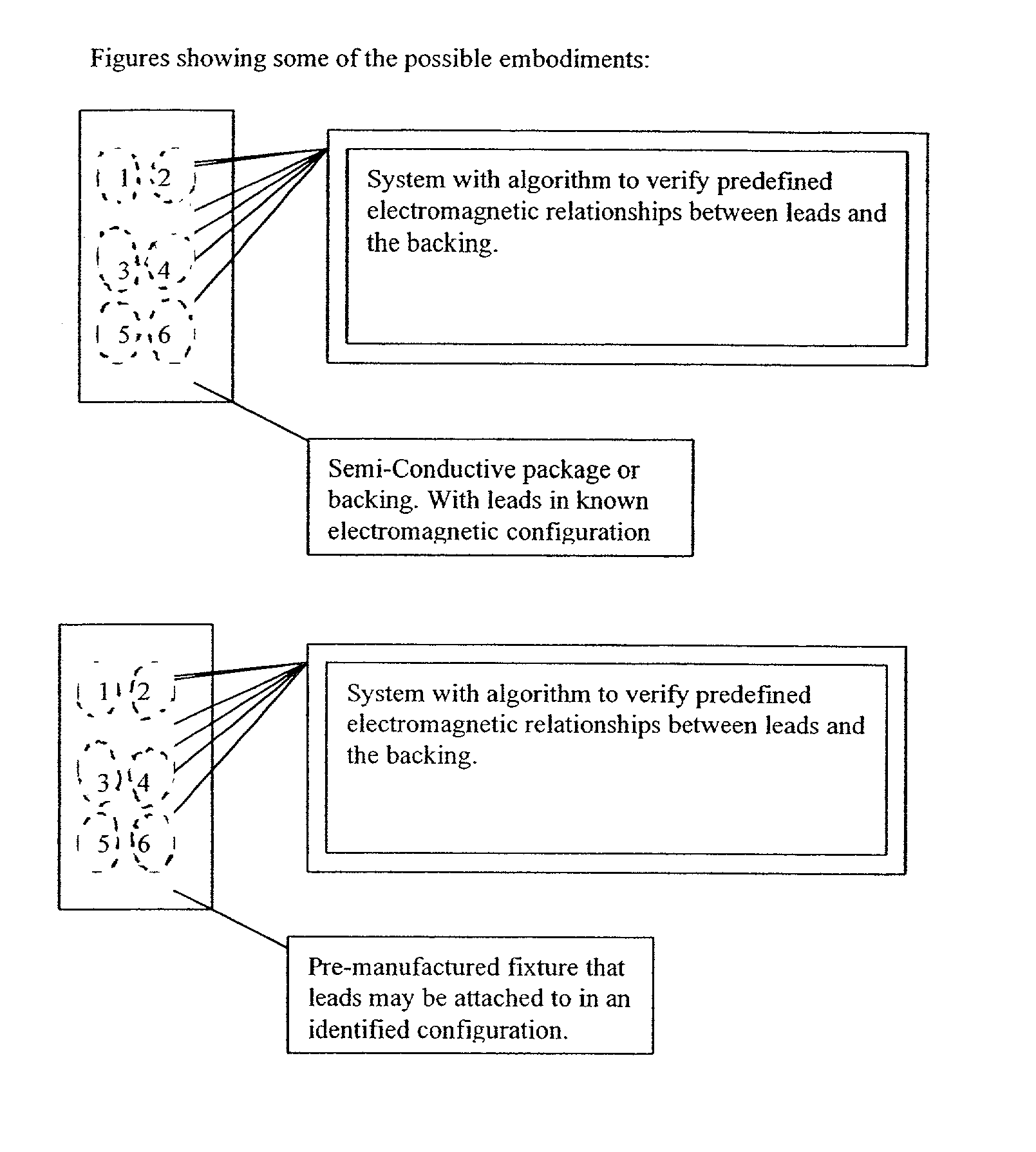
Apparatus and method for detecting lead adequacy and quality
InactiveUS7171265B2Preserve sterilityConvenient verificationElectrocardiographyHeart defibrillatorsTest fixtureInstrumentation
Owner:HARBINGER MEDICAL
System and method for out-of-band pairing of sterile device with non-sterile device
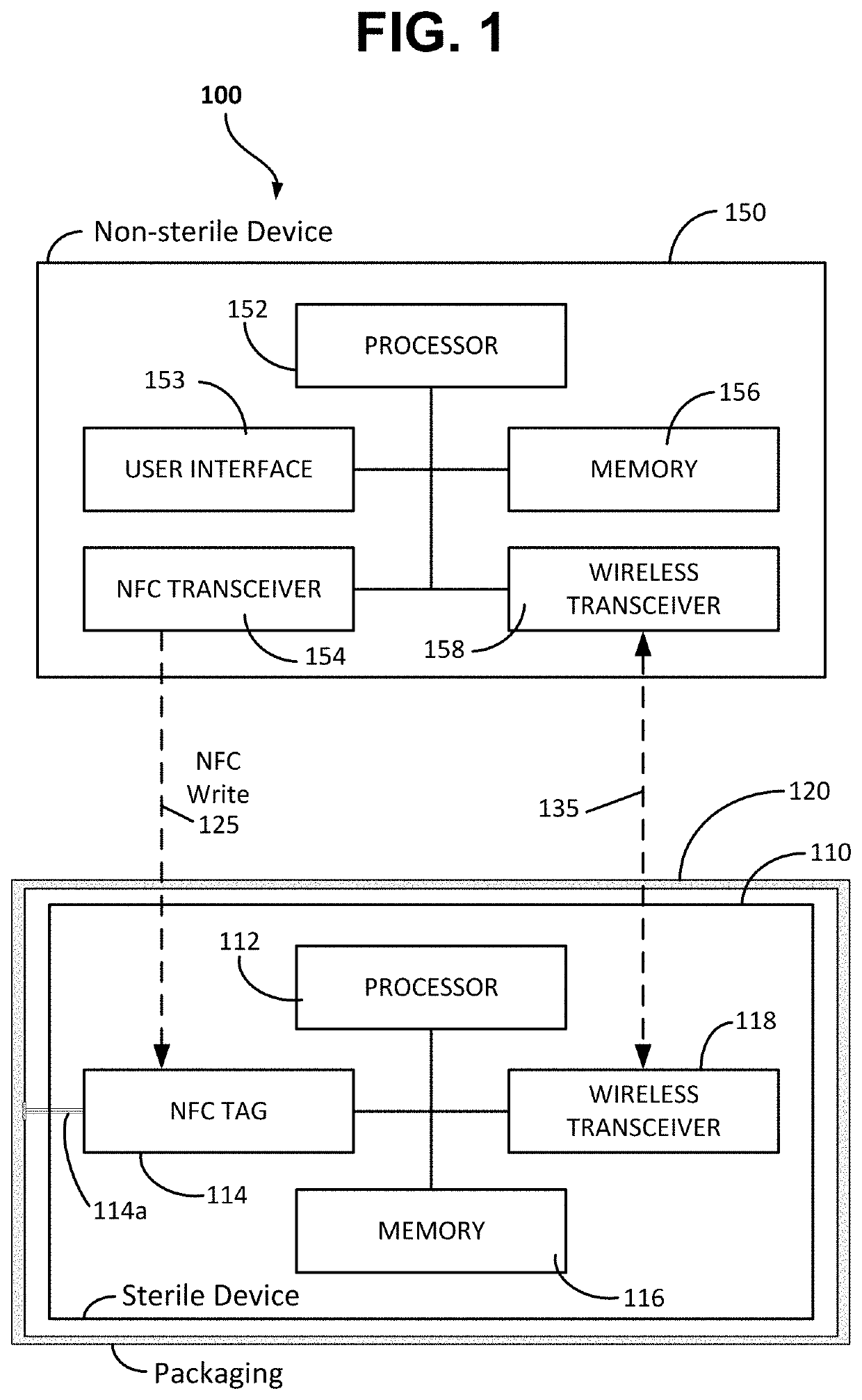
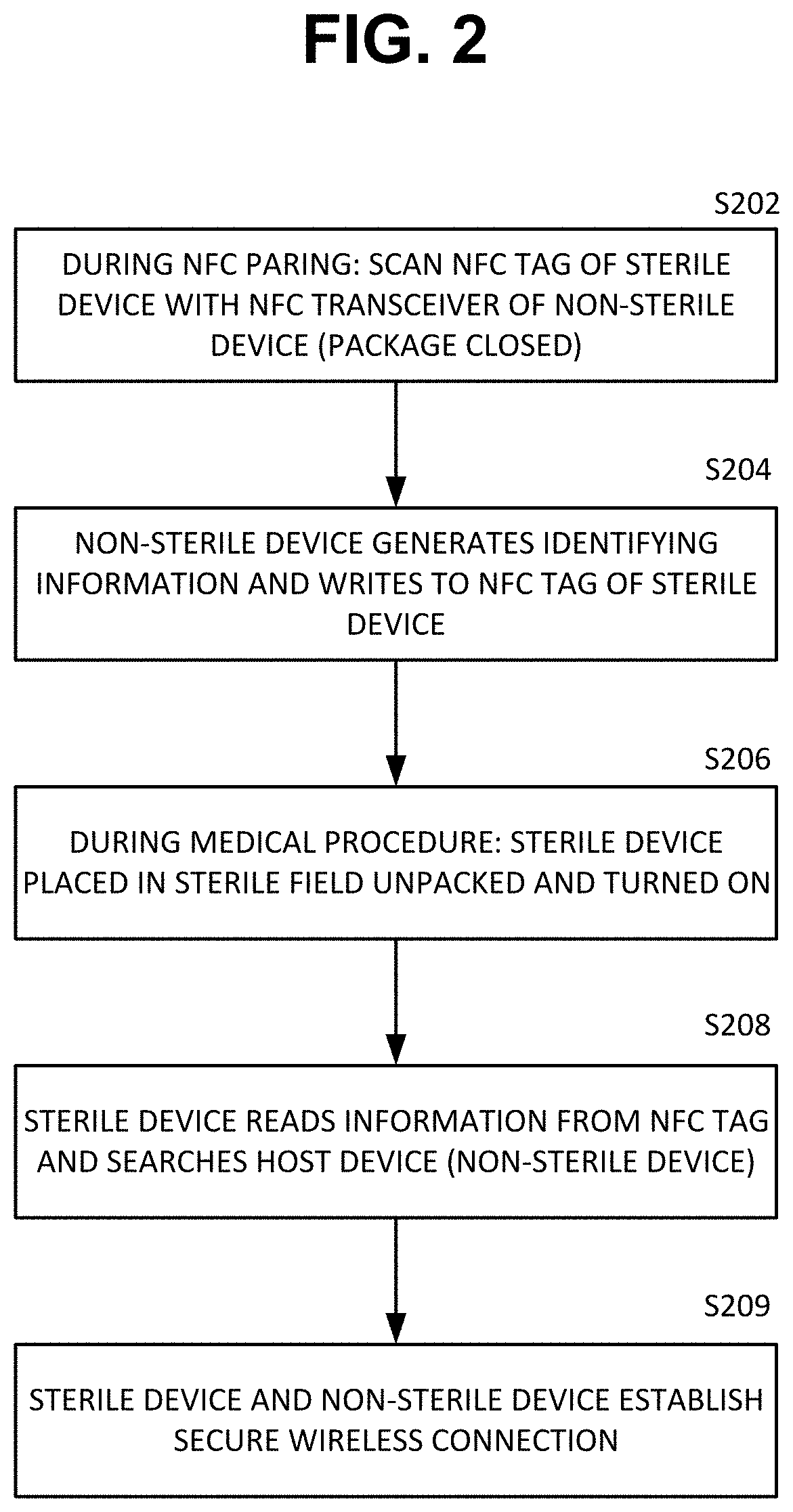
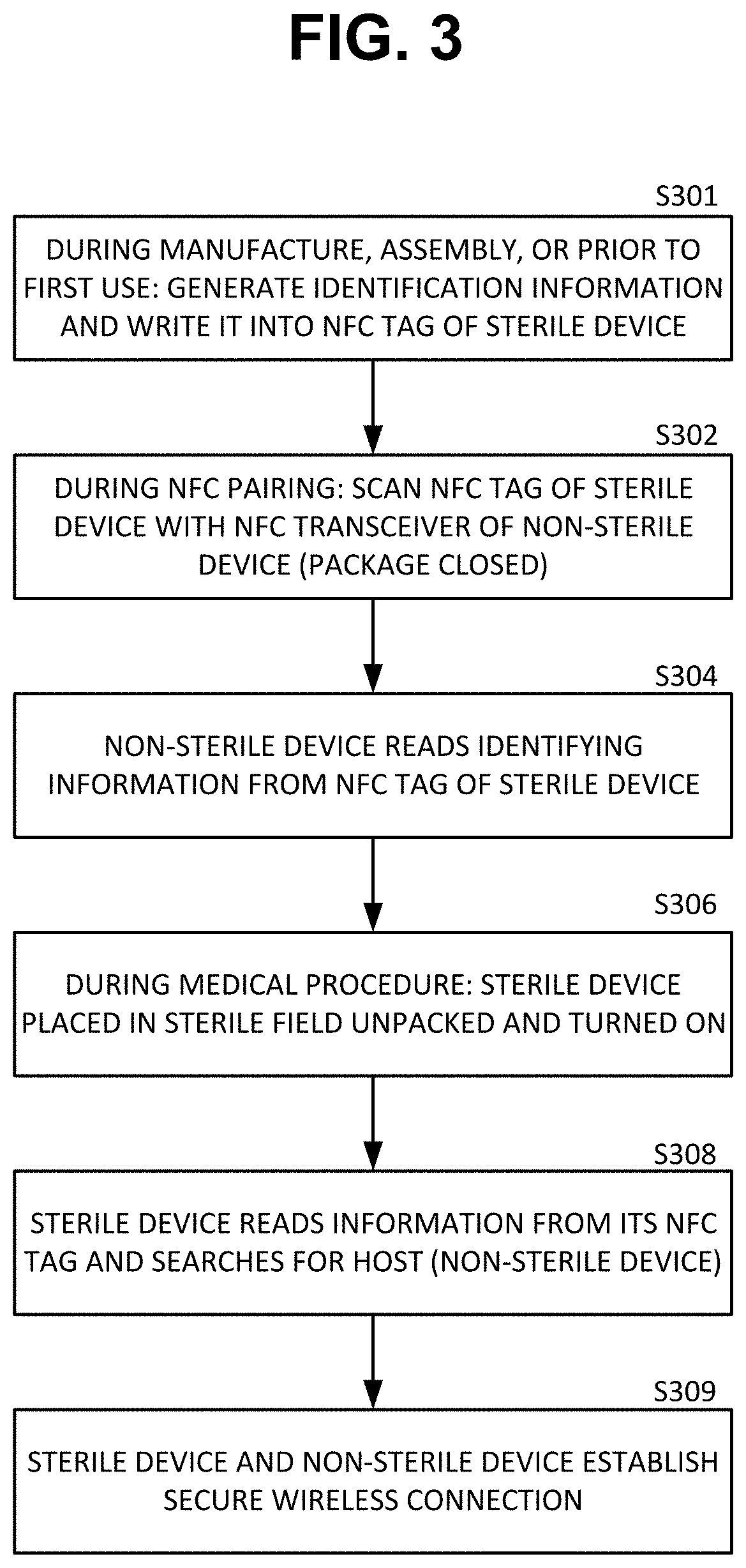
ActiveUS20210084700A1Without compromising sterilityPreserve sterilitySurgical furnitureUser identity/authority verificationMedical equipmentNeedle guidance
System and methods for out-of-band pairing sterile medical device with non-sterile devices without compromising sterility thereof. A system includes a sterile medical device; a non-sterile computing device; at least one near field communication (NFC) tag; and a sterile packaging enclosing the sterile medical device. In one example, a sterile percutaneous needle guidance device needs to pair and communicate with a non-sterile computer. The sterile device has an NFC tag embedded in the sterile device and an NFC tag embedded in the sterile packaging. The two NFC tags include identification information duplicate of each other. Before opening the sterile packaging either NFC tag can be scanned with the non-sterile device to initiate wireless pairing. If the sterile package is opened before pairing, the NFC tag contained in the packaging can be brought out of the sterile field and scanned with the non-sterile computer thus preserving the sterility of the sterile device.
Owner:CANON USA
Adhesive bandage with barrier tear away tabs
ActiveUS20100222731A1Preserve sterilityAvoid cross contaminationPlastersAdhesive dressingsPressure sensitiveBiomedical engineering
