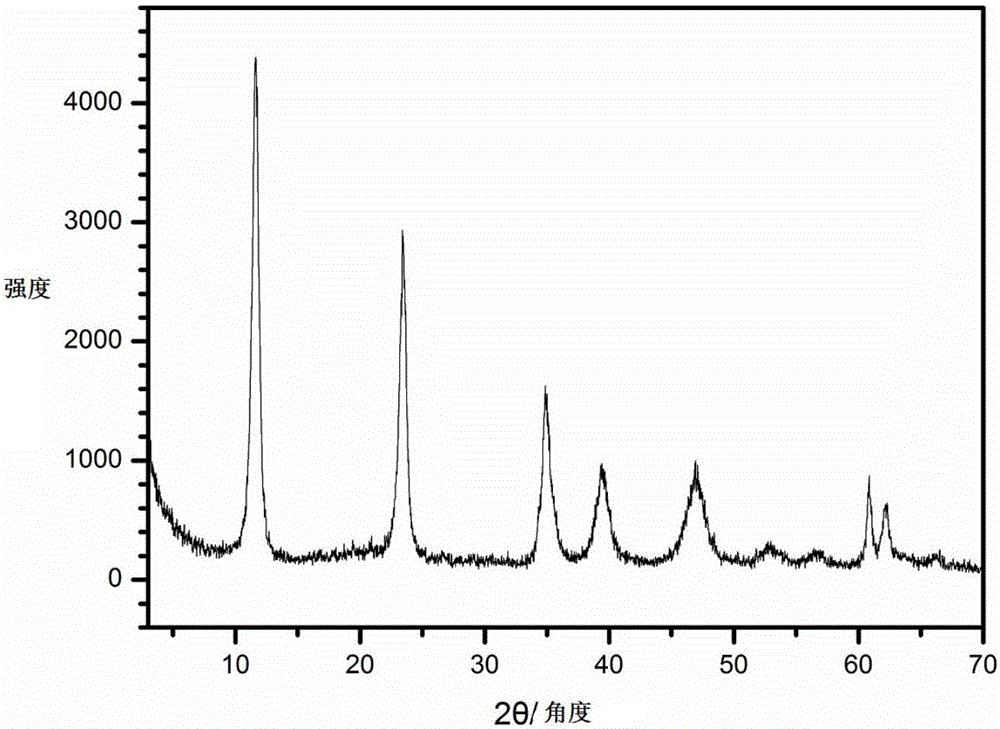
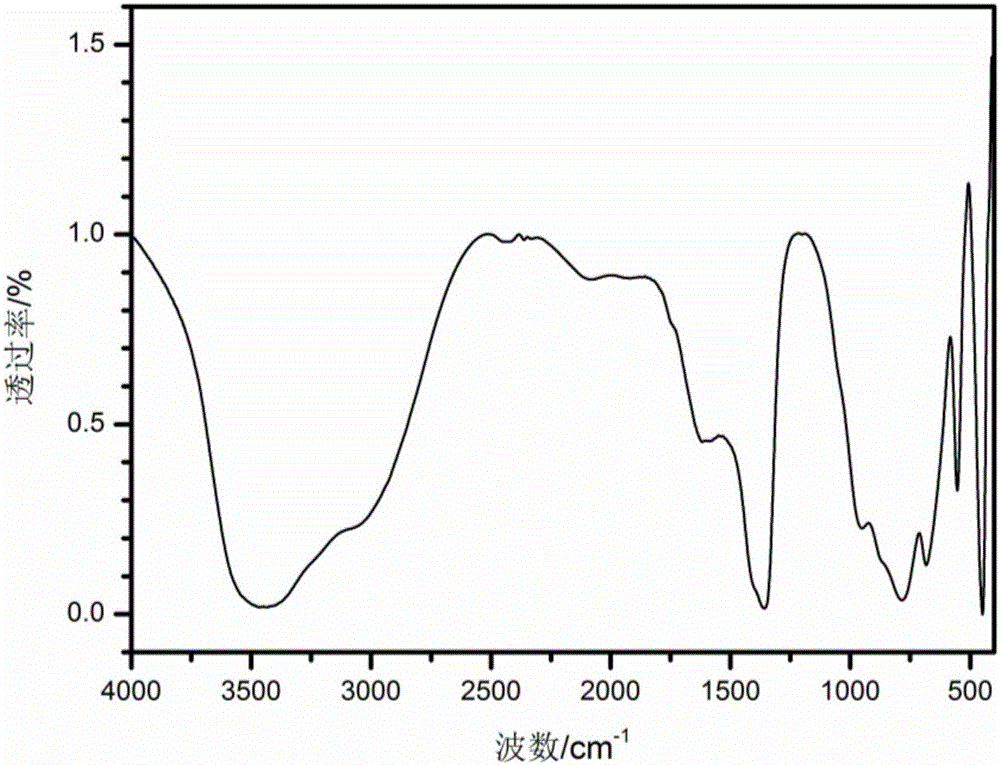
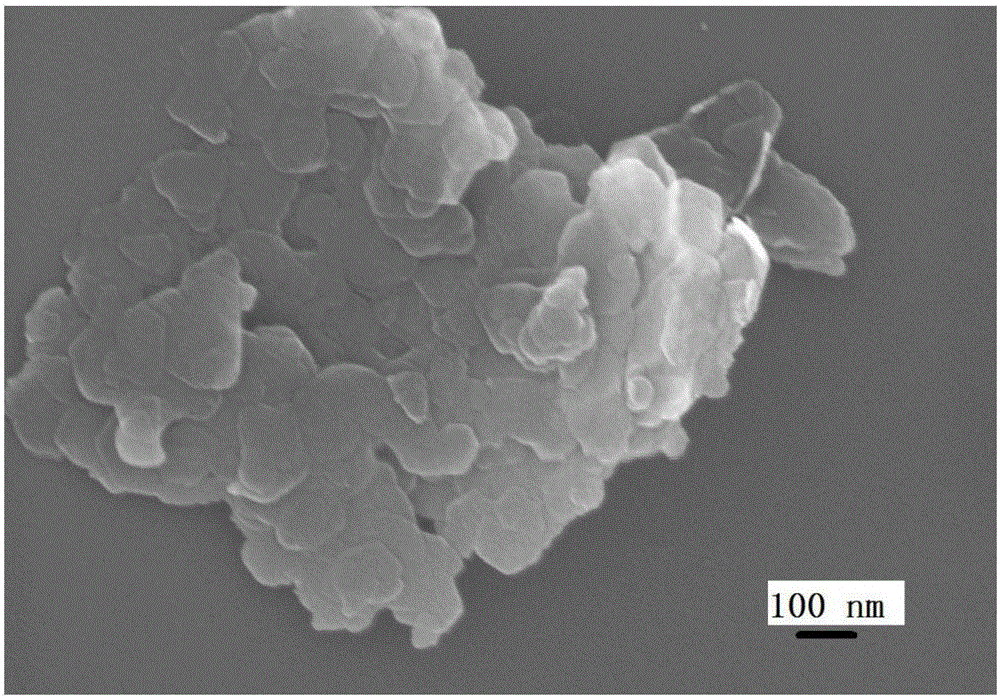
Preparation method of LDHs (magnesium-based layered double hydroxides)
A composite hydroxide and magnesium hydroxide technology, which is applied in chemical instruments and methods, aluminum compounds, zinc compounds, etc., can solve the problems of high energy consumption, high reaction temperature, and long heating time, and achieve simple preparation process and high reaction efficiency. Mild Conditions, Inexpensive Effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Step A: Weigh 3.36g of calcium oxide and add it into 50g of deionized water, grind it in a ball mill for 5 minutes, and prepare A slurry.
[0026] Step B: Weigh 15.384gMgCl 2 . 6H 2 O was added to 100ml deionized water to prepare a solution, and the MgCl 2 The solution was added to the A slurry within 10 minutes, and then continued to stir and react for 2 hours. After the reaction, the magnesium hydroxide precipitate was filtered and washed until the filtrate contained no calcium ions.
[0027] Step C: take by weighing 7.5gAl(NO 3 ) 3 . 9H 2 O was added to 100ml deionized water to prepare C solution.
[0028] Step D: the magnesium hydroxide filter cake that step B obtains is formulated into the slurry that solid content is 5%, weighs 1.92g (NH 4 ) 2 CO 3 Prepare a 50ml solution and add it to the magnesium hydroxide slurry, stir evenly to obtain D slurry.
[0029] Step E: Add C solution dropwise to D slurry under stirring state, and the dropwise addition is co...
Embodiment 2
[0031] Step A, B: same as embodiment 1.
[0032] Step C: take by weighing 2.975gZn (NO 3 ) 2 . 6H 2 O and 7.5gAl(NO 3 ) 3 . 9H 2 O was added to 50ml deionized water to prepare C solution.
