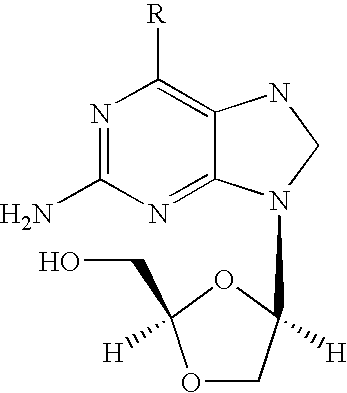
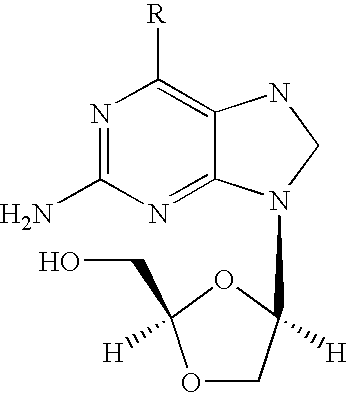
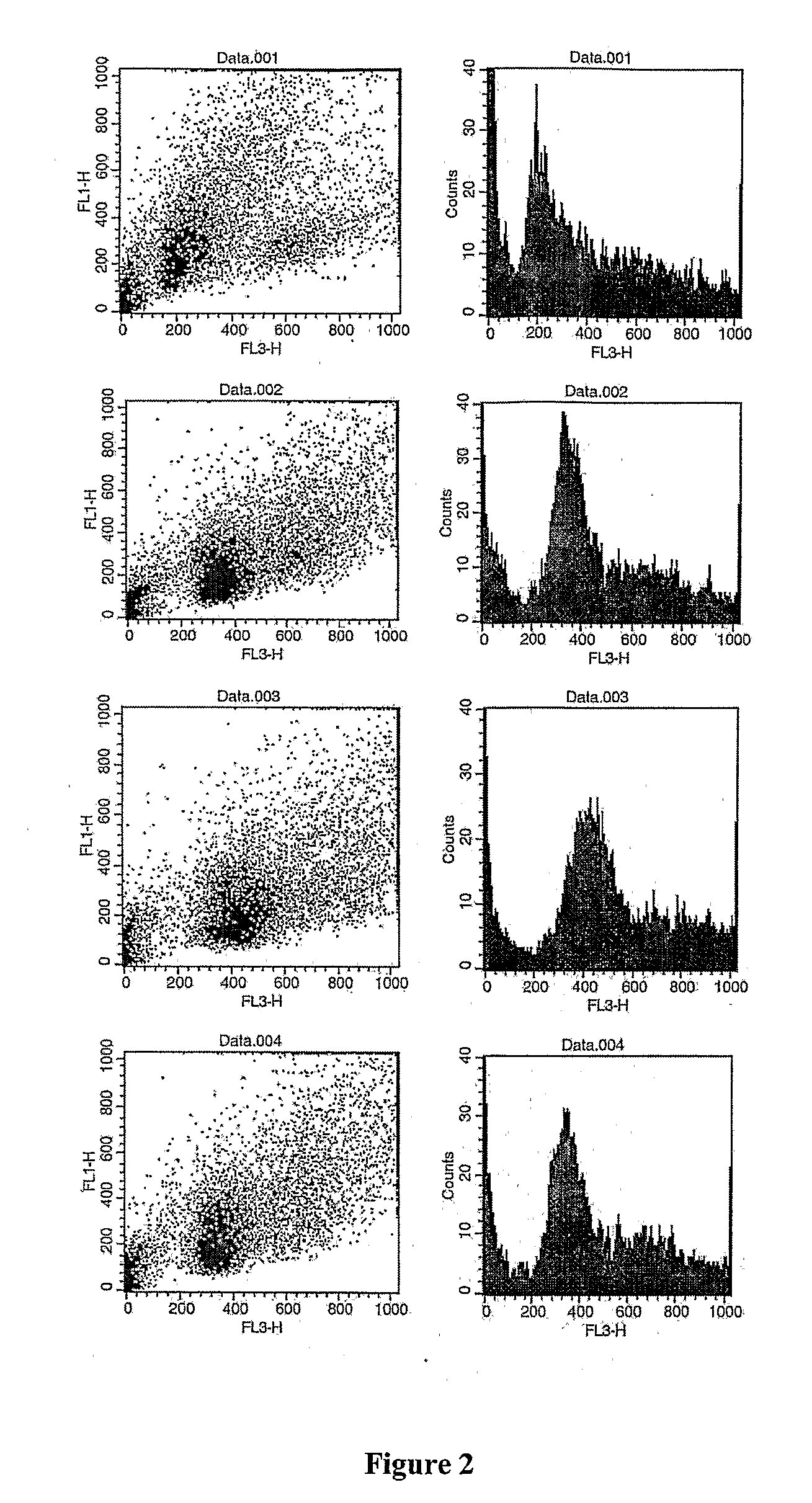
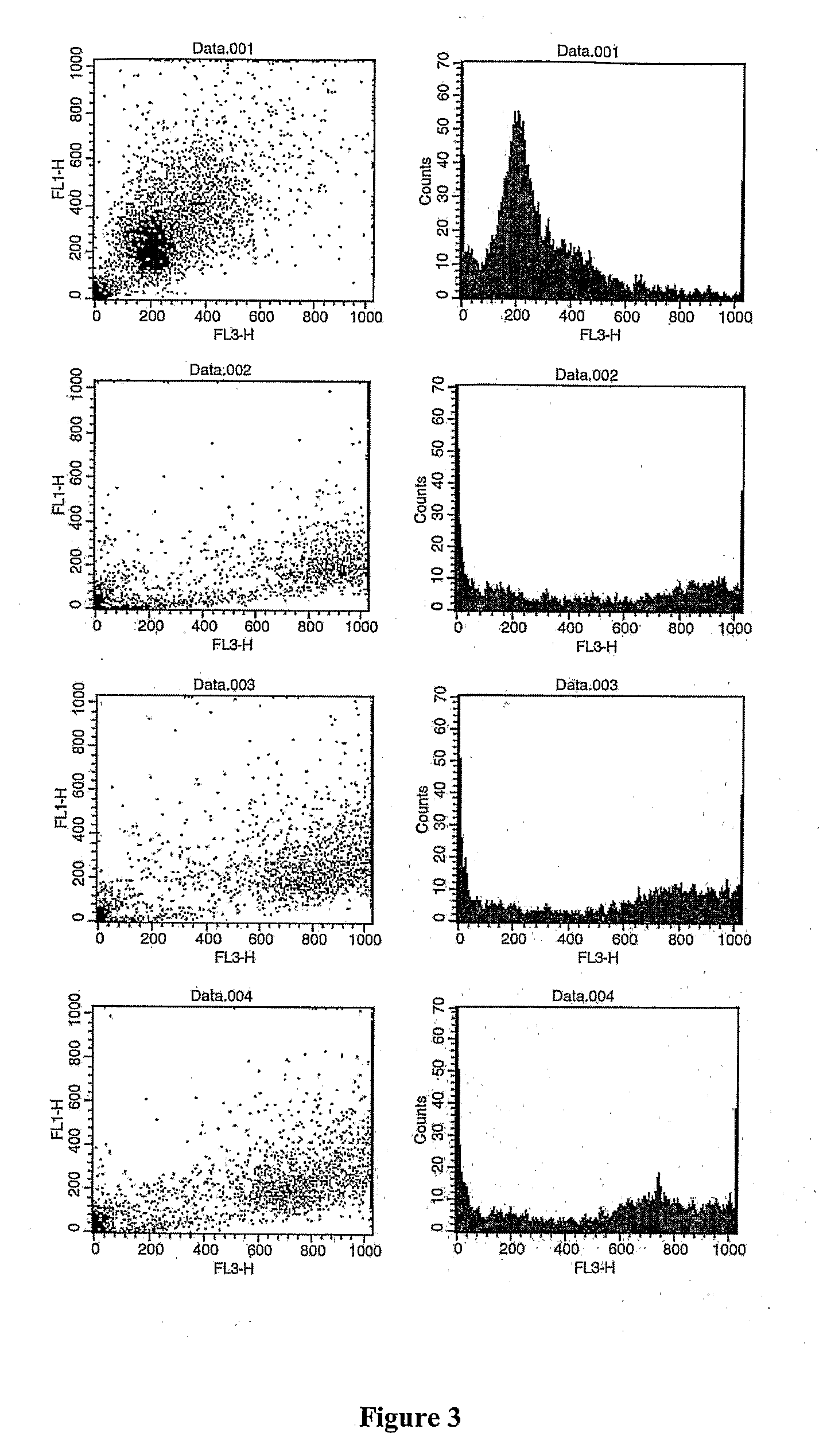
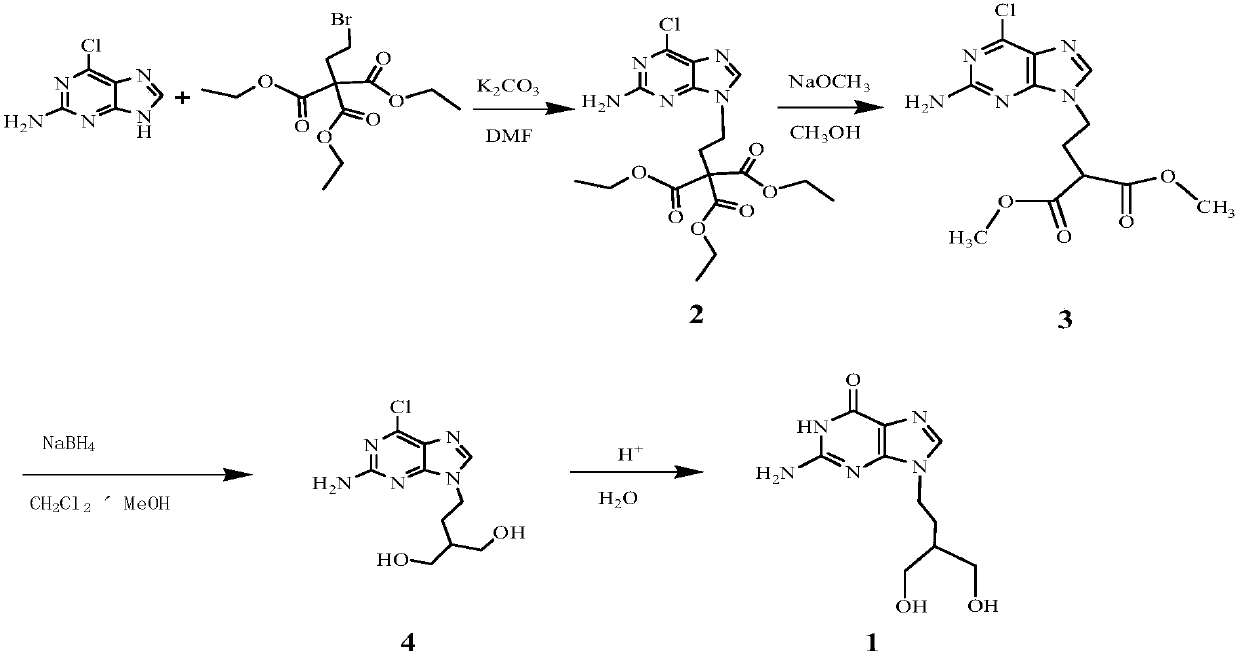
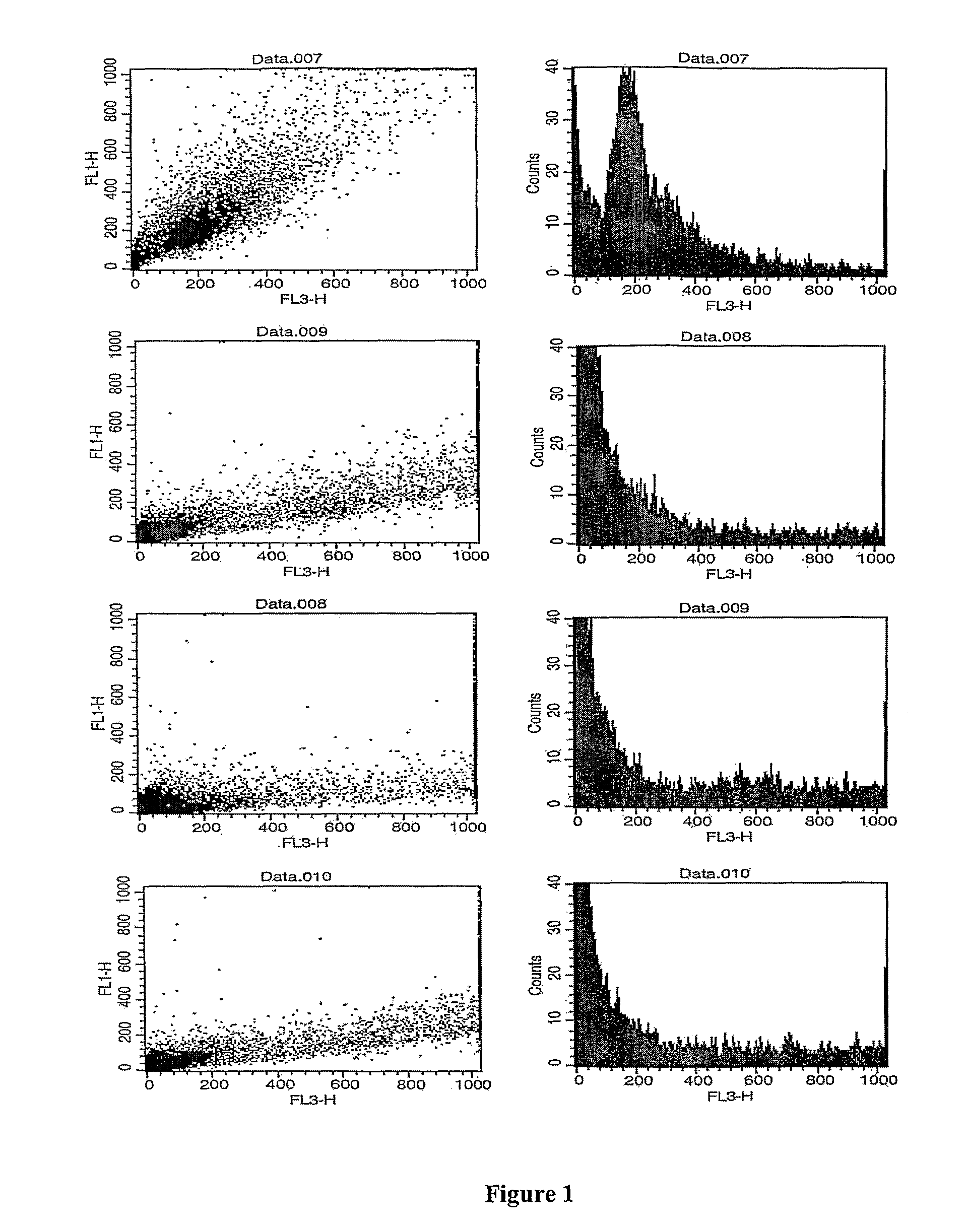
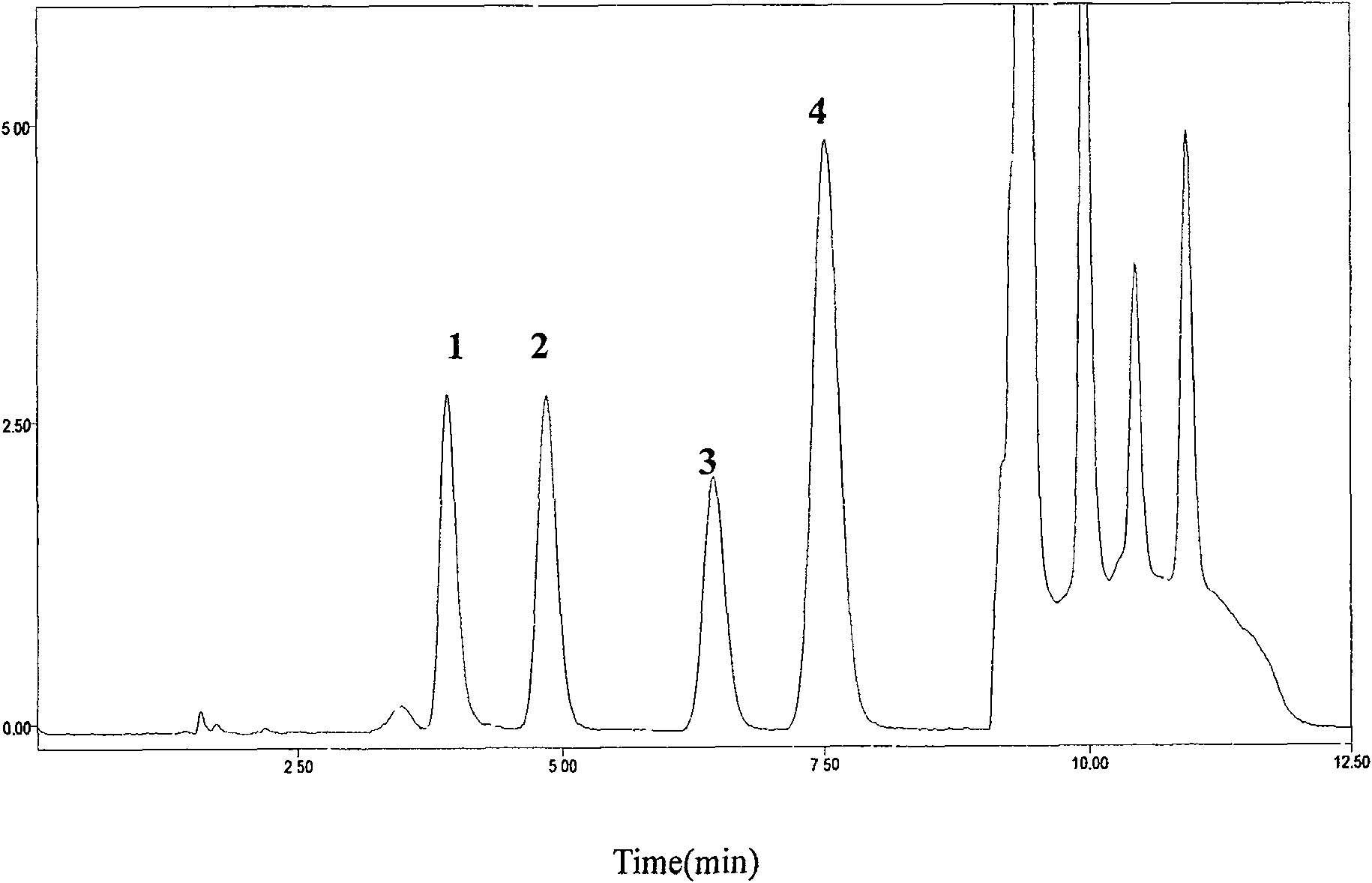
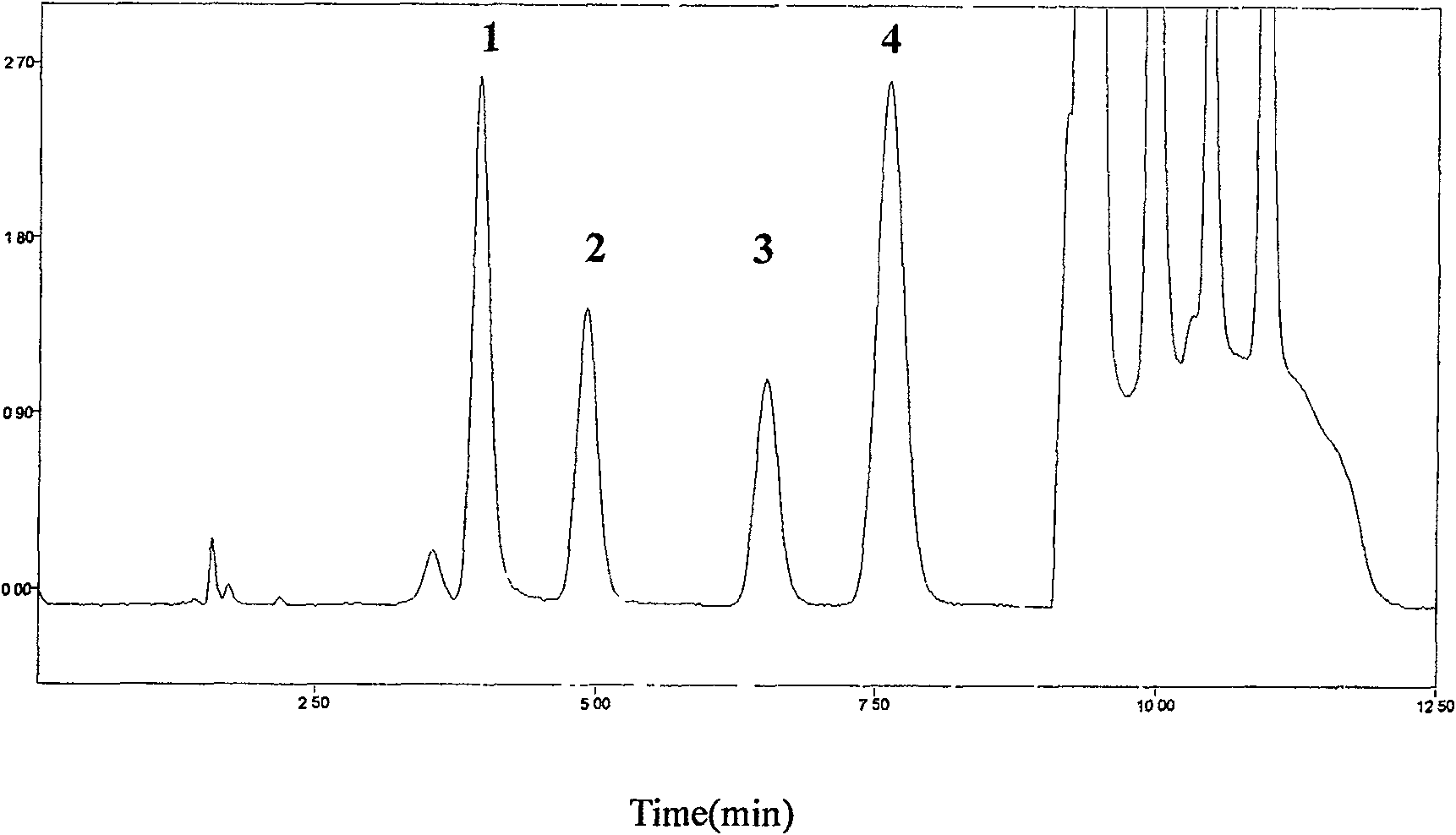
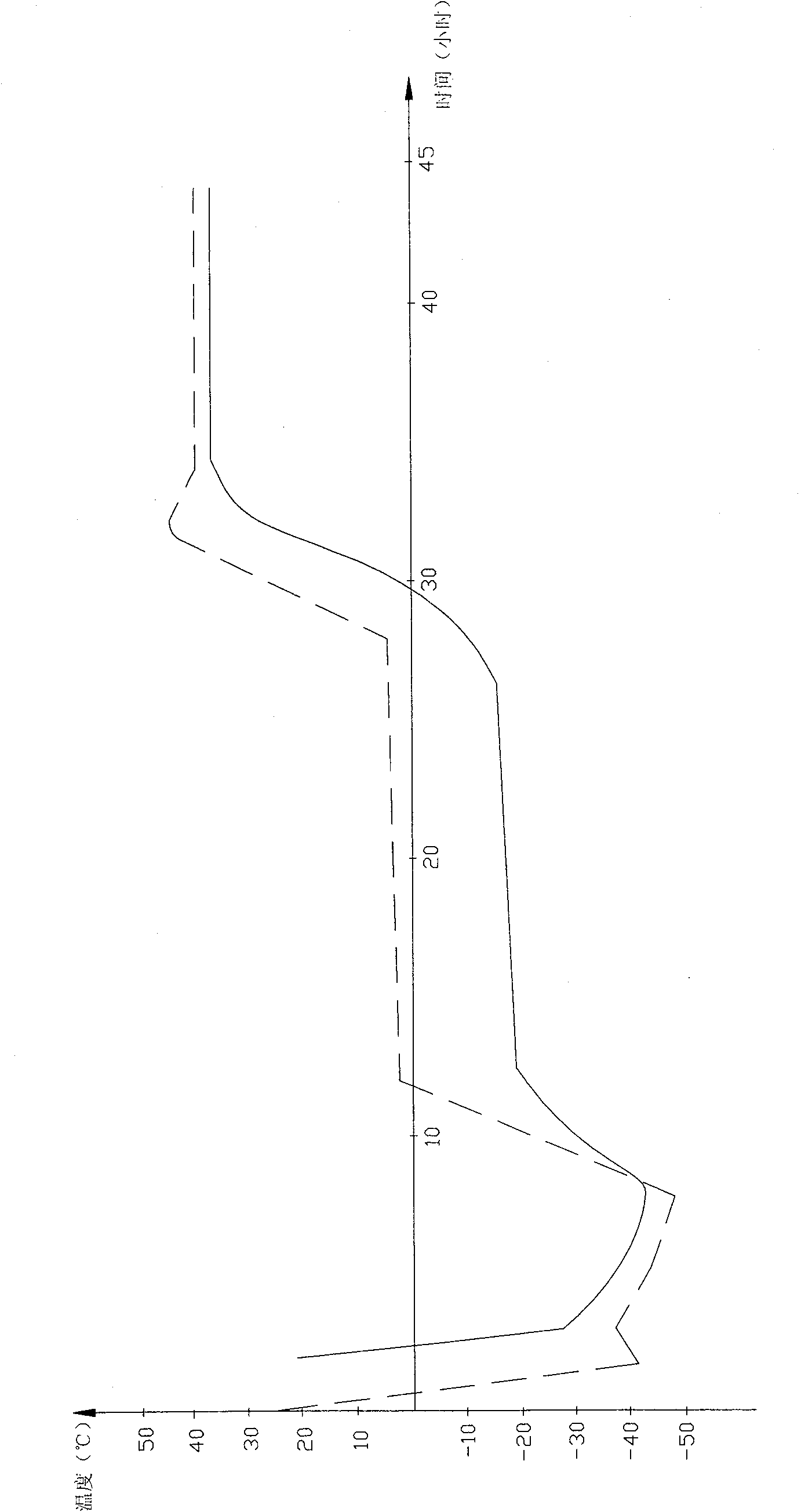
Patents
Literature
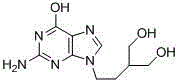
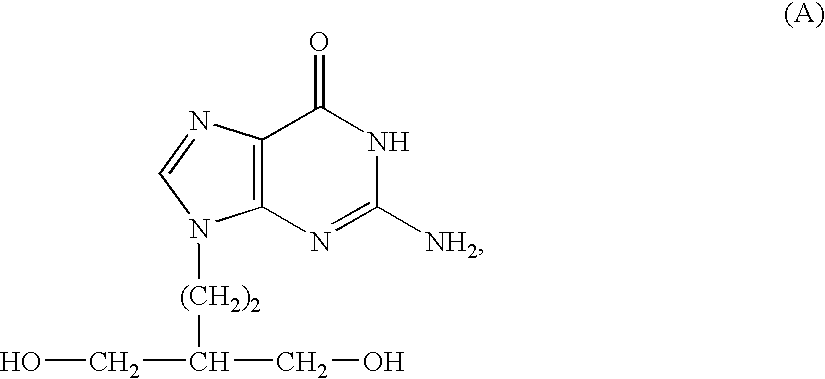
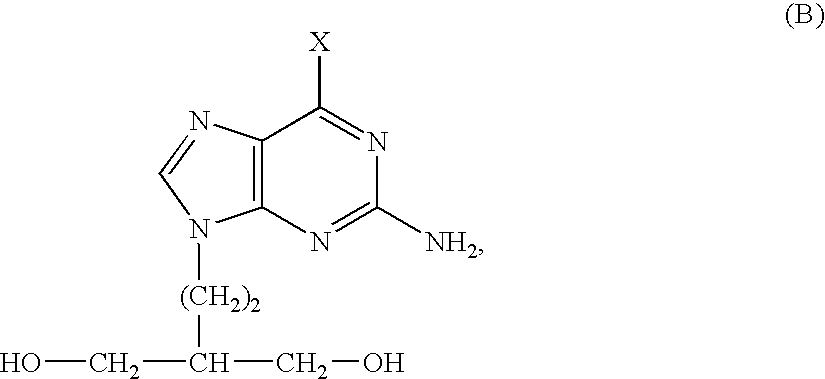
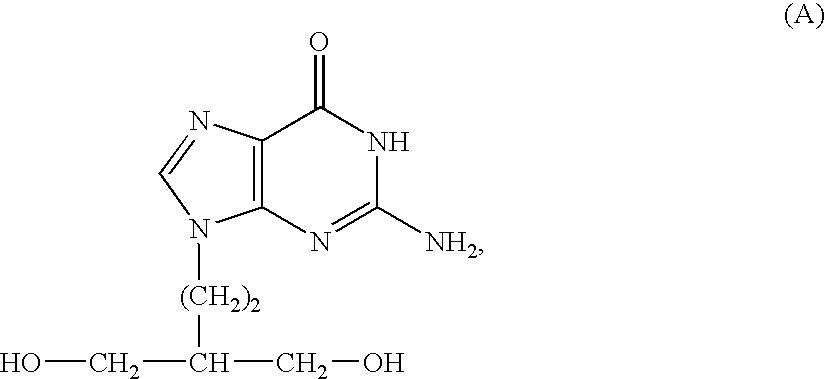
50 results about "Penciclovir" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
This medication is used to treat "cold sores/fever blisters" (herpes labialis).
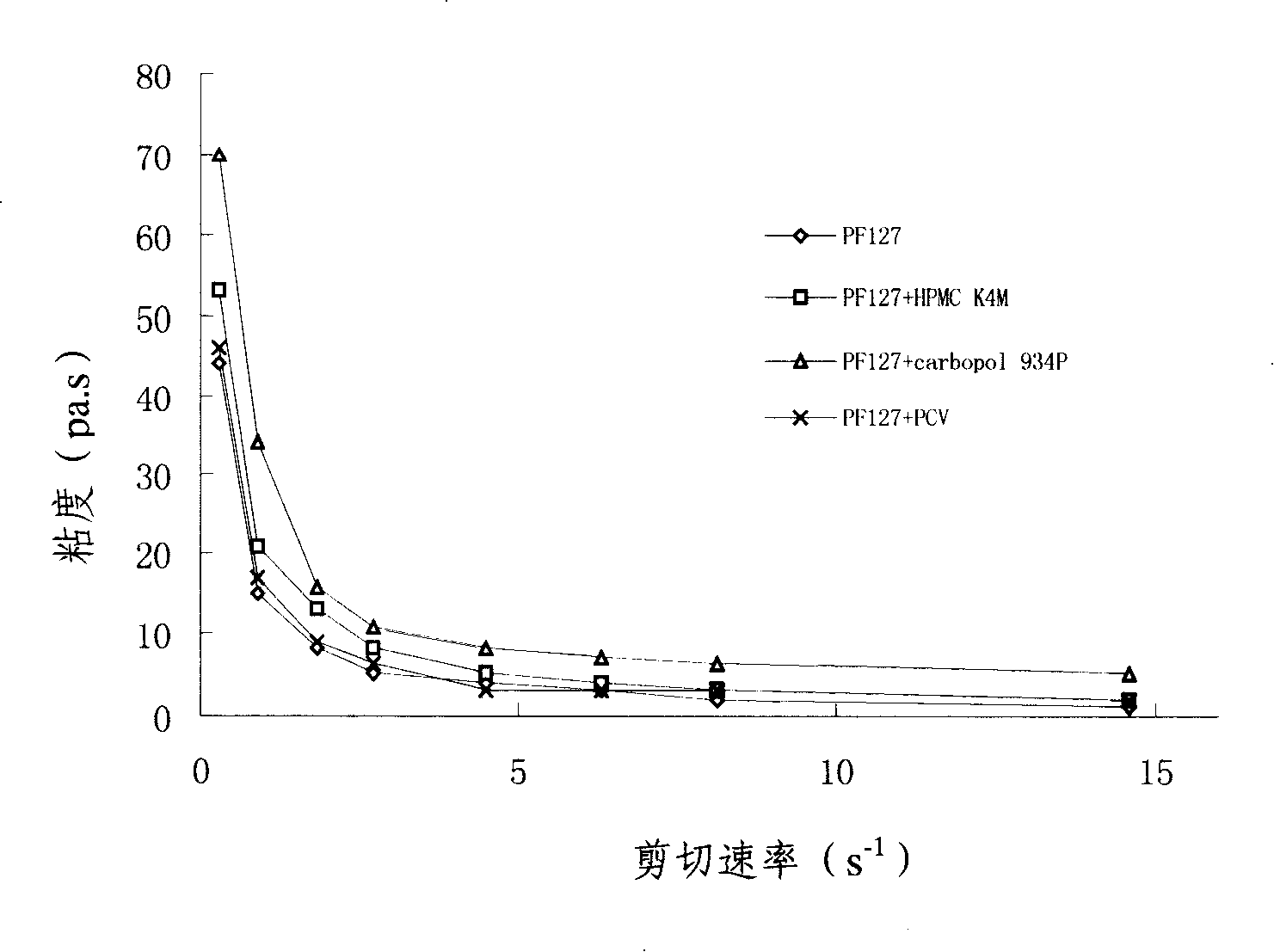
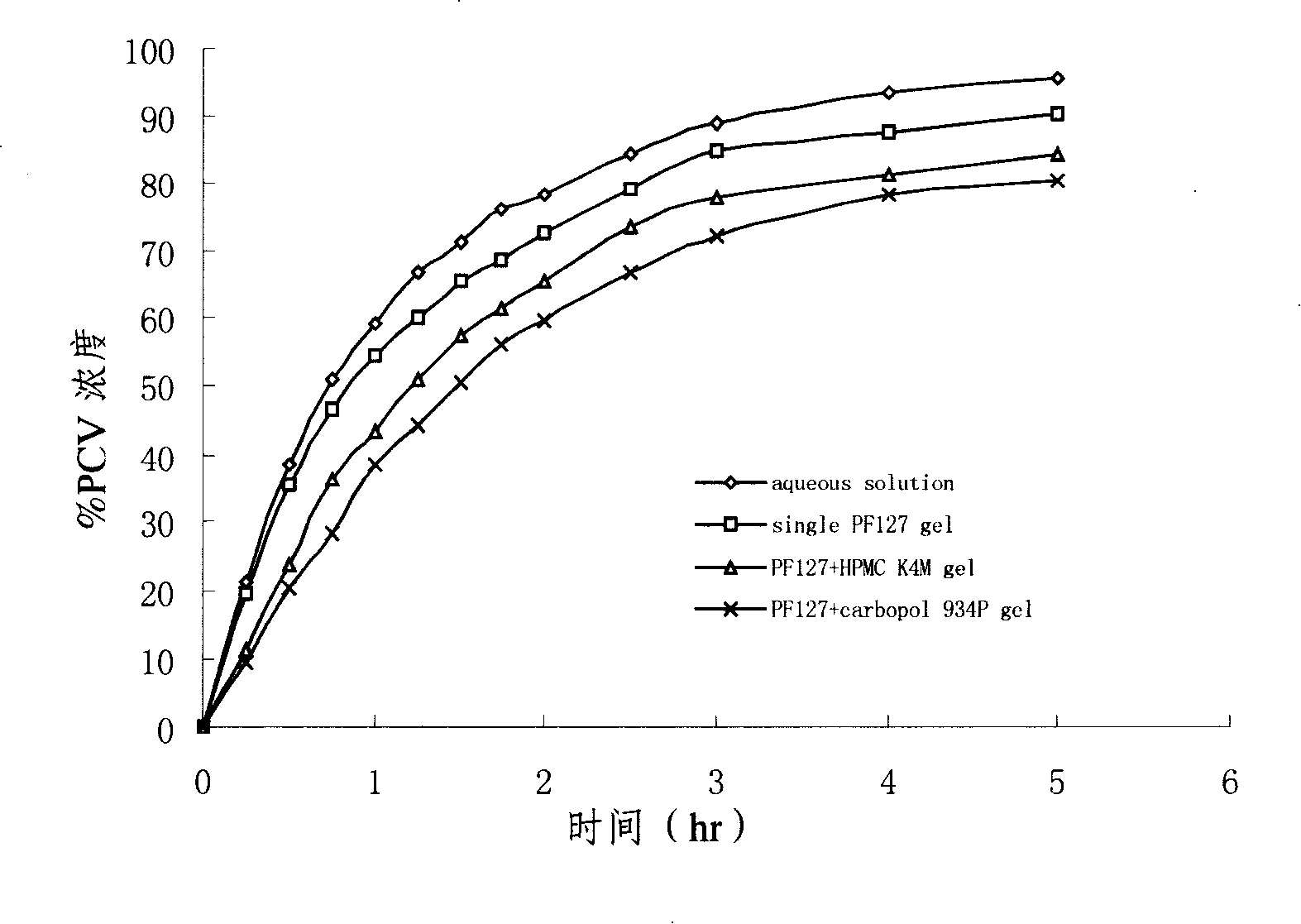
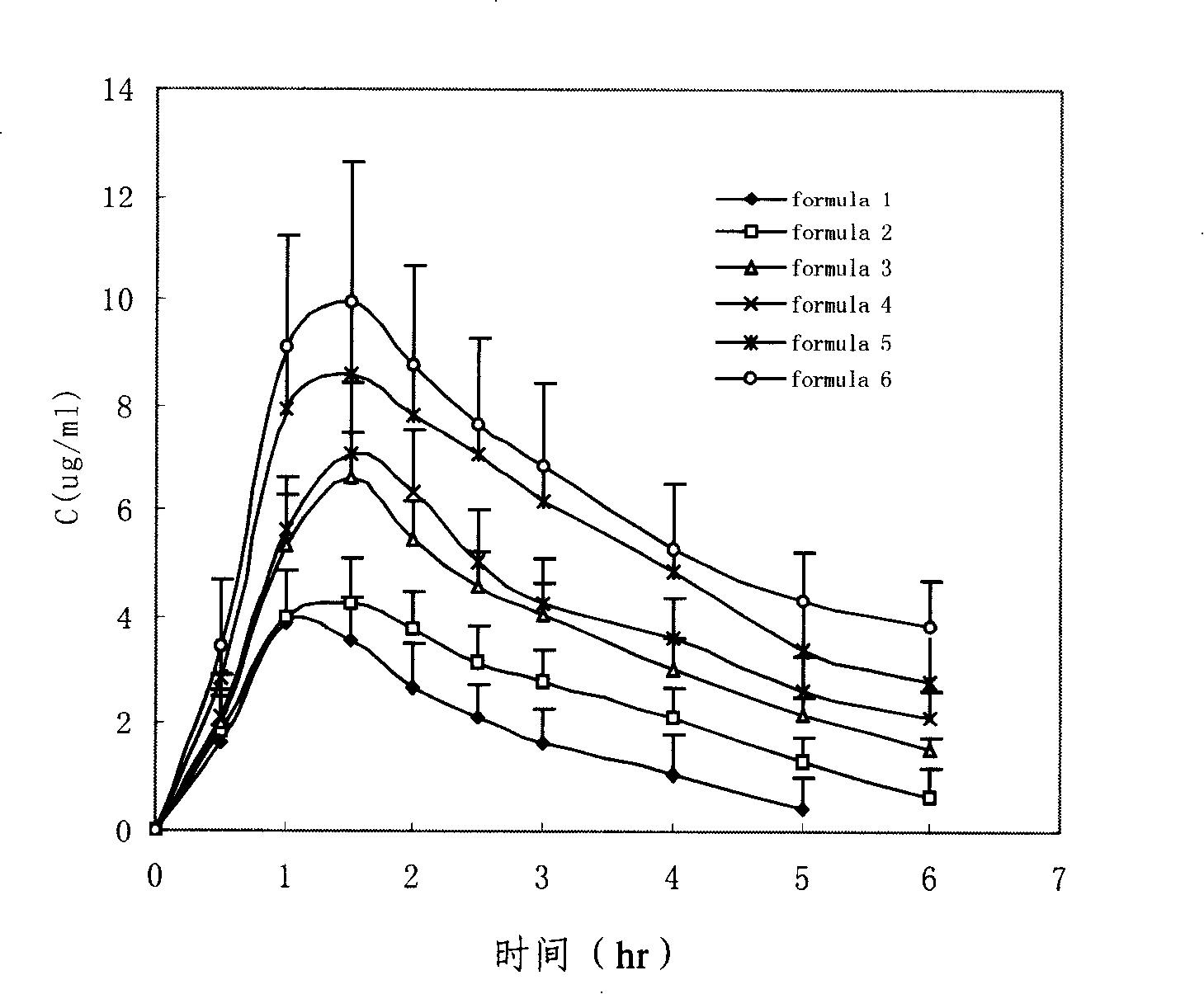
Penciclovir ophthalmic temperature sensitivity in situ gel preparation and preparation method thereof
InactiveCN101185650AGood biocompatibilityMedication convenienceSenses disorderPharmaceutical delivery mechanismRetention timeBiocompatibility Testing
The invention provides an in situ eye gel preparation of penciclovir. The compositions and weight percentages thereof are 0.01-2.0wt% of penciclovir, 5-40wt% of poloxamer, 0.1-5wt% of tackifier high polymer base, 0.8-10wt% of osmotic regulation agent, 0.001-0.5wt% of antiseptics and