The adhesive bandage consists of a support with one surface having pressure sensitive adhesive and a wound covering pad placed centrally on it, which are covered by two protective detachable tear away tabs. The tear away tab consists of a single layer over the adhesive surface of the support and becomes two layered over the pad region. The two layers are fused at the zone of fusion near the margin of the pad, but are otherwise free from each other. The layer close to the pad, called inner barrier layer, completely covers the pad area. The outer layer, also called grasping layer, partially covers the barrier layer and the pad, and is folded upon itself to give sufficient length. When the outer layer is grasped to pull apart the releasable tear away tabs the inner barrier layer prevents contact of the finger to the pad. As one pulls apart the tear away tabs the barrier layers unfold and prevent contact of the fingers with the wound and wound exudates, preventing cross contamination. The bandage is enclosed in an envelope with the free ends of uneven dimension to have free part of the surface of both leaves, which are easy to grasp and separate.
Owner:GAJIWALA KALPESH JAYANTKUMAR
Flat Packaging of Petri Dishes for Prolonged Preservation and Method of Producing the Same
ActiveUS20120261277A1Keep sterileProlong lifeBioreactor/fermenter combinationsBiological substance pretreatmentsVisibilityPetri dish
The present invention provides an improved flat package of petri dishes and method of producing the same for prolonging shelf life and preservation of sterility of the petri dishes. The flat package is comprised of an optically clear, high-barrier, moisture-, gas- and microbial-resistant pouch. A plurality of petri dishes located adjacent one another are placed in an interior cavity of the pouch, after which the pouch is vacuum-packed, optionally flushed with an inert gas and then heat sealed. The placement and immobilization of the petri dishes in the pouch allow for prolonged shelf life and preservation of sterility, greatly reduced breakage rates, and enhanced visibility of the petri dishes by a user.
Owner:BARNHIZER BRET T
Heat treatment device
InactiveUS20150032192A1Infection controlPromote wound healingDiagnosticsSurgeryPower flowTherapeutic treatment