[0033] Step D: the magnesium hydroxide filter cake that step B obtains is formulated into the slurry that solid content is 8%, weighs 1.44g (NH 4 ) 2 CO 3 Prepare a 50ml solution and add it to the magnesium hydroxide slurry, stir evenly to obtain D slurry.
[0034] Step E: Add C solution dropwise to D slurry under agitation, and the dropwise addition is completed in 90 minutes, then heat the reaction system to 95°C and continue to stir and react for 5 hours. After the reaction, filter and wash the slurry 4 times to obtain composite hydroxide The product filter cake was dried in an oven at 100°C for 12 hours to obtain the LDH product. Elemental analysis shows that the chemical composition formula of the product is: Mg 0.5 Zn 0.167 al 0.333 (OH) 2 (CO 3 ) 0.167 0.6H 2 O.
Embodiment 3
[0036] Step A, B: same as embodiment 1.
[0037] Step C: take by weighing 5.95gZn (NO 3 ) 2 . 6H 2 O and 7.5gAl(NO 3 ) 3 . 9H 2 O was added to 150ml deionized water to prepare C solution.
[0038] Step D: the magnesium hydroxide filter cake that step B obtains is mixed with the slurry that solid content is 10%, weighs 1.85g (NH 4 ) 2 CO 3 After preparing 80ml solution, add it to the magnesium hydroxide slurry, stir evenly to obtain D slurry.
[0039] Step E: Add C solution dropwise to D slurry under stirring state, and the dropwise addition is completed in 30 minutes, then heat the reaction system to 80°C and continue to stir and react for 8 hours. After the reaction is completed, filter and wash the slurry 4 times to obtain composite hydroxide The product filter cake was dried in an oven at 100°C for 12 hours to obtain the LDH product. Elemental analysis shows that the chemical composition formula of the product is: Mg 0.333 Zn 0.333 al 0.333 (OH) 2 (CO 3 ) ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com