the rest is distilled water. Meanwhile, the preparation method is also provided. The in situ gel preparation has good biocompatibility and glutinousness; compared with a liquid preparation, the invention can increase retention time of drug in the eyes and delay elimination, thus improving the bioavailability of the eyes and reducing the times of administration; meanwhile, the invention avoids weaknesses that operation is inconvenient during gel administration, the preparation is not easy to spread in eyes, the dosage can not be controlled accurately, etc.
Owner:MEDICINE & BIOENG INST OF CHINESE ACAD OF MEDICAL SCI
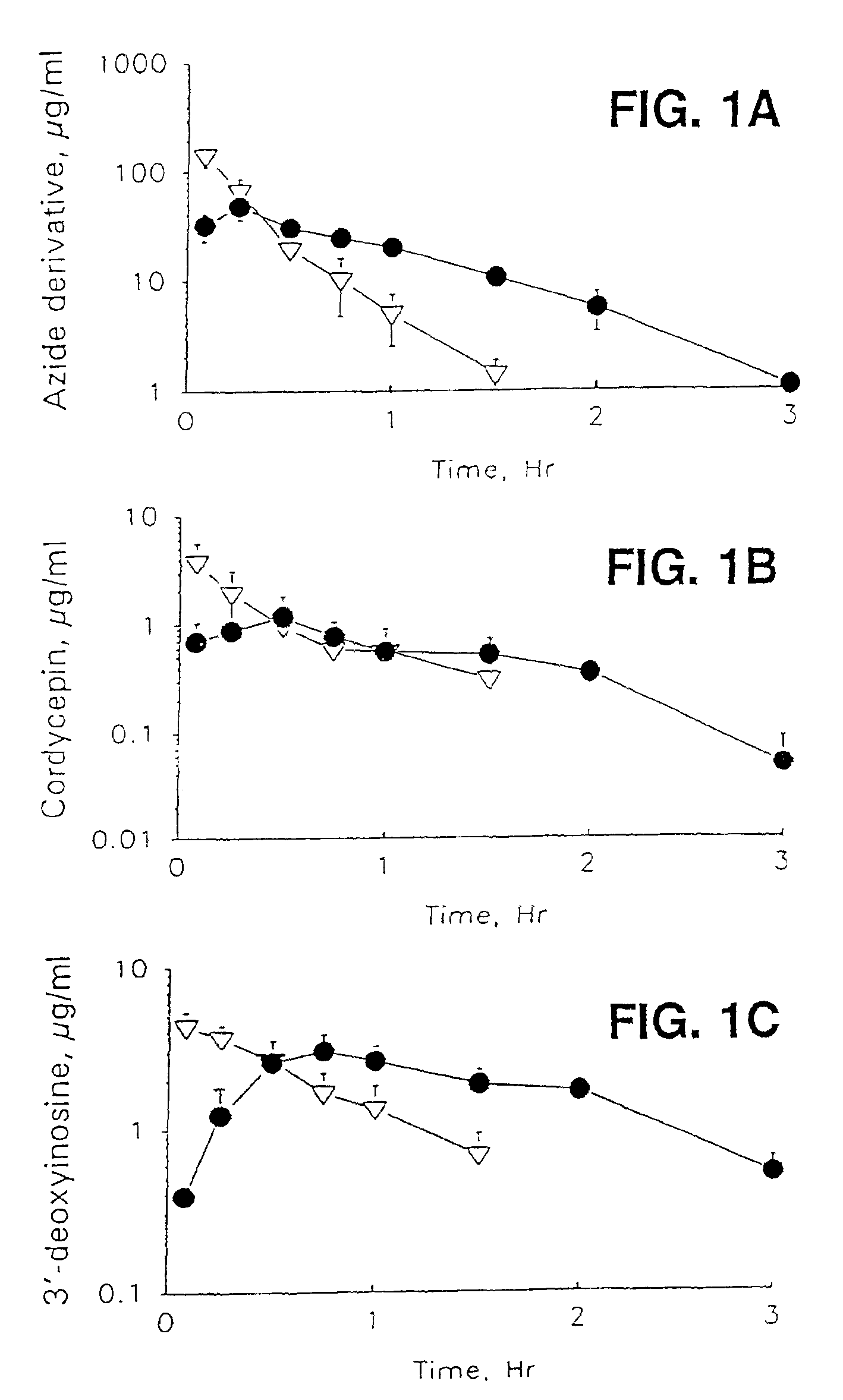
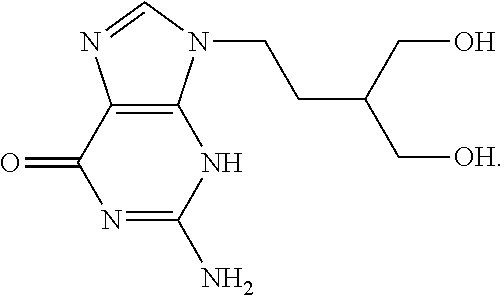
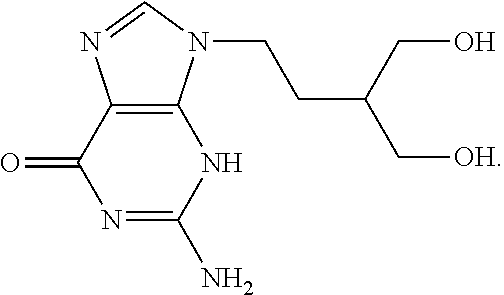
Therapeutic azide compounds
InactiveUS6949521B2Extended half-lifeDestroy effectivenessBiocideSugar derivativesHalf-lifeSide chain
Pharmaceutical prodrug compositions are provided comprising azide derivatives of drugs which are capable of being converted to the drug in vivo. Azide derivatives of drugs having amine, ketone and hydroxy substituents are converted in vivo to the corresponding drugs, increasing the half-life of the drugs. In addition azide prodrugs are often better able to penetrate the blood-brain barrier than the corresponding drugs. Especially useful are azide derivatives of cordycepin, 