A heat treatment device with a handle portion, a tip portion, a power source, and a heating element. The tip portion is heatable to a desired temperature and can be positioned against an affected area for therapeutic treatment. The device can also have control circuitry that can adjust a current flowing to the heating element to regulate the treatment temperature.
Owner:EMPIRE TECH DEV LLC
Apparatus and method for detecting lead adequacy and quality
InactiveUS20020128685A1Preserve sterilityConvenient verificationElectrocardiographyHeart defibrillatorsPackage testingEngineering
A system and method for detecting lead adequacy and quality is disclosed. The system includes leads attached to a package having known electrical or optical characteristics. The package is adapted to interface with a testing device that allows the operator to ascertain whether the leads are appropriate for the desired task. This allows the testing of the lead set without the need to remove it from the package. The system of the present invention generally includes packaging of known electrical or optical characteristics, a package testing interface, and a lead testing assembly including hardware and / or software to determine whether the leads in question fulfill the desired characteristics. The lead testing assembly may be freestanding or may be incorporated into an existing testing instrument.
Owner:HARBINGER MEDICAL
Compress with cooling effect in sterile pack
The invention relates to a compress with a cooling effect, based on particles (6) of a polymer that has a high water absorption capacity, which comes enclosed with a reserve of water to activate it in an item (2) which comprises, inside a watertight outer sachet (3), on the one hand an inner pouch (4) filled with water and sealed in a watertight manner by a wall comprising a frangible region (10) and, on the other hand, the compress (1) produced in the form of a wrapper (5) at least partially permeable to water and containing the polymer particles (6) in the dry state. The sachet (3) is made of a material of a nature able to keep the pouch (4) and the compress (1) sterile.
