Preparation method and new clinic application of penciclovir injection solution
A technology for penciclovir and injection, which is applied in the direction of medical preparations of non-active ingredients, pharmaceutical formulas, powder delivery, etc., and can solve the problem of production and clinical application of injection liquid injections and penciclovir injections that have not yet been seen. The treatment of condyloma acuminatum has not been reported, and the pain caused by patients has achieved the effect of controlling subclinical infection, increasing the safety of medication, and reducing pain
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Exploration experiment of embodiment 1 penciclovir and amino acid formula
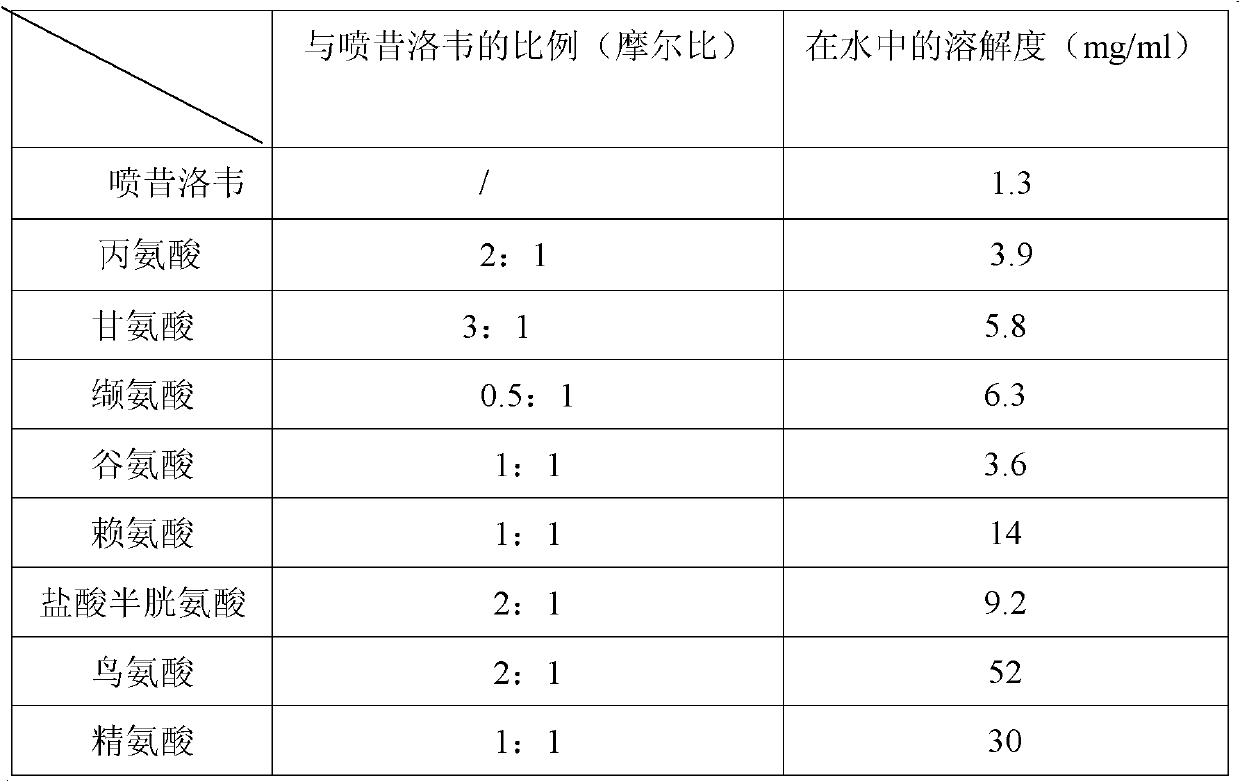
[0031] This experiment studies the solubility of penciclovir alone or in combination with amino acids in pure water. We experimented with amino acids of different types and molar ratios, and found that the combination of different types of amino acids produced significantly different solubilization effects. The specific results are shown in Table 1.
[0032] Table 1 Solubility of Penciclovir alone or in combination with different kinds of amino acids
[0033]
[0034] According to the above experiments, considering the cost of medication and other factors, the comprehensive effect of arginine, ornithine and glycine is the best.
Embodiment 2
[0035] The preparation of embodiment 2 penciclovir sodium chloride injection
[0036] prescription:
[0037] Penciclovir 25g
[0039] Arginine 34.4g
[0040] 6mol / L hydrochloric acid appropriate amount
[0041]
[0042] Water for injection (add to) 25L sub-package of 100 bottles
[0043] Preparation method:
[0044] The sodium chloride of prescription quantity adds water for injection and is made into 10% (weight) solution, adds the arginine of prescription quantity, after stirring and dissolving, add 25g gac and stir and boil for 30 minutes, cool, filter and remove carbon. Add 10L of water for injection to the filtrate, add the prescribed amount of penciclovir, heat and stir at 80°C to dissolve, then cool to room temperature, adjust the pH value to 5.03 with 6mol / L hydrochloric acid solution, then make up water for injection to 25L, and then Pass through 0.45μm and 0.22μm filter membranes to obtain a c...
