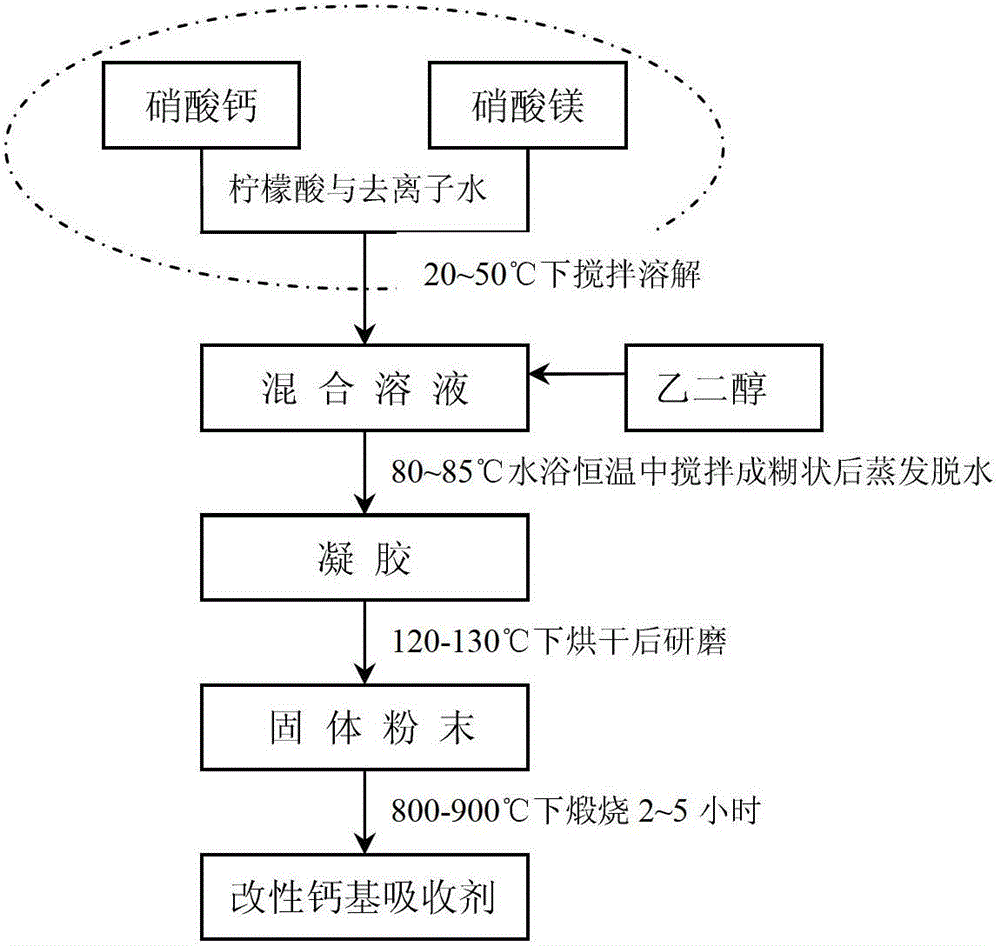
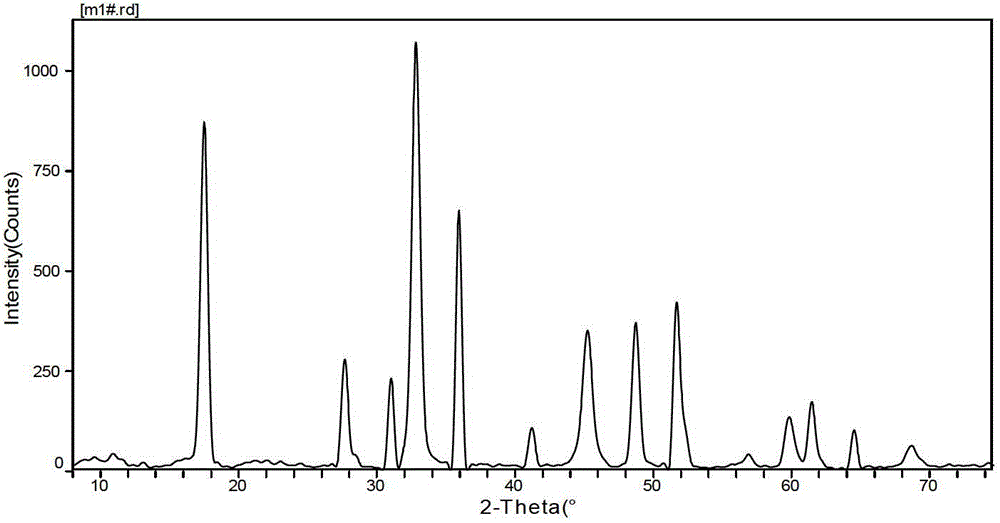
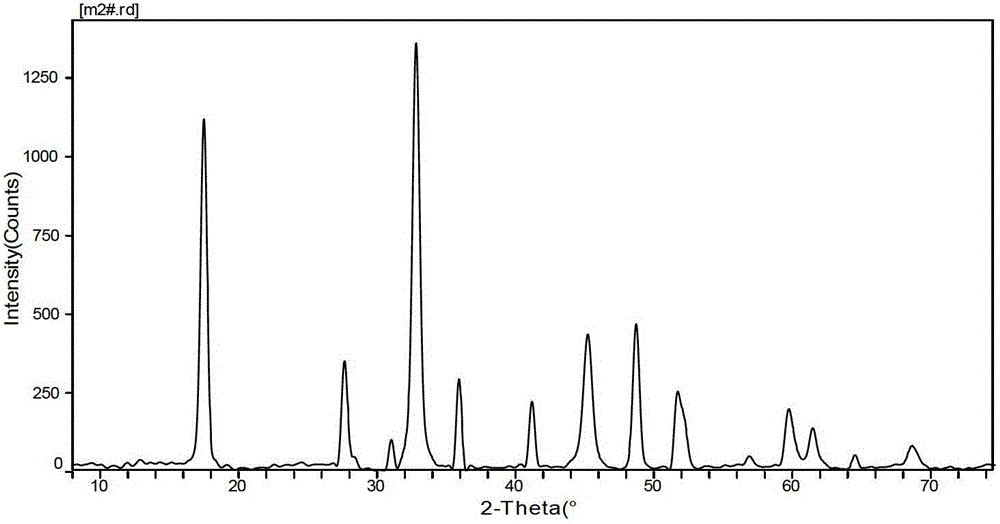
Preparation method of modified carbon dioxide calcium-based absorbent
A calcium-based absorbent and carbon dioxide technology, applied in the field of environmental emission control, can solve the problems of reduced carbonation conversion rate, porosity, and surface area, and achieve strong anti-sintering performance, good pore structure distribution, and high cycle absorption efficiency effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Weigh an appropriate amount of calcium nitrate and magnesium nitrate according to the molar ratio of calcium metal ion and magnesium metal ion as 10: 0.5 (converted into the corresponding CaO and MgO mass according to the molar ratio of calcium and magnesium ions, and then converted into the content of MgO, such as this example The calcium-magnesium molar ratio of 10:0.5 in the formula can be converted into 560g of CaO and 20g of MgO, then the content of MgO in this example is 20 / (560+20)=3.5%), according to calcium-magnesium metal ions, citric acid The molar ratio to ethylene glycol is 0.8:1:1.5 Weigh an appropriate amount of citric acid and ethylene glycol, dissolve the weighed calcium nitrate, magnesium nitrate and citric acid in deionized water, and stir at 20°C until completely Dissolve to form a mixed solution, add the weighed ethylene glycol into the mixed solution, and stir in a constant temperature water bath at 80°C until it becomes a paste, then evaporate and ...
Embodiment 2