Owner:ALKANTIS
Heat treatment device
InactiveUS10085879B2Infection controlPromote wound healingDiagnosticsSurgeryTherapeutic treatmentControl circuit
A heat treatment device with a handle portion, a tip portion, a power source, and a heating element. The tip portion is heatable to a desired temperature and can be positioned against an affected area for therapeutic treatment. The device can also have control circuitry that can adjust a current flowing to the heating element to regulate the treatment temperature.
Owner:EMPIRE TECH DEV LLC
Adhesive bandage with barrier tear away tabs
ActiveUS8822751B2Preserve sterilityAvoid cross contaminationPlastersAdhesive dressingsBiomedical engineeringTears
The adhesive bandage consists of a support with one surface having pressure sensitive adhesive and a wound covering pad placed centrally on it, which are covered by two protective detachable tear away tabs. The tear away tab consists of a single layer over the adhesive surface of the support and becomes two layered over the pad region. The two layers are fused at the zone of fusion near the margin of the pad, but are otherwise free from each other. The layer close to the pad, called inner barrier layer, completely covers the pad area. The outer layer, also called grasping layer, partially covers the barrier layer and the pad, and is folded upon itself to give sufficient length. When the outer layer is grasped to pull apart the releasable tear away tabs the inner barrier layer prevents contact of the finger to the pad. As one pulls apart the tear away tabs the barrier layers unfold and prevent contact of the fingers with the wound and wound exudates, preventing cross contamination. The bandage is enclosed in an envelope with the free ends of uneven dimension to have free part of the surface of both leaves, which are easy to grasp and separate.
Owner:GAJIWALA KALPESH JAYANTKUMAR
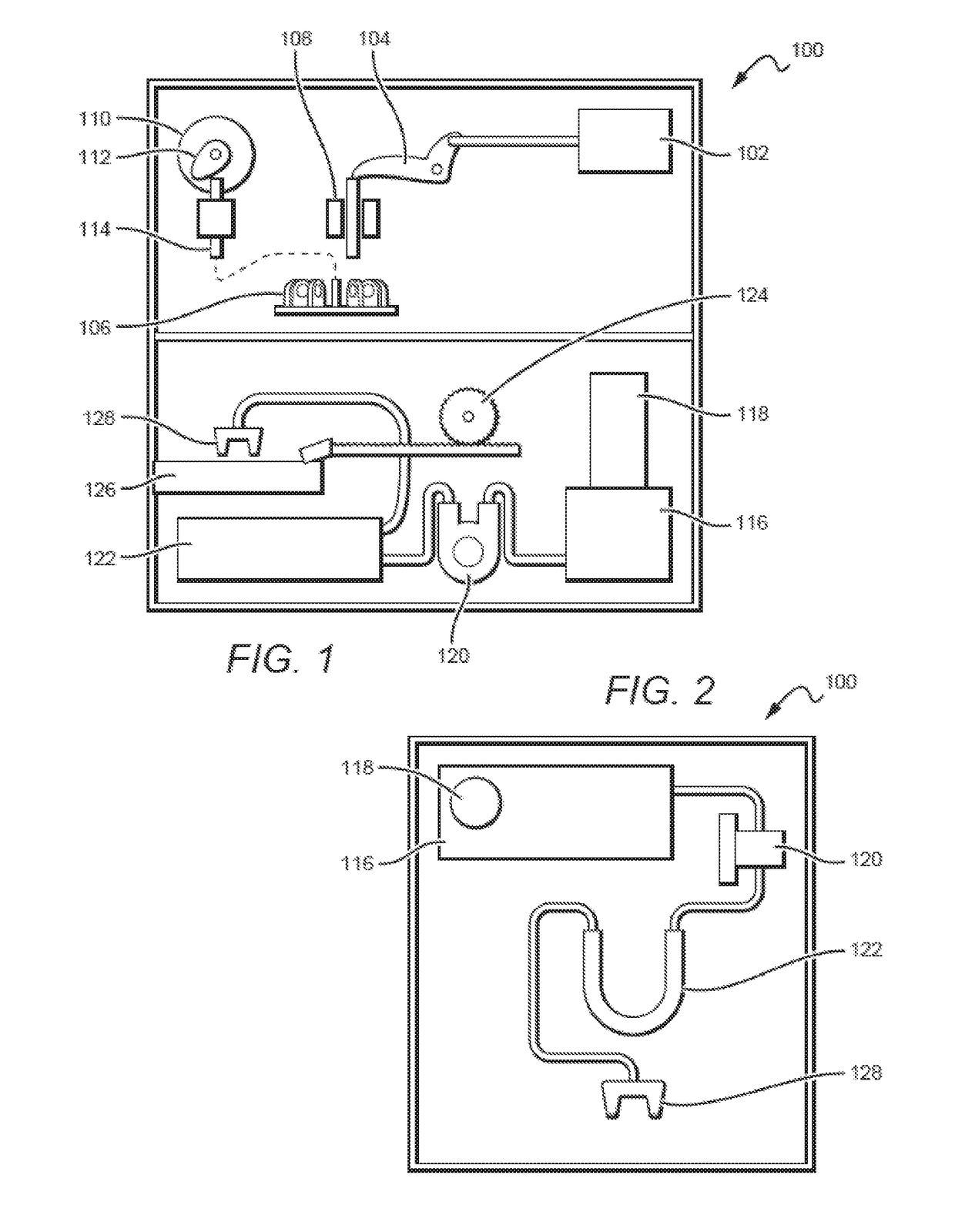
Device for dispensing sterile on-demand, heated towelettes
InactiveUS20190029476A1Preserve sterilityAvoid pollutionDomestic applicationsTowelettesBiomedical engineering
A wet towelette dispensing device is provided. The device is operable in conjunction with a container storing a plurality of dried, compressed, individually wrapped, preferably sealed towelettes. The device includes a pump configured to transfer a predetermined amount of liquid from a liquid reservoir to a heat exchanger to heat the liquid to a predetermined temperature, a plunger configured to release a first one of the towelettes from its individual wrapping. The device further includes a liquid dispense unit configured to deliver the heated liquid to the released towelette.