2′-F-ara-ddI, AraA, acyclovir, penciclovir and related drugs. Useful azide prodrugs are azide derivatives of therapeutic alicyclic amines, ketones, and hydroxy-substituted compounds, including aralkyl, heterocyclic aralkyl, and cyclic aliphatic compounds, where the amine or oxygen moiety is on the ring, or where the amine or oxygen moiety is on an aliphatic side chain, as well as therapeutic purines and pyrimidines, nucleoside analogs and phosphorylated nucleoside analogs.
Owner:UNIV OF GEORGIA RES FOUND INC +1
Anti-infective compositions, methods and systems for treating pathogen-induced disordered tissues
InactiveUS20060135464A1Stimulates rapid immunological attackExtraordinary therapeutic effectBiocidePharmaceutical delivery mechanismCidofovirMetabolite
Compositions, methods and systems for treating disordered epithelial tissues, such as is caused by pathogens and / or by toxins produced thereby. The invention relates to the use of an anti-infective and / or antimicrobial active agent in a carrier, with vigorous agitation of the disordered epithelial tissue for topical treatment thereof under such conditions sufficient to achieve clinically discernable improvement of the disordered epithelial tissue. The preferred anti-infective and / or antimicrobial active agent comprises a nucleoside, such as acyclovir, valcyclovir, penciclovir, famciclovir, ganciclovir, cidofovir, adefovir, and tenofovir, and derivatives, analogs, or metabolites thereof, or a mixture thereof, or 1-docosanol, optionally in combination with an organohalide. The inventive compositions and methods may employ the use of an applicator adapted for use in promoting the penetration of the treatment composition and / or the vigorous agitation of the disordered tissue.
Owner:QUADEX PHARMA
Penciclovir microemulsion gel preparation and preparing method thereof
InactiveCN101229121ANon-irritatingEasy to administerAntiviralsEmulsion deliveryGel preparationOil phase
The invention relates to a penciclovir micro-emulsified gel and preparation process, belonging to medicine technology field, which comprises penciclovir surfactant, assistant surfactant, oil phase, water and carbomer and is used for transdermal drug delivery, the processing method is simple, the quality is easy to be controlled, and the stability of the gel is good, compared with present cream, the unit area accumulation infiltration amount of drugs can be improved obviously, and detained dosage in epidermis and dermis can be improved, moreover the invention has certain sustained-release function so that drug treating times and drug amounts can be reduced, and the using process of the drug is convenient, no pollution is caused to clothes, so the medicine is suitable for clinical wide application.
