Preparation method of penciclovir
A technology of penciclovir and chloropurine is applied in the field of preparation of purine derivative penciclovir, which can solve problems such as being unsuitable for large-scale production, reducing the yield of N9-position product, and achieving low cost, good product purity and high yield. high rate effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
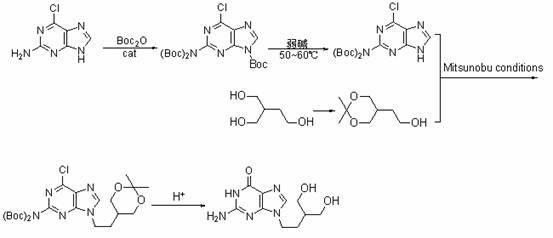
[0021] 1) Preparation of 2-(di-tert-butoxycarbonylamino)-6-chloro-9H-9-tert-butoxycarbonylpurine
[0022] Under the protection of N2, 2.00g (11.8mmol) of 2-amino-6-chloropurine and 0.22g (1.48mmol) of 4-pyrrolidinylpyridine were dissolved in 112mL of anhydrous ether, stirred evenly by magnetic force, and then added to Boc 2 O 5.20g (23.6mmol), react at room temperature for 8h, distill off ether, dissolve the solid in 400mL AcOEt, wash with aqueous hydrochloric acid (2 N, 1 × 30 mL), take the organic phase and wash with water (2 × 50 mL ), after standing for stratification, the organic phase was used Na 2 SO 4 Drying and distillation under reduced pressure gave 5.43 g (98.2%) of 2-(di-tert-butoxycarbonylamino)-6-chloro-9H-9-tert-butoxycarbonylpurine as a white solid.
[0023] 2) Preparation of 2-(di-tert-butoxycarbonylamino)-6-chloropurine
[0024] Dissolve 14.0 g (30 mmol) of 2-(di-tert-butoxycarbonylamino)-6-chloro-9H-9-tert-butylcarboxypurine in 177 mL of ethanol, then a...
Embodiment 2
[0032] 1) Preparation of 2-(di-tert-butoxycarbonylamino)-6-chloro-9H-9-tert-butoxycarbonylpurine
[0033] Under the protection of N2, 2.00g (11.8mmol) of 2-amino-6-chloropurine and 0.14g (1.18mmol) of 4-dimethylaminopyridine were dissolved in 100mL of anhydrous THF, stirred evenly by magnetic force, and then added Boc2O 10.30g (47.2mmol), reacted at room temperature for 6h, distilled off THF, dissolved the solid in 400mL AcOEt, washed with aqueous hydrochloric acid (2 N, 1 × 30 mL), took the organic phase and washed with water (2 × 50 mL) , after standing and stratifying, the organic phase was washed with Na 2 SO 4 Dry and distill under reduced pressure to obtain 5.40 g (97.5%) of white solid 2-(di-tert-butoxycarbonylamino)-6-chloro-9H-9-tert-butoxycarbonylpurine.
[0034] 2) Preparation of 2-(di-tert-butoxycarbonylamino)-6-chloropurine
[0035] Dissolve 14 g (30 mmol) of 2-(di-tert-butoxycarbonylamino)-6-chloro-9H-9-tert-butylcarboxypurine in 265 mL of methanol, then add 5...
Embodiment 3
[0043] 1) Preparation of 2-(di-tert-butoxycarbonylamino)-6-chloro-9H-9-tert-butoxycarbonylpurine
[0044] Under the protection of N2, 2.00g (11.8mmol) of 2-amino-6-chloropurine and 0.13g (0.98mmol) of 1-hydroxybenzotriazole were dissolved in 117mL of anhydrous 2-methyltetrahydrofuran, and stirred by magnetic force Mix well, then add Boc 2 O 15.43g (70.8mmol), reacted at room temperature for 4h, distilled off 2-methyltetrahydrofuran, dissolved the solid in 400mL AcOEt, washed with aqueous hydrochloric acid (2 N, 1 × 30 mL), and washed the organic phase with water (2 × 50 mL), after static separation, the organic phase was washed with Na 2 SO 4 Drying and distillation under reduced pressure gave 5.36 g (96.8%) of 2-(di-tert-butoxycarbonylamino)-6-chloro-9H-9-tert-butoxycarbonylpurine as a white solid.
[0045] 2) Preparation of 2-(di-tert-butoxycarbonylamino)-6-chloropurine
[0046] Dissolve 14 g (30 mmol) of 2-(di-tert-butoxycarbonylamino)-6-chloro-9H-9-tert-butylcarboxypu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com