Owner:MCCARTHY MELANIE H +2
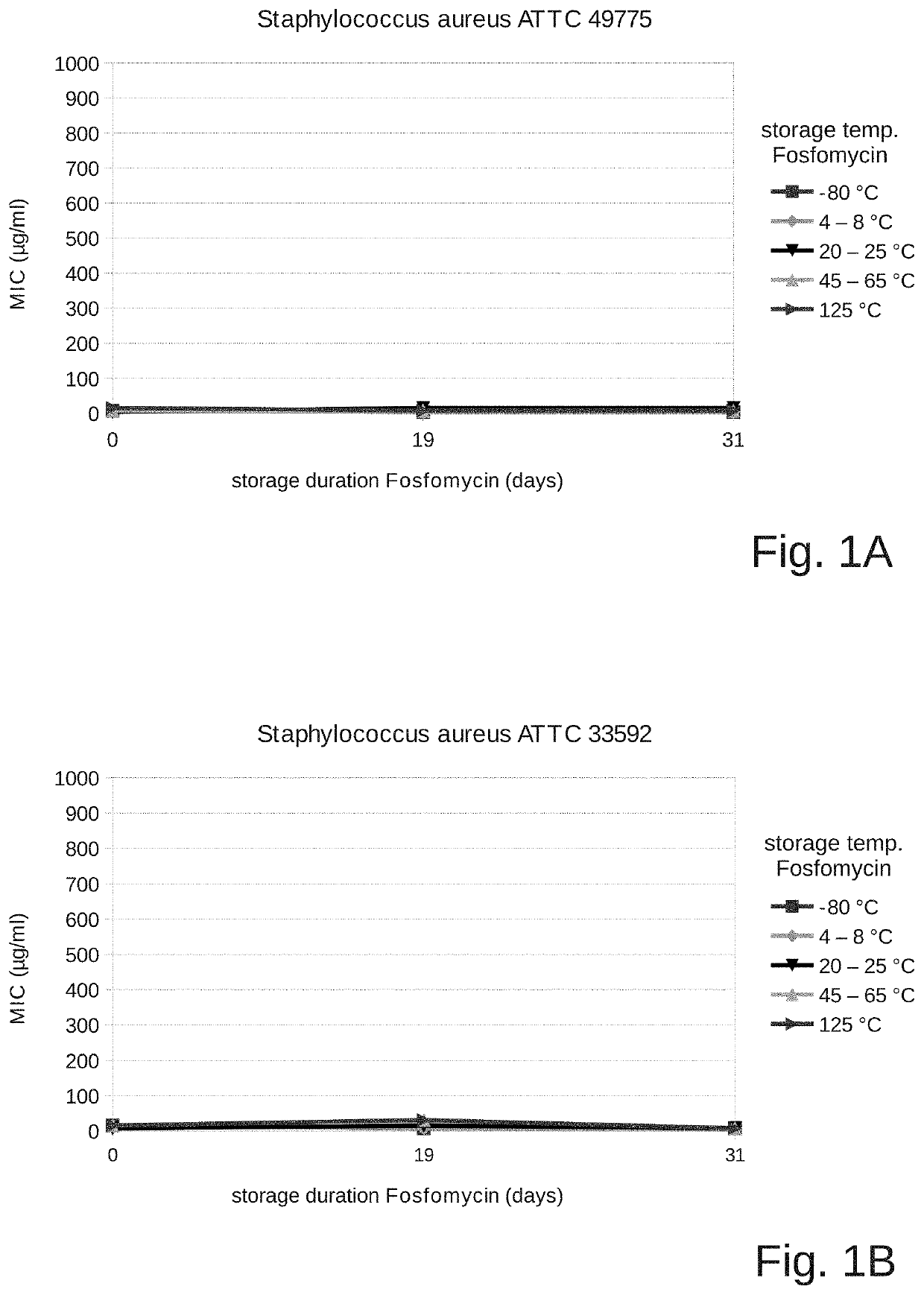
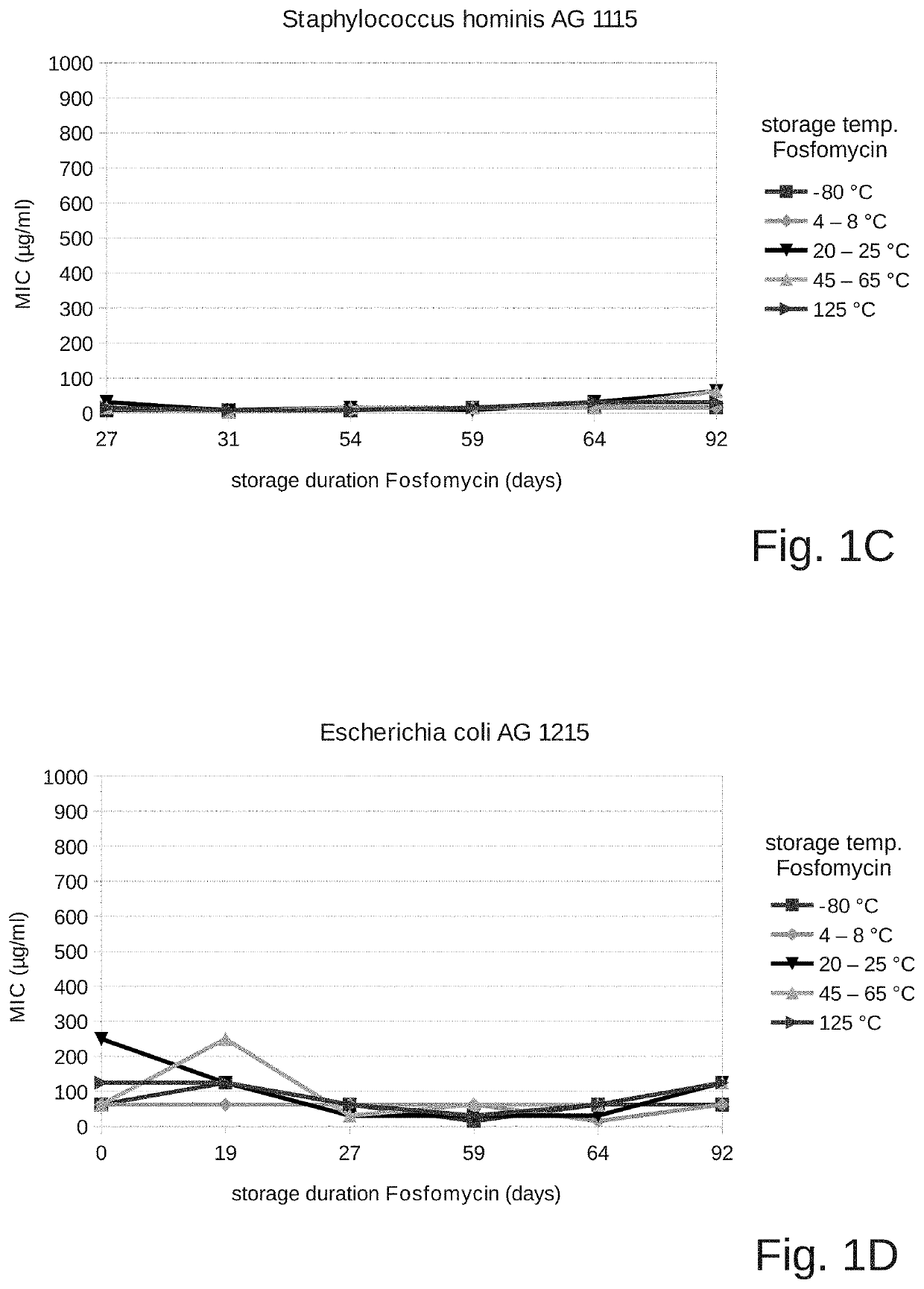
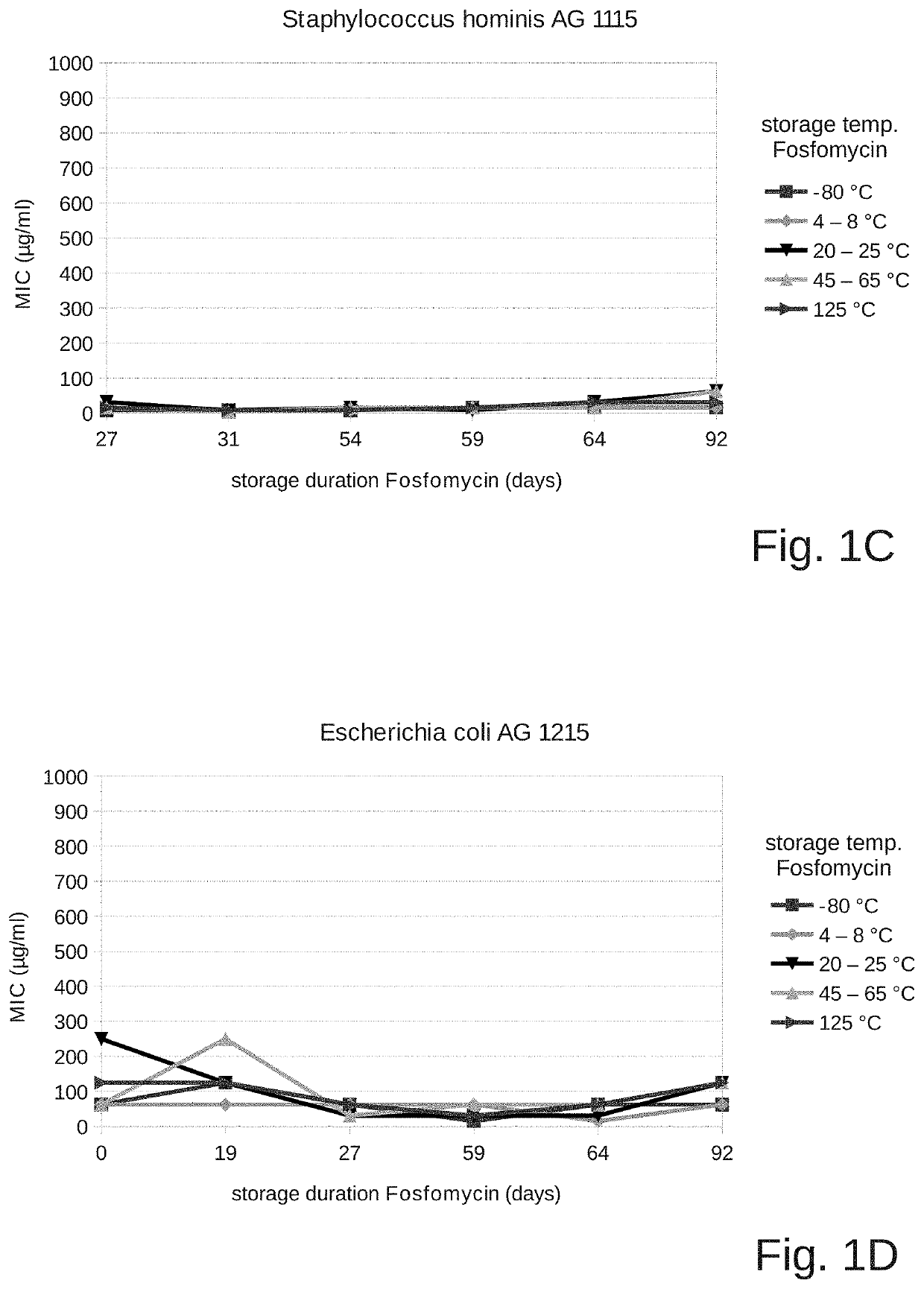
Fosfomycin formulation for parenteral administration
ActiveUS10993951B2Easy to administerEasy to produceOrganic active ingredientsPharmaceutical containersPharmacyHealth risk
Owner:GEORGOPOULOS APOSTOLOS +2
Flat packaging of petri dishes for prolonged preservation and method of producing the same
ActiveUS8413800B2Prolong lifeImprove preservationBioreactor/fermenter combinationsBiological substance pretreatmentsVisibilityPetri dish
The present invention provides an improved flat package of petri dishes and method of producing the same for prolonging shelf life and preservation of sterility of the petri dishes. The flat package is comprised of an optically clear, high-barrier, moisture-, gas- and microbial-resistant pouch. A plurality of petri dishes located adjacent one another are placed in an interior cavity of the pouch, after which the pouch is vacuum-packed, optionally flushed with an inert gas and then heat sealed. The placement and immobilization of the petri dishes in the pouch allow for prolonged shelf life and preservation of sterility, greatly reduced breakage rates, and enhanced visibility of the petri dishes by a user.