Owner:SHANDONG UNIV
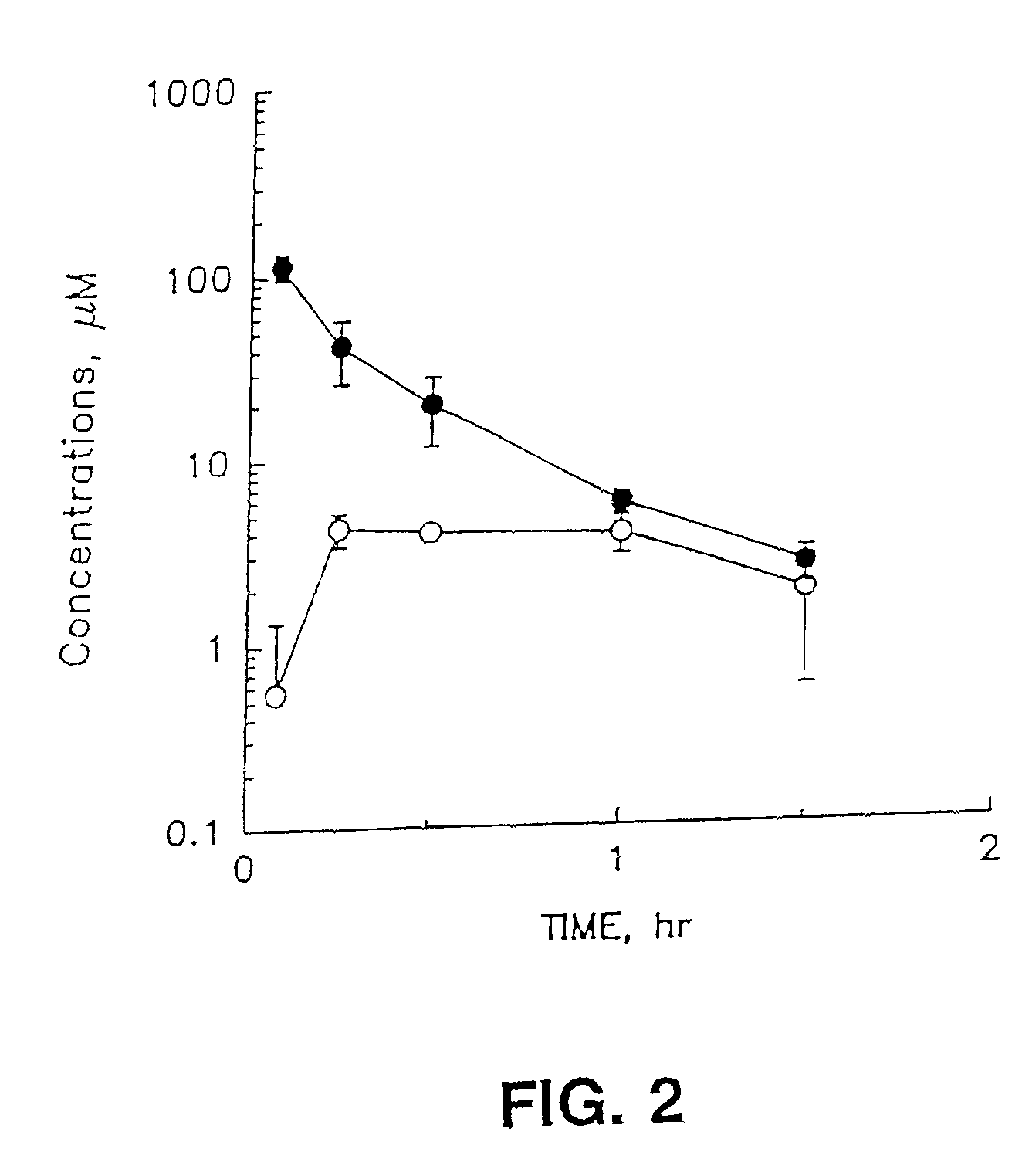
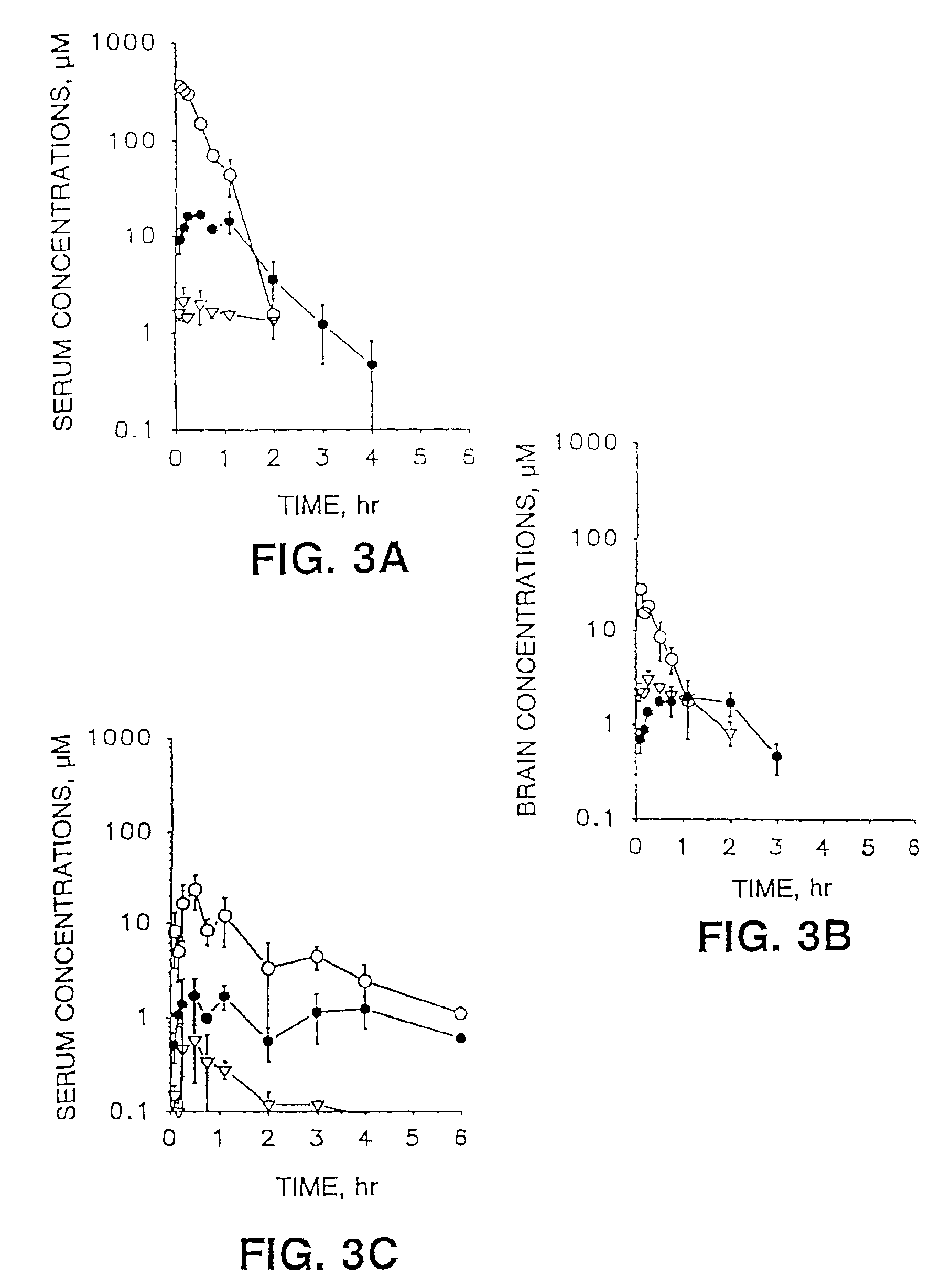
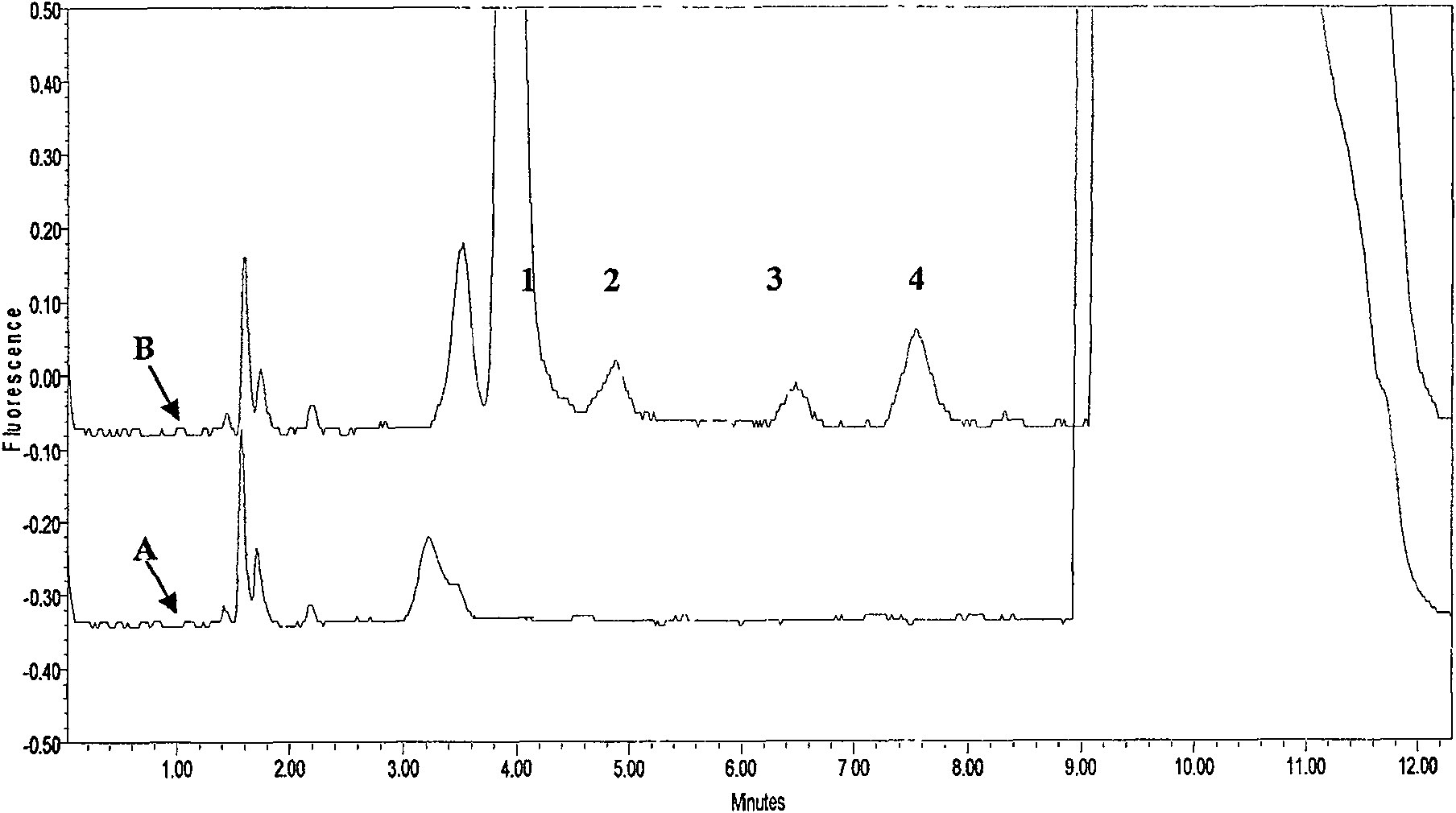
Method for determining human plasma antiviral drug concentration
InactiveCN101105478ASimple and fast operationEasy to operateComponent separationFluorescence/phosphorescenceAntiviral drugMedicine
The invention belongs to medical detection field, relates to an analysis detection method of drug in the body of a person, and specifically relates to the method that the densities of antiviral drugs in blood plasma of the person such as acyclovir, ganciclovir and penciclovir can be detected at the same time. The method in the invention is characterized in that pilot sample is pretreated; as acyclovir, ganciclovir and penciclovir have the character of strong fluorescence absorption, acyclovir, ganciclovir and penciclovir can be separated from each other in an acidity flowing phase chromatographic column and be detected by a fluorescence detector. The method in the invention has the advantages of little sample, simple, swift and sensitive pretreatment, short analysis period and low cost; furthermore, the invention doesn't need expensive equipment and reagent and is suitable for the detection of clinical conventional blood drug density of acyclovir, ganciclovir and penciclovir.
Owner:AFFILIATED HUSN HOSPITAL OF FUDAN UNIV
Therapeutic composition to treat lesions caused by herpes simplex virus
InactiveUS20110065655A1Shorten the construction periodAvoid problemsBiocidePharmaceutical delivery mechanismPenciclovirBULK ACTIVE INGREDIENT
The present invention is generally directed toward therapeutic compositions for treating infections caused by Herpes Simplex Virus (“HSV”). The therapeutic compositions meet a long felt need in the art of providing a treatment for lesions that result from HSV that drastically reduce the duration of a cold sore when vesicles have already appeared and a treatment that will prevent the outbreak of a lesion and formation of vesicles when applied in the prodormal stage. The therapeutic compound comprises a mixture of Acyclovir (“ACV”), Penciclovir (“PCV”), and 2-Deoxy-D-Glucose (“2-DDG”). The therapeutic compositions of the present invention include multiple formulations of the three active ingredients and may also include inactive ingredients.