[0029] Take by weighing an appropriate amount of calcium nitrate and magnesium nitrate according to the molar ratio of calcium metal ion and magnesium metal ion as 10:0.7, and take an appropriate amount of lemon by weighing the molar ratio of calcium magnesium ion, citric acid and ethylene glycol as 0.9:1:1.5 Acid and ethylene glycol, dissolve the weighed calcium nitrate, magnesium nitrate and citric acid in deionized water, stir at 30°C until completely dissolved to form a mixed solution, add the weighed ethylene glycol into the mixed solution , and stirred in a constant temperature water bath at 80°C until it became a paste, then evaporated and dehydrated to form a gel, put the formed gel into an oven at 120°C for drying, dry and grind to obtain a solid powder, and then calcined at 850°C for 4 hours to prepare A secondary modified carbon dioxide calcium-based absorbent is obtained.
Embodiment 3
[0031] Take by weighing an appropriate amount of calcium nitrate and magnesium nitrate by calcium metal ion and magnesium metal ion molar ratio is 10: 1.6, by weighing an appropriate amount of lemon acid and ethylene glycol, dissolve the weighed calcium nitrate, magnesium nitrate and citric acid in deionized water, stir at 50°C until completely dissolved to form a mixed solution, add the weighed ethylene glycol into the mixed solution , and stirred in a constant temperature water bath at 80°C until it became a paste, evaporated and dehydrated to form a gel, put the formed gel in an oven at 125°C for drying, dry and grind to obtain a solid powder, and then calcined at 900°C for 5 hours to prepare A secondary modified carbon dioxide calcium-based absorbent is obtained.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com