Owner:BARNHIZER BRET T
Pad of Dispensable Tape Segments
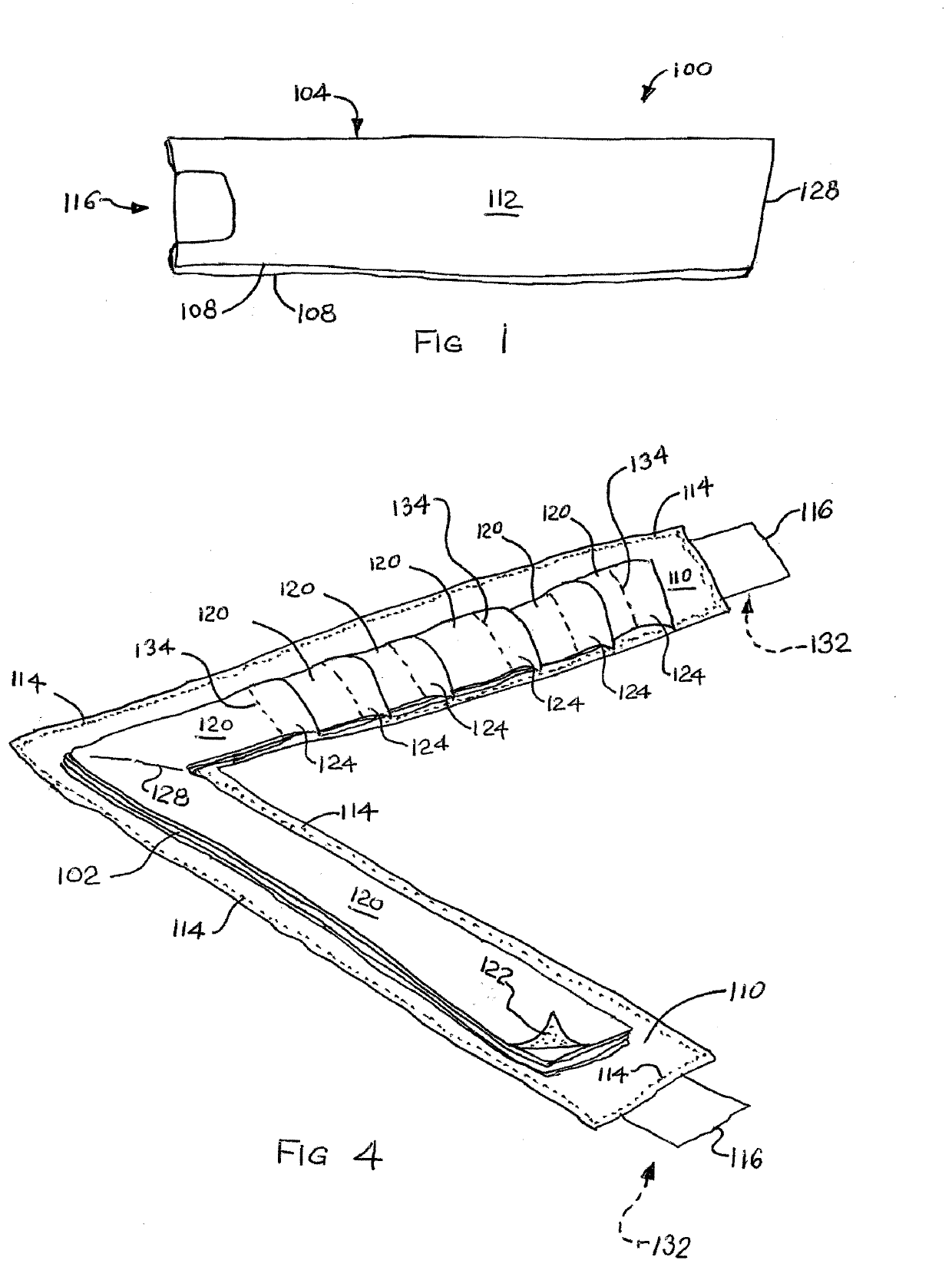
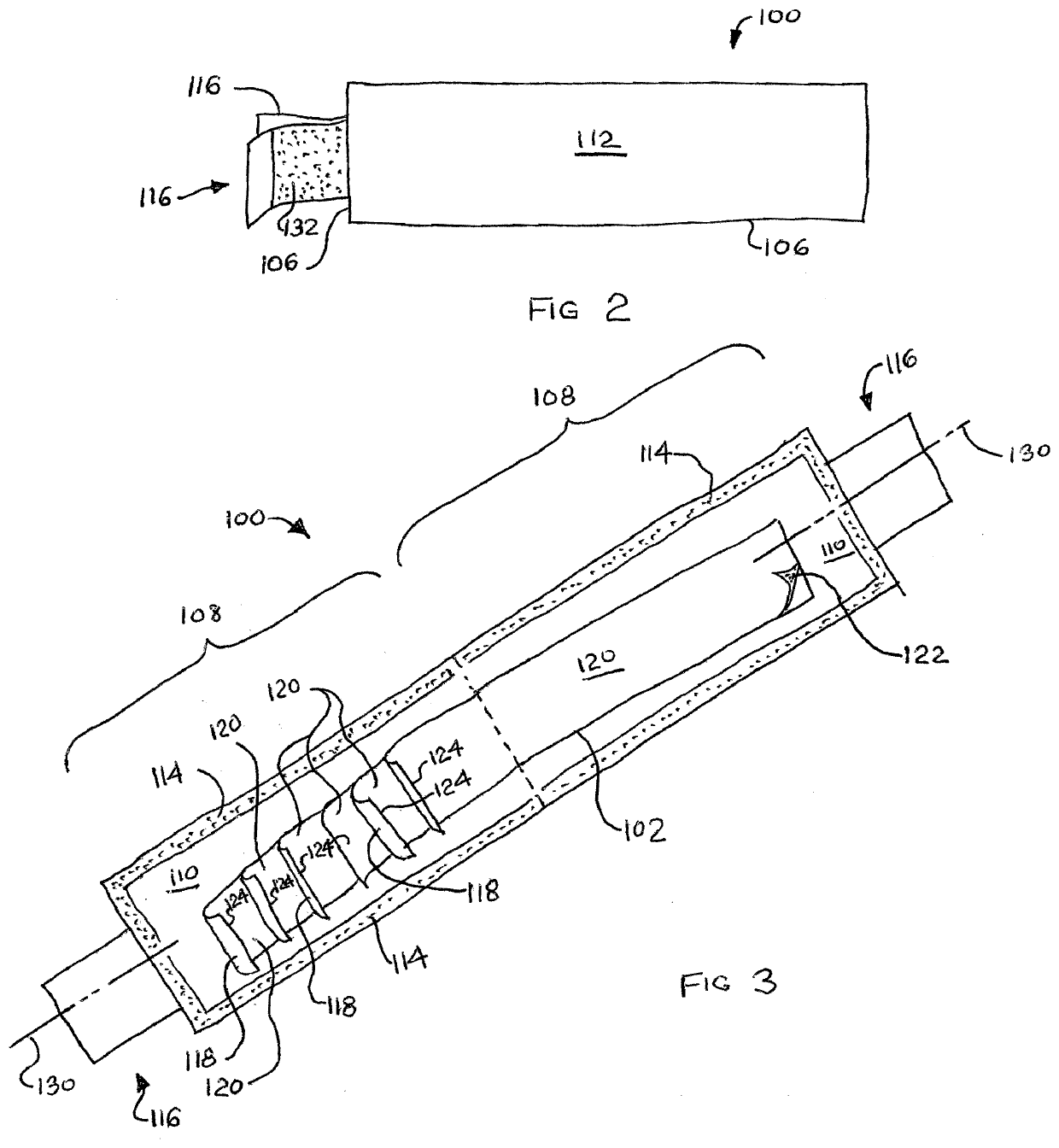
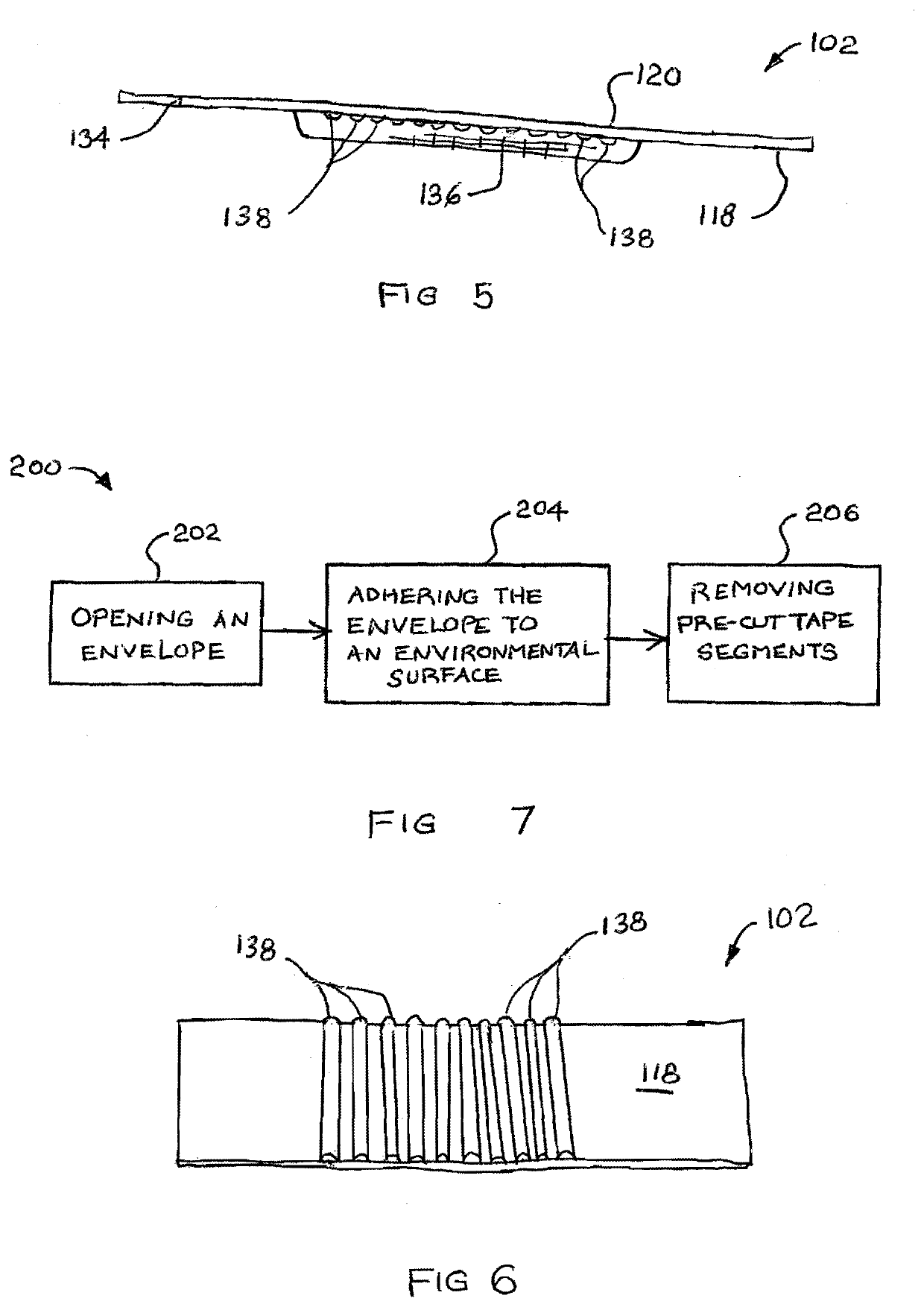
ActiveUS20210179382A1Reduce demandEasy retrievalSynthetic resin layered productsCellulosic plastic layered productsAdhesive beltBiomedical engineering
A pad of dispensable adhesive tape segments is shown and described. The pad includes a protective envelope and a number of tape segments. The tape segments are arrayed such that detachable pull tabs for each are exposed for grasp. The envelope may include a strip folded over onto itself and adhered in place. The envelope is pulled open and may be adhered to an environmental surface. Tape segments have adhesive of weaker adherence than the adhesive of the envelope so that when pulled by a pull tab, each tape segment preferentially releases from the next, but the envelope maintains adherence to the environmental surface. Adhesive lined tabs initially holding the envelope closed may also be used for adherence to the environmental surface. Preferably used for medical tape, the envelope is waterproof to maintain sterility of tape segments.
Owner:CHOULJIAN PARSIG
Medical Preparation and Method for Enhancing Tissue Oxygenation in Case of Diabetic Foot
ActiveUS20200237850A1Improve stabilityPreserve sterilityDipeptide ingredientsMetabolism disorderDipeptideActive agent
The present invention proposes a new use of dipeptide L-glutamic-L-tryptophan acid (L-Glu-L-Trp) for enhancing tissue oxygenation by suppression (reducing a synthesis) of HIF-1α factor in case of diabetic foot, a medical preparation including an effective amount of the dipeptide L-Glu-L-Trp as an active agent and a pharmaceutically acceptable carrier, and a method of treating a diabetic foot including locally administering the mentioned