Owner:G2L TOUCH
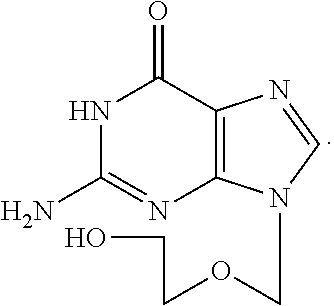
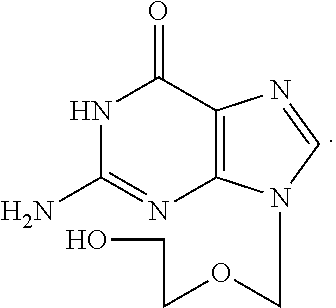
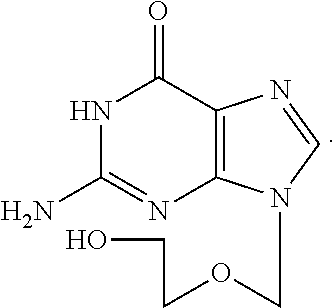
Water solution of penciclovir and its prepn. method
InactiveCN1461643AImprove solubilityExpand the scope of clinical applicationAntiviralsHeterocyclic compound active ingredientsDiseaseHerpes zoster virus
A water soluble of Penxiluowei contains the Penxiluowei or its salt, solubilizer, cosolvent and pH regulator. It can be used to prepare injection, eye drops and nose drops for treating the diseases caused by herpes simple virus, herpes zoster virus and hepatitis B virus.
Owner:XICHUAN INST OF ANTIBIOTIC IND NAT MEDICINE SUPERVISION BUREAU
Preparation method and use of sodium Penciclovir sodium hydrate
InactiveCN1429826ASimple preparation processReduce manufacturing costOrganic chemistryAntiviralsSolubilityOrganic solvent
A monohydrated Penxiluowei sodium used for preparing injection or exterior-applied medicine is prepared through dissolving Penxiluowei in the solution of sodium hydroxide, regulating pH value, addinginertial organic solvent dissolved with water, stirring, laying aside, filter, washing, and vacuum drying the deposit at 40-90 deg.C. Its advantages are low cost, high purity and high water solubility.
Owner:HUBEI KYLIN BIOLOGICAL SCI & TECH DEV
Penciclovir gel and its preparing method
InactiveCN1857270ADefinite curative effectQuality is easy to controlPharmaceutical delivery mechanismAntiviralsHydrophilic matrixPenciclovir
The present invention provides a kind of new penciclovir preparation form, penciclovir gel. The penciclovir gel consists of penciclovir, matrix, pH regulator and water. The preparation process of the penciclovir gel includes dissolving penciclovir and pH regulator in water through heating and stirring to obtain penciclovir solution; mixing with the hydrophilic matrix; adding pure water to required amount; and vacuumizing to eliminate bubble, filling and sealing to obtain penciclovir gel product. Compared with available cream, the penciclovir gel is comfortable and has no greasy feeling.
Owner:陈益智
Penciclovir freeze dried and method of manufacturing the same
ActiveCN101108169AImprove solubilityImprove stabilityPowder deliveryAntiviralsSolubilityFreeze-drying
The invention relates to the technical field of medical technology, in particular to a penciclovir lyophilized royal jelly powder injection and its preparation method, which comprises penciclovir and excipient. The powder injection is made by making penciclovir into penciclovir sodium salt, add the excipient and then freeze out. The weight ratio of the penciclovir and the excipient is: 1:0.2 to 3; the excipient comprises low molecular dextran. The invention resolves the problem of defective water-solubility in the prior art. Besides, with the freezing out technology and by adding low molecular dextran as the excipient, the invention enhances the solubility and stability of penciclovir, resolves the problem of low biologic utilization rate of penciclovir and ensures the safety and convenience of medication. What is more, the invention also provides the preparation method for the penciclovir lyophilized royal jelly powder injection.
Owner:ZHEJIANG JIANFENG PHARM CO LTD
Therapeutic composition to treat lesions caused by herpes simplex virus
ActiveUS20120071498A1Shorten the construction periodAvoid problemsBiocideOrganic chemistryAdditive ingredientBULK ACTIVE INGREDIENT
The present invention is generally directed toward therapeutic compositions for treating infections caused by Herpes Simplex Virus (“HSV”). The therapeutic compositions meet a long felt need in the art of providing a treatment for lesions that result from HSV that drastically reduce the duration of a cold sore when vesicles have already appeared and a treatment that will prevent the outbreak of a lesion and formation of vesicles when applied in the prodromal stage. The therapeutic compound comprises a mixture of Acyclovir (“ACV”), Penciclovir (“PCV”), and dimethyl sulfoxide (“DMSO”). The therapeutic compositions of the present invention include multiple formulations of the three active ingredients and may also include inactive ingredients and / or additional active ingredients.
Owner:G2L TOUCH
Penciclovir emulsifiable paste and its preparation
InactiveCN1919169AEasy to acceptFunctional impact is smallAerosol deliveryOintment deliveryAlcoholPreservative
The invention relates to a Penciclovir dispersible paste which comprises Penciclovir 0.5-2.0 wt%, emulsifying agent 1.0-10.0 wt%, methyl glycol 5.0-20.0 wt%, antiseptic agent 0.01-0.5 wt%, glyceryl monostearate 1.0-5.0 wt%, hexadecyl alcohol 5.0-15.0 wt%, dahurian angelica root pieces 5.0-15.0 wt%, liquid paraffin 5.0-15.0 wt%, and balancing water. The invention also discloses the process for preparing the dispersible paste.
Owner:湖北科益药业股份有限公司
Therapeutic composition to treat lesions caused by herpes simplex virus
ActiveUS8853223B2Shorten the construction periodAvoid problemsBiocideOrganic chemistryVesicle/vacuoleBULK ACTIVE INGREDIENT
The present invention is generally directed toward therapeutic compositions for treating infections caused by Herpes Simplex Virus (“HSV”). The therapeutic compositions meet a long felt need in the art of providing a treatment for lesions that result from HSV that drastically reduce the duration of a cold sore when vesicles have already appeared and a treatment that will prevent the outbreak of a lesion and formation of vesicles when applied in the prodromal stage. The therapeutic compound comprises a mixture of Acyclovir (“ACV”), Penciclovir (“PCV”), and dimethyl sulfoxide (“DMSO”). The therapeutic compositions of the present invention include multiple formulations of the three active ingredients and may also include inactive ingredients and / or additional active ingredients.
Owner:G2L TOUCH
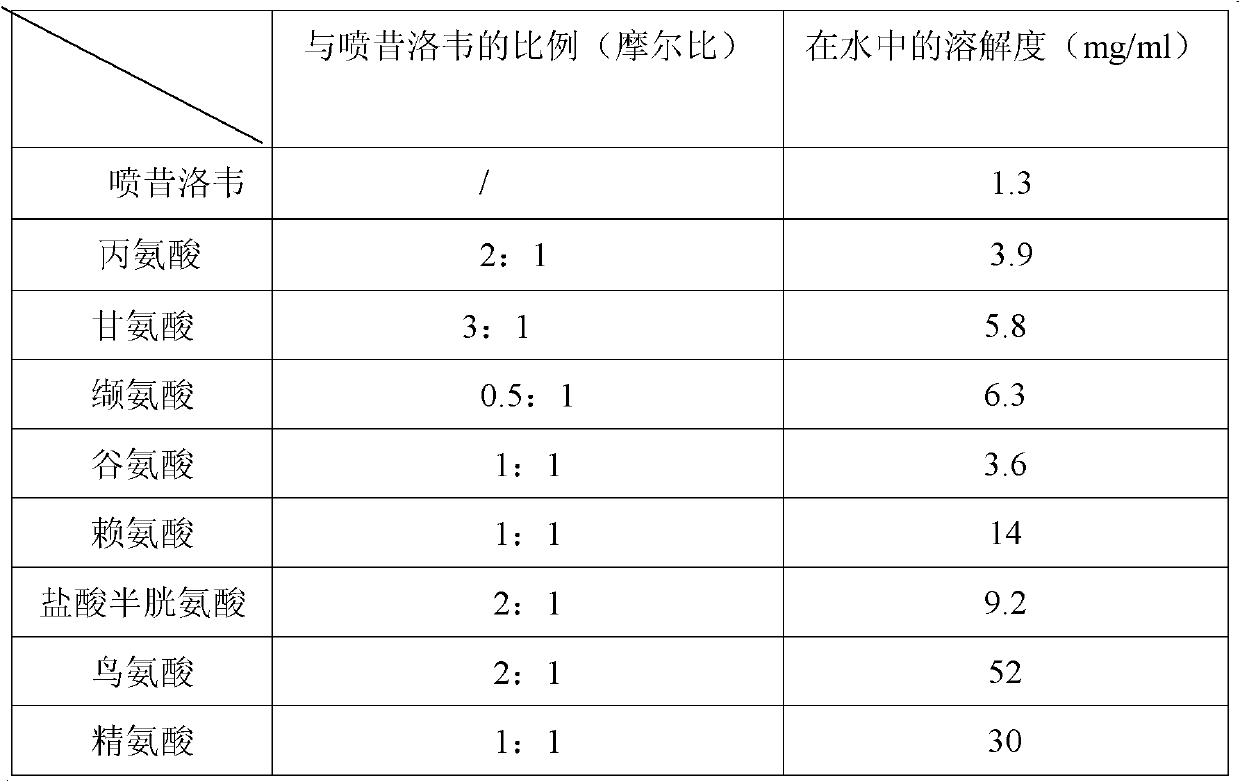
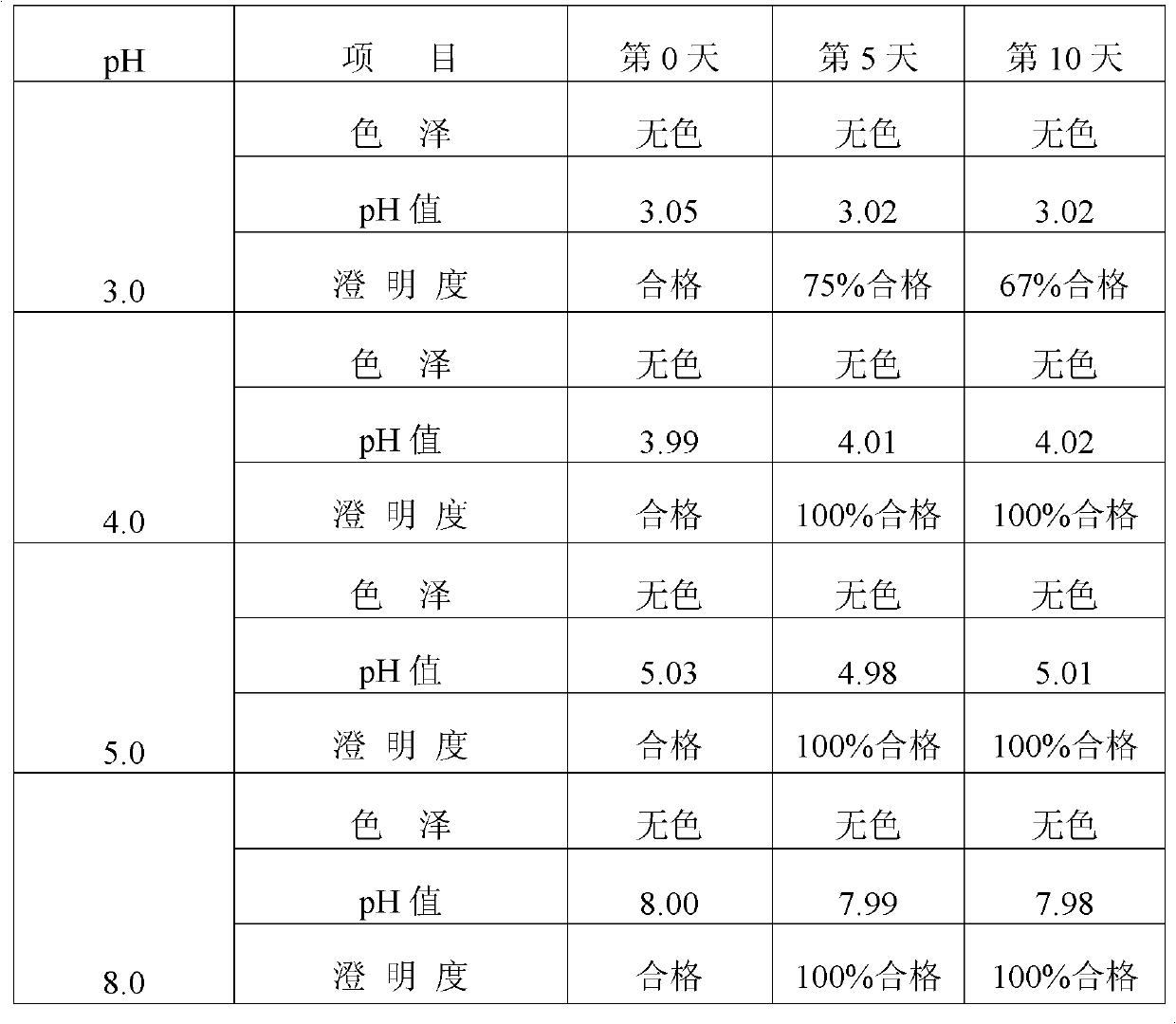
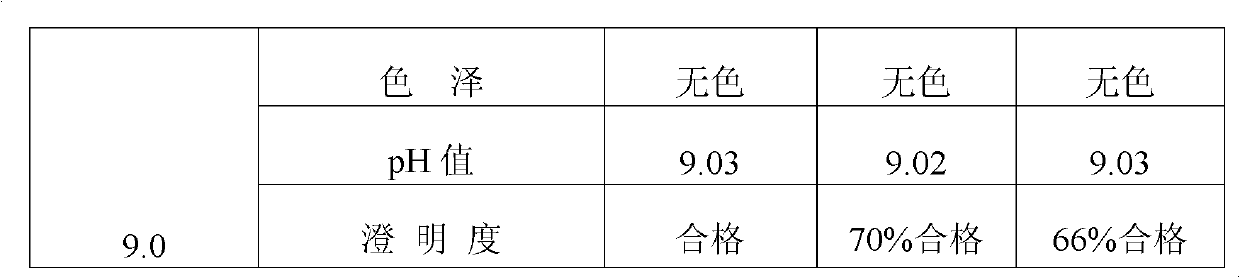
Preparation method and new clinic application of penciclovir injection solution
The invention discloses a preparation method and new clinic application of a penciclovir injection solution. The preparation method comprises the steps of: (1) mixing and dissolving penciclovir, one or more amino acids selected from alanine, glycocoll, cysteine hydrochloride, valine, glutamic acid, lysine and arginine, and pharmaceutically acceptable injection auxiliaries in water, wherein the concentration of the penciclovir is larger than 1.5mg / ml; (2) adjusting the pH value to be 4.0-8.0, preferably 4.5-6.5; and (3) adding water to adjust the concentration of the penciclovir to be 0.1-1.2mg / ml, preferably 0.4-1.1mg / ml, or randomly adjusting the pH value and then carrying out freeze-drying. The penciclovir injection solution can be used for safely, effectively and rapidly preventing and treating venereal diseases, such as herpes progenitalis, condyloma acuminatum and the like.
