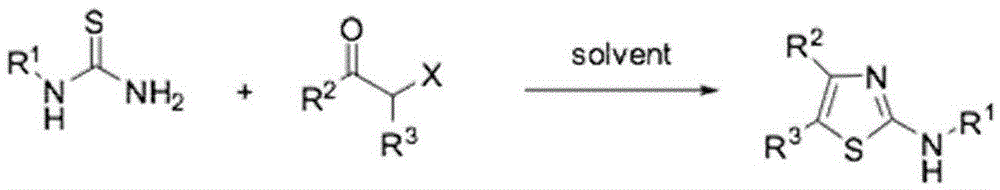
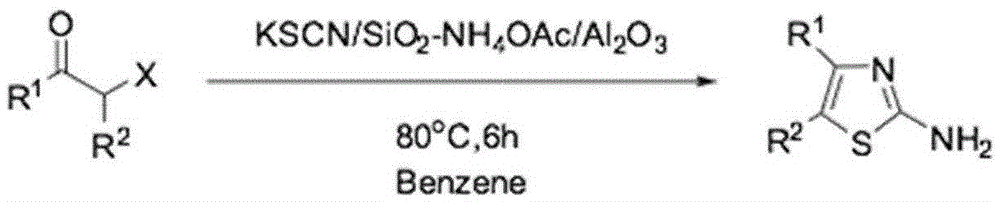
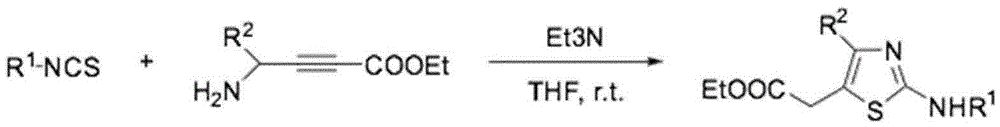Preparation method of 4,5-disubstituted-2-substituted aminothiazole compound
A technology for aminothiazoles and compounds, which is applied in the field of compound synthesis, can solve the problems of harsh reaction conditions, cumbersome operations, and poor applicability of different functional groups, and achieve the effects of mild reaction conditions, less environmental pollution, and easy-to-operate steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0056] Embodiment 1, 4-methyl-5-phenyl-2-aminothiazole (m1)
[0057] Add 179.1 mg (1 mmol) of 2-methyl-2-nitro-3-phenyloxirane and 152.2 mg (2 mmol) of thiourea into the reaction flask, and then add K 2 CO 3 212.0mg (2mmol), methanol 5.0ml, after the addition was complete, the reaction was stirred at room temperature (25°C) for 6-8 hours, and the reaction was detected by TLC (petroleum ether: ethyl acetate = 20:1 volume ratio). When the TLC detection reaction result is that 2-methyl-2-nitro-3-phenyloxirane disappears, it means that the reaction has ended.
[0058] After the reaction was finished, the solvent was evaporated by a rotary evaporator, 60ml of water was added, extracted three times with 3×20mL ethyl acetate, the organic layer (located on the upper layer) was combined and washed three times with 3×20mL saturated brine, and then washed with anhydrous sodium sulfate ( 2.0 g) was dried for 30 minutes, and concentrated by a rotary evaporator until there was basically n...
Embodiment 2
[0084] Embodiment 2, 4-ethyl-5-phenyl-2-aminothiazole (m2)
[0085] Replace 2-methyl-2-nitro-3-phenyloxirane with 2-ethyl-2-nitro-3-phenyloxirane, the molar weight is constant, and the rest are equal to Example 1 . 180.9 mg of 4-ethyl-5-phenyl-2-aminothiazole was obtained as a white powder, with a yield of 88.7%.
[0086] Its structural formula is:
[0087]
[0088] Whitepowder; mp: 128.3~129.0℃; 1 HNMR (500MHz, CDCl 3 )δ7.41–7.31(m,4H),7.28(dd,J=10.5,3.9Hz,1H),5.12(s,2H),2.62(q,J=7.5Hz,2H),1.25(t,J =7.5Hz,3H). 13 CNMR (126MHz, CDCl 3 )δ165.51(s),149.39(s),132.68(s),129.05(s),128.58(s),126.99(s),120.93(s),77.29(s),77.04(s),76.78( s),22.94(s),14.19(s).HRMS(ESI):m / zcalcdforC 11 h 13 N 2 S[M+H] + :205.0794,found:205.0797.
Embodiment 3
[0089] Example 3, 4-methyl-5-(4-fluoro-phenyl)-2-aminothiazole (m3)
[0090] Replace 2-methyl-2-nitro-3-phenyloxirane with 2-methyl-2-nitro-3-(4-fluoro-phenyl)-oxirane, the molar weight remains unchanged , all the other are with embodiment 1. 199.1 mg of 4-methyl-5-(4-fluoro-phenyl)-2-aminothiazole was obtained as a white powder product, with a yield of 95.7%.
[0091] Its structural formula is:
[0092]
[0093] Whitepowder; mp: 165.5~167.0℃; 1 HNMR (500MHz, CDCl 3 )δ7.35–7.28(m,2H),7.10–7.02(m,2H),5.18(s,2H),2.27–2.25(m,3H). 13 CNMR (126MHz, CDCl 3 ( s),77.30(s),77.04(s),76.79(s),15.85(s).HRMS(ESI):m / zcalcdforC 10 h 10 FN 2 S[M+H] + :209.0543,found:209.0544.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com