medical preparation comprising the dipeptide L-Glu-L-Trp in a dose of 1.0 μg per kg-10.0 μg per kg of body weight at least once a day for a period necessary to achieve a therapeutic effect. The technical result of invention includes providing a peptide preparation, which exerts an antihypoxic effect in diabetes mellitus of both types.
Owner:OMNIPEP ESTAB
Fosfomycin Formulation for Parenteral Administration
ActiveUS20200261478A1Easy to administerEasy to produceOrganic active ingredientsPharmaceutical containersPharmacyHealth risk
The field of the present invention is that of fosfomycin formulations for parenteral administration, in particular for intravenous administration. Formulations of the prior art provide fosfomycin as a powder to be diluted directly prior to the administration. The aim of the invention is to provide a fosfomycin formulation for parenteral administration which is easier to produce and administer or which lowers the risk of puncture injuries for healthcare professionals and the health risks for the patients (for example due to contamination or incorrect dosages) by preventing additional processing steps. During the course of the invention, it was surprisingly shown that fosfomycin is much more stable in an aqueous solution than what is commonly assumed. The invention therefore provides a closed container which contains an aqueous solution for parenteral administration, wherein at least one pharmaceutically acceptable salt of fosfomycin, in particular fosfomycin disodium salt, and a pharmaceutically acceptable acid, in particular succinic acid, are dissolved in the solution. Preferred containers are breakable ampoules made of plastic or glass, puncturable vials, infusion bags, or syringes ready for injection.
Owner:GEORGOPOULOS APOSTOLOS +2
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com