Owner:SHANDONG DANHONG PHARMA
Combination therapy to treat hepatitis B virus
The present invention is directed to a method for treating hepatitis B virus infection in humans comprising administering a synergistically effective amount of agents having known anti-hepatitis B virus activity in combination or alternation. Specifically, the invention is directed to a method for treating hepatitis B virus infection comprising administering FTC in combination or alternation with penciclovir, famciclovir or Bis-POM-PMEA. Additionally, the invention is directed to a method for treating hepatitis B virus infection comprising administering L-FMAU in combination or alternation with DAPD, penciclovir or Bis-POM-PMEA. The invention is further directed to a method for treating hepatitis B virus infection comprising administering DAPD in combination or alternation with Bis-POM-PMEA.
Owner:GILEAD SCI INC
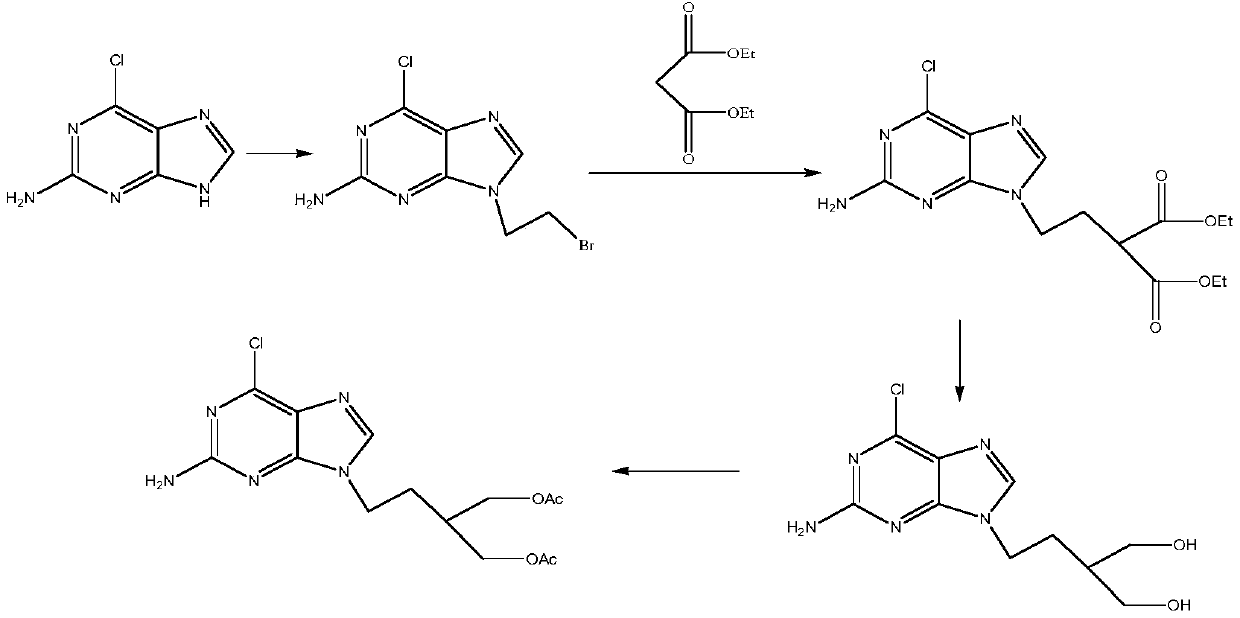
Preparation method of penciclovir
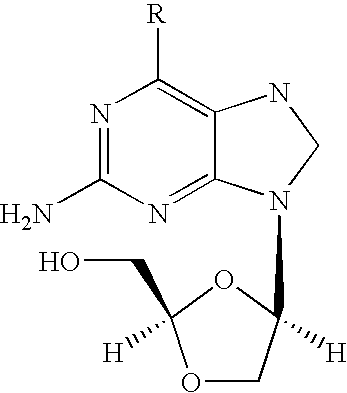
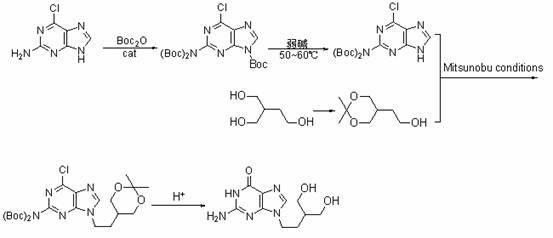
InactiveCN102070636AHigh yieldShort routeOrganic chemistryPtru catalystTert-Butyloxycarbonyl protecting group
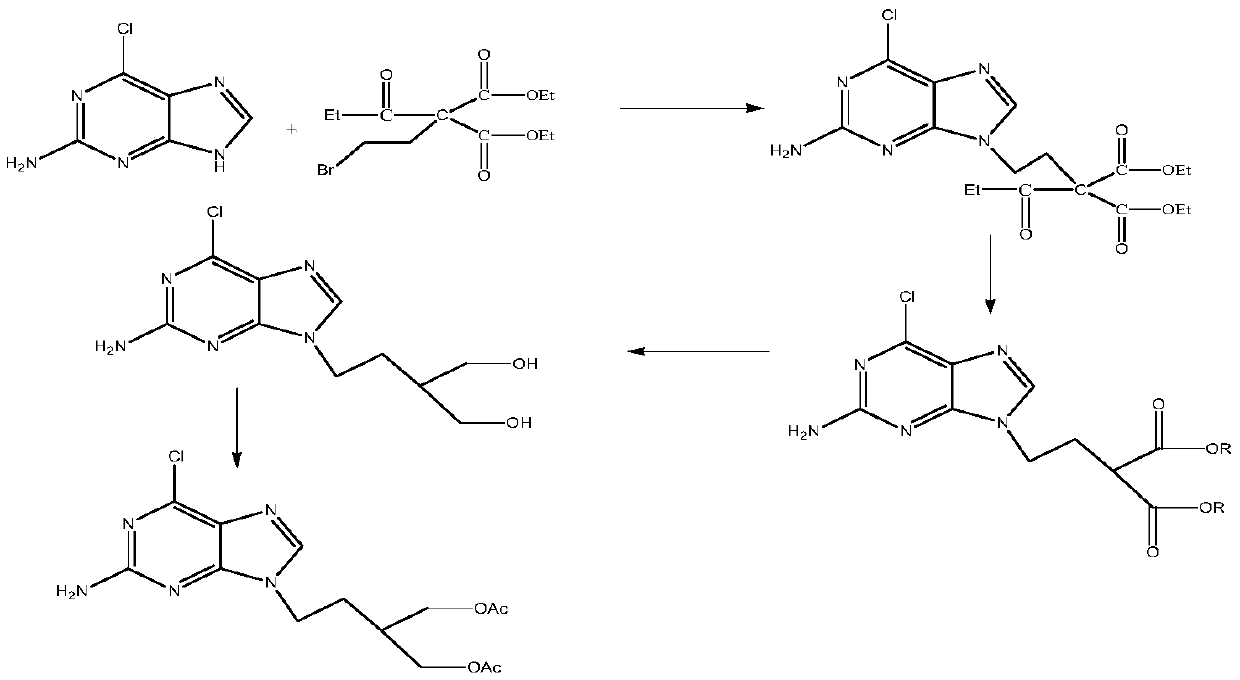
The invention discloses a preparation method of penciclovir. The preparation method comprises the following steps: in the presence of a catalyst, reacting 2-amino-6-dichloropurine with butoxycarbonyl (Boc) anhydride to generate 2-(dibutoxycarbonylamino)-6-chloro-9H-9-butoxycarbonyl-purine; subsequently, removing 9-butoxycarbonyl under the action of weak base so as to generate 2-(dibutoxycarbonylamino)-6-choropurine; coupling with 5-(2-hydroxyethyl)-2,2-dimethyl-1,3-dipxane under the conditions of Mitsunobu reaction to obtain intermediate 2-(dibutoxycarbonylamino)-6-chloro-9-[2-(2,3-dimethyl-1,3-dioxin-5-yl)ethyl]purine in which N is positioned at the ninth site; and carrying out de-Boc protection by the one pot method, and carrying out side chain ring opening and hydrolytic dechlorination to obtain penciclovir. The preparation method of the penciclovir in the invention has the advantages of short route, high yield, good product purity and low cost.
Owner:ZHEJIANG UNIV
Antiviral formulation
A topical antiviral composition comprising acyclovir, penciclovir and / or omaciclovir in a glucocorticoid-free pharmaceutical carrier comprising 15 to 25 weight % propylene glycol and 10 to 25 weight % isopropyl C12-C22 alkanoic ester. The compositions have utility in the treatment or prophylaxis of herpesvirus infections. Clinical results demonstrate that treatment commencing at the prodromal stage can prevent the development of a classic cold sore lesion in a large proportion of patients.
Owner:VIATRIS GMBH & CO KG
Pharmacological modulation of telomere length in cancer cells for prevention and treatment of cancer
InactiveUS20090137503A1Particular utilityUncontrolled cell growthBiocideDispersion deliveryTelomeraseCancer cell
Acyclic nucleoside analogs such as acyclovir, ganciclovir, penciclovir and the corresponding pro-drugs, i.e., valacyclovir, valganciclovir and famciclovir, respectively have been identified as inhibitors or antagonists of both telomerase (encoded by TERT) and reverse transcriptase encoded by L-1 (LINE-1) RT, and as useful for treating or preventing cancers induced or mediated by the two enzymes. Method of treating or preventing such cancers in patients involves administration of a therapeutically effective amount of a composition having an inhibitor or antagonist of the reverse transcriptases in cells of the patients. The inhibitor or antagonist blocks lengthening of telomeres in telomerase positive and telomerase negative cells. Methods and kits for detecting pathologically proliferating cells expressing TERT and L1RT are also disclosed.
Owner:ALT SOLUTIONS INC
Preparation method of penciclovir
The invention discloses a preparation method of penciclovir. An existing synthetic route mainly has the following defects that the N-7 site by-products need to be removed through column chromatography, a large amount of three wastes are generated, and the yield of N-9 site products is not high. According to the technical scheme adopted by the invention, the preparation method comprises the following steps: carrying out alkylation on 2-amino-6-chloropurine and triethyl bromopropane under an alkaline condition to introduce an N-9 site side chain, carrying out decarboxylation and transesterification in a methanol solution of sodium methoxide to generate the 2-amino-6-chloro-9-(3,3-dimethoxycarbonyl-1-propyl)purine; then reducing the 2-amino-6-chloro-9-(3,3-dimethoxycarbonyl-1-propyl)purine togenerate 2-amino-6-chloro-9-(3-hydroxymethyl-4-hydroxy-1-butyl)purine with sodium borohydride; and finally, directly hydrolyzing under acidic conditions to obtain penciclovir. According to the method, the target product is obtained by directly hydrolyzing the reduction product, ester group protection and deprotection are not needed, and the method has the advantages of few reaction steps, good product quality, simplicity and convenience in operation, suitability for industrial production and the like.