Embodiment 3
[0045] The quality influence of embodiment 3 different pH values to penciclovir sodium chloride injection
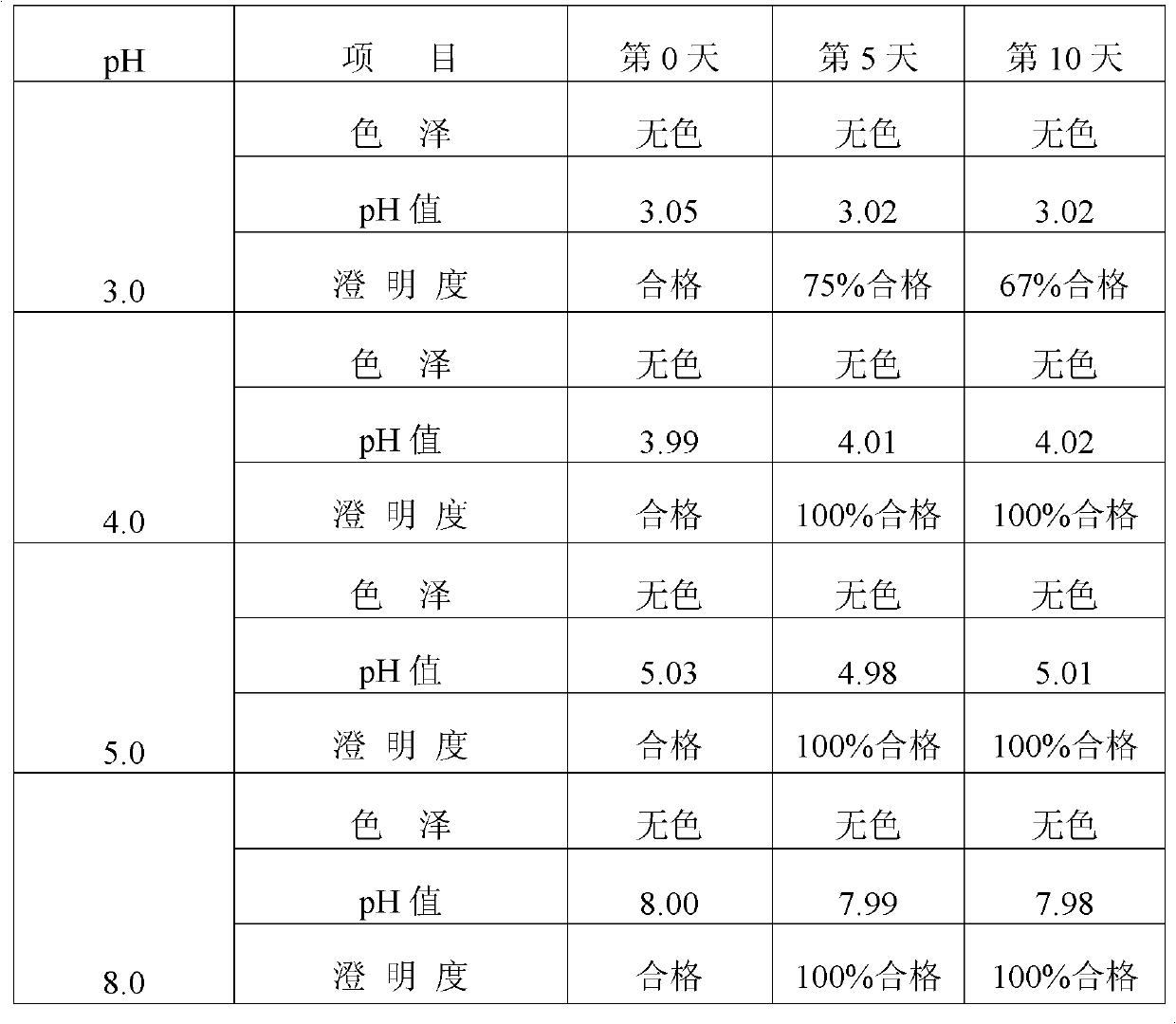
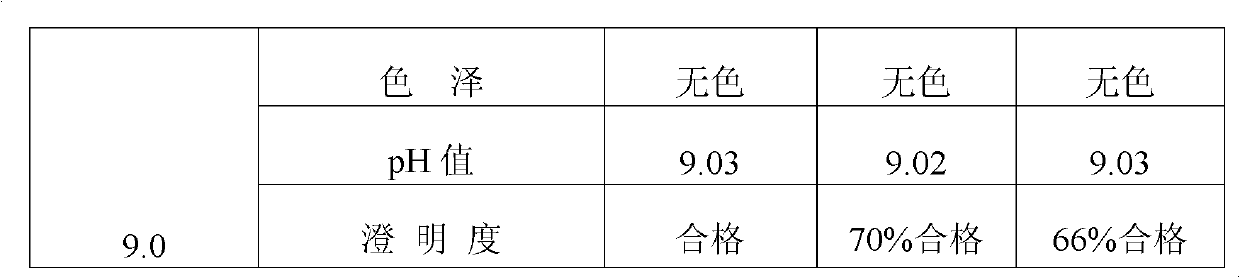
[0046] The prescription in Example 2 is adjusted to four different pH values (3.0, 4.0, 5.0, 8.0, 9.0) with a pH regulator (hydrochloric acid or sodium hydroxide), prepared as an injection, and subjected to an accelerated test at a temperature of 80°C , the stability of the injection was investigated through items such as color, clarity, pH value, content, and related substances. The specific results are shown in Table 2.
[0047] Table 2 Different pH values affect the quality of Penciclovir Sodium Chloride Injection (80°C)
[0048]
[0049]
[0050] As can be seen from Table 2, the quality of penciclovir sodium chloride injection is relatively stable in the pH range of 4.0 to 8.0, but within this pH range, the solubility of penciclovir itself is relatively low, so it is not suitable for preparation For injection, it is especially not suitable to be formulat...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com