Owner:ZHEJIANG MEDICINE CO LTD XINCHANG PHAMACEUTICAL FACTORY +1
Pharmacological modulation of telomere length in cancer cells for prevention and treatment of cancer
InactiveUS8609623B2Particular utilityUncontrolled cell growthBiocideDispersion deliveryTelomeraseCancer cell
Acyclic nucleoside analogs such as acyclovir, ganciclovir, penciclovir and the corresponding pro-drugs, i.e., valacyclovir, valganciclovir and famciclovir, respectively have been identified as inhibitors or antagonists of both telomerase (encoded by TERT) and reverse transcriptase encoded by L-1 (LINE-1) RT, and as useful for treating or preventing cancers induced or mediated by the two enzymes. Method of treating or preventing such cancers in patients involves administration of a therapeutically effective amount of a composition having an inhibitor or antagonist of the reverse transcriptases in cells of the patients. The inhibitor or antagonist blocks lengthening of telomeres in telomerase positive and telomerase negative cells. Methods and kits for detecting pathologically proliferating cells expressing TERT and L1RT are also disclosed.
Owner:ALT SOLUTIONS INC
Water solution of penciclovir, and its prepn. method
InactiveCN1230177CImprove solubilityExpand the scope of clinical applicationAntiviralsHeterocyclic compound active ingredientsDiseaseHerpes zoster virus
Owner:XICHUAN INST OF ANTIBIOTIC IND NAT MEDICINE SUPERVISION BUREAU
Ointment for reducing side effects of penciclovir cream
InactiveCN106581147ASignificant effectGood treatment effectAntipyreticAerosol deliverySide effectMedicine
The invention discloses an ointment for reducing side effects of penciclovir cream, and belongs to the technical field of medicine ointments. The ointment for reducing side effects of penciclovir cream comprises a component A and a component B, the component A is the penciclovir cream, and the component B is Chinese herbal medicine extract. The ointment for reducing side effects of penciclovir cream is obtained through fully mixing and uniformly stirring the component A and the component B according to a weight ratio of 1:3. Clinic experiments prove that the ointment has a substantial cure effect on verruca plana, and overcomes the side effects of the penciclovir cream in clinic use.
Owner:沂南县迎辉农业开发有限公司
Purification method of penciclovir
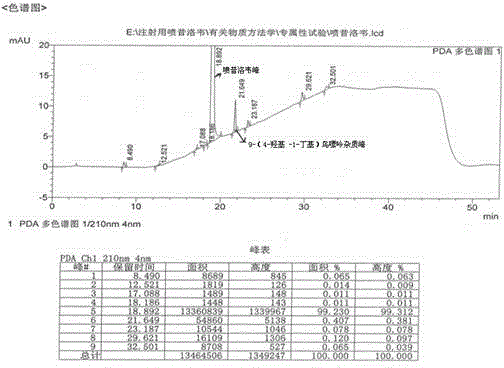
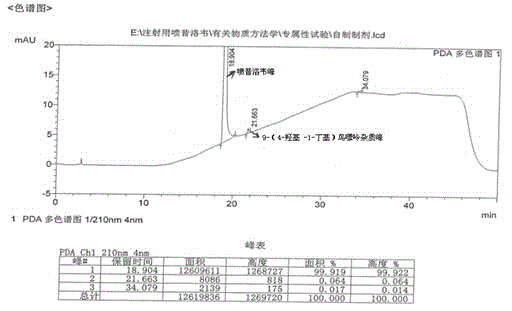
The invention provides a purification method of penciclovir. The purification process comprises the following steps of heating and dissolving a crude penciclovir product with dimethyl sulfoxide, then adding aluminum oxide, stirring, filtering, adding a low-basic alcohol solvent to the filtrate, crystallizing, filtering and drying to obtain penciclovir. By virtue of the purification process disclosed by the invention, a penciclovir finished product of which the purity reaches above 99.9% and in which the content of 9-(4-hydroxy-1-butyl) guanine as an impurity is less than 0.10% can be obtained; the purification method has the advantages of simple operation, strong repeatability, no pollution, low cost and relatively high yield and is suitable for industrial production.
Owner:JINAN KANGHE MEDICAL TECH
Famciclovir for the treatment of recurrent herpes labialis using a one-day treatment
A method for the treatment of recurrent herpes labialis in mammals, including humans, which method comprises administering to the mammal in need of such treatment, an effective amount of penciclovir or famciclovir, or a pharmaceutically acceptable salt thereof for a period of one day.
Owner:BILLSTEIN STEPHAN ANTHONY +2
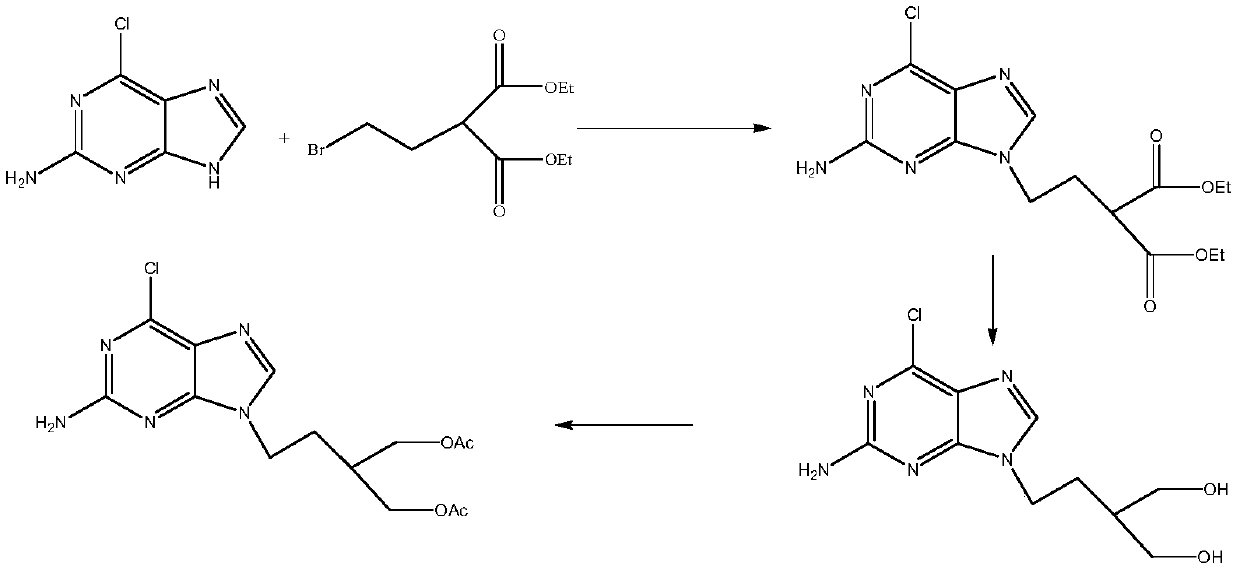
A kind of preparation method of 2-amino-6-chloro-9-(4-acetoxy-3-acetoxymethylbutyl)purine
The invention relates to a preparation method for 2-amino-6-chloro-9-(4-acetoxy-3-acetoxymethylbutyl)purine. The method comprises the following steps: performing a reaction on 1,3-dichloro-2-propanoland an acetate to obtain 1,3-diacetoxy-2-propanol, performing a bromination reaction to obtain 1,3-diacetoxy-2-bromopropane, performing a reaction on the 1,3-diacetoxy-2-bromopropane and zinc powder to obtain 1,3-diacetoxy-2-zinc bromide propane, performing a coupling reaction on the 1,3-diacetoxy-2-zinc bromide propane and 2-bromoethanol protected by hydroxyl, and performing a de-protection reaction to obtain 1,3-diacetoxy-2-(2-hydroxyethyl)propane, and performing a Mitsunobu reaction on the 1,3-diacetoxy-2-(2-hydroxyethyl)propane and 2-amino-6-chloropurine to obtain the 2-amino-6-chloro-9-(4-acetoxy-3-acetoxymethylbutyl)purine. According to the method provided by the invention, the 2-amino-6-chloro-9-(4-acetoxy-3-acetoxymethylbutyl)purine prepared by the method has purity of 99.5% or more detected by HPLC, and can be used to prepare famciclovir and penciclovir.
Owner:LIVZON GROUP CHANGZHOU KONY PHARMA
Method for determining human plasma antiviral drug concentration
InactiveCN100580447CEasy pretreatmentSuitable for routine testingComponent separationFluorescence/phosphorescenceAntiviral drugMedicine
The invention belongs to medical detection field, relates to an analysis detection method of drug in the body of a person, and specifically relates to the method that the densities of antiviral drugs in blood plasma of the person such as acyclovir, ganciclovir and penciclovir can be detected at the same time. The method in the invention is characterized in that pilot sample is pretreated; as acyclovir, ganciclovir and penciclovir have the character of strong fluorescence absorption, acyclovir, ganciclovir and penciclovir can be separated from each other in an acidity flowing phase chromatographic column and be detected by a fluorescence detector. The method in the invention has the advantages of little sample, simple, swift and sensitive pretreatment, short analysis period and low cost; furthermore, the invention doesn't need expensive equipment and reagent and is suitable for the detection of clinical conventional blood drug density of acyclovir, ganciclovir and penciclovir.
Owner:AFFILIATED HUSN HOSPITAL OF FUDAN UNIV
Penciclovir freeze dried and method of manufacturing the same
ActiveCN100594889CSolve the technical defect of low solubilityEasy to masterPowder deliveryAntiviralsSolubilityFreeze-drying
The invention relates to the technical field of medical technology, in particular to a penciclovir lyophilized royal jelly powder injection and its preparation method, which comprises penciclovir andexcipient. The powder injection is made by making penciclovir into penciclovir sodium salt, add the excipient and then freeze out. The weight ratio of the penciclovir and the excipient is: 1:0.2 to 3;the excipient comprises low molecular dextran. The invention resolves the problem of defective water-solubility in the prior art. Besides, with the freezing out technology and by adding low moleculardextran as the excipient, the invention enhances the solubility and stability of penciclovir, resolves the problem of low biologic utilization rate of penciclovir and ensures the safety and convenience of medication. What is more, the invention also provides the preparation method for the penciclovir lyophilized royal jelly powder injection.
Owner:ZHEJIANG JIANFENG PHARM CO LTD
Penciclovir microemulsion gel preparation and preparing method thereof
InactiveCN101229121BNon-irritatingEasy to administerAntiviralsEmulsion deliveryGel preparationOil phase
The invention relates to a penciclovir micro-emulsified gel and preparation process, belonging to medicine technology field, which comprises penciclovir surfactant, assistant surfactant, oil phase, water and carbomer and is used for transdermal drug delivery, the processing method is simple, the quality is easy to be controlled, and the stability of the gel is good, compared with present cream, the unit area accumulation infiltration amount of drugs can be improved obviously, and detained dosage in epidermis and dermis can be improved, moreover the invention has certain sustained-release function so that drug treating times and drug amounts can be reduced, and the using process of the drug is convenient, no pollution is caused to clothes, so the medicine is suitable for clinical wide application.
Owner:SHANDONG UNIV
Antiviral formulation
Owner:VIATRIS GMBH & CO KG
Antivirus transdermal emulsifiable paste containing penciclovir, liquorice, astragalus, cassia nomame and basil
InactiveCN101433579BRegulate immune functionImprove solubilityOil/fats/waxes non-active ingredientsHeterocyclic compound active ingredientsSolubilityCuticle
The invention relates to a penciclovir ganhuang soap antiviral transdermal cream. The cream is characterized in that a formulation comprises the following compositions in mixture ratio (weight portion): 0.5 to 1.0 portion of penciclovir, 1 to 2 portions of novel water-soluble azone and 1 to 2 portions of menthol. The penciclovir is western antiviral medicine and is compatible with the water-soluble azone and other strong cosolvents and transdermal agents in order to be favorable for the penciclovir in a junction phase among an oil phase, a water phase and a mixed phase; and at least the creamincreases solubility in five phases and achieves the treatment function through permeating epidermis, corium, subcutis, nerve ending and nerve fiber tissue of skin. Chinese and western medicines permeate each layer and subcutis of the skin through multiphase dissolution, multiphase permeation and high concentration, thereby implementing the functions of inhibiting and killing virus and adjusting immunity.
Owner:谭国梁
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com