Method for determining human plasma antiviral drug concentration
An antiviral drug, human plasma technology, applied in the field of medical testing, can solve the problems of unsuitable for conventional treatment drug concentration monitoring, cumbersome and time-consuming operation, high analysis cost, and achieve the effects of low cost, improved detection sensitivity, and less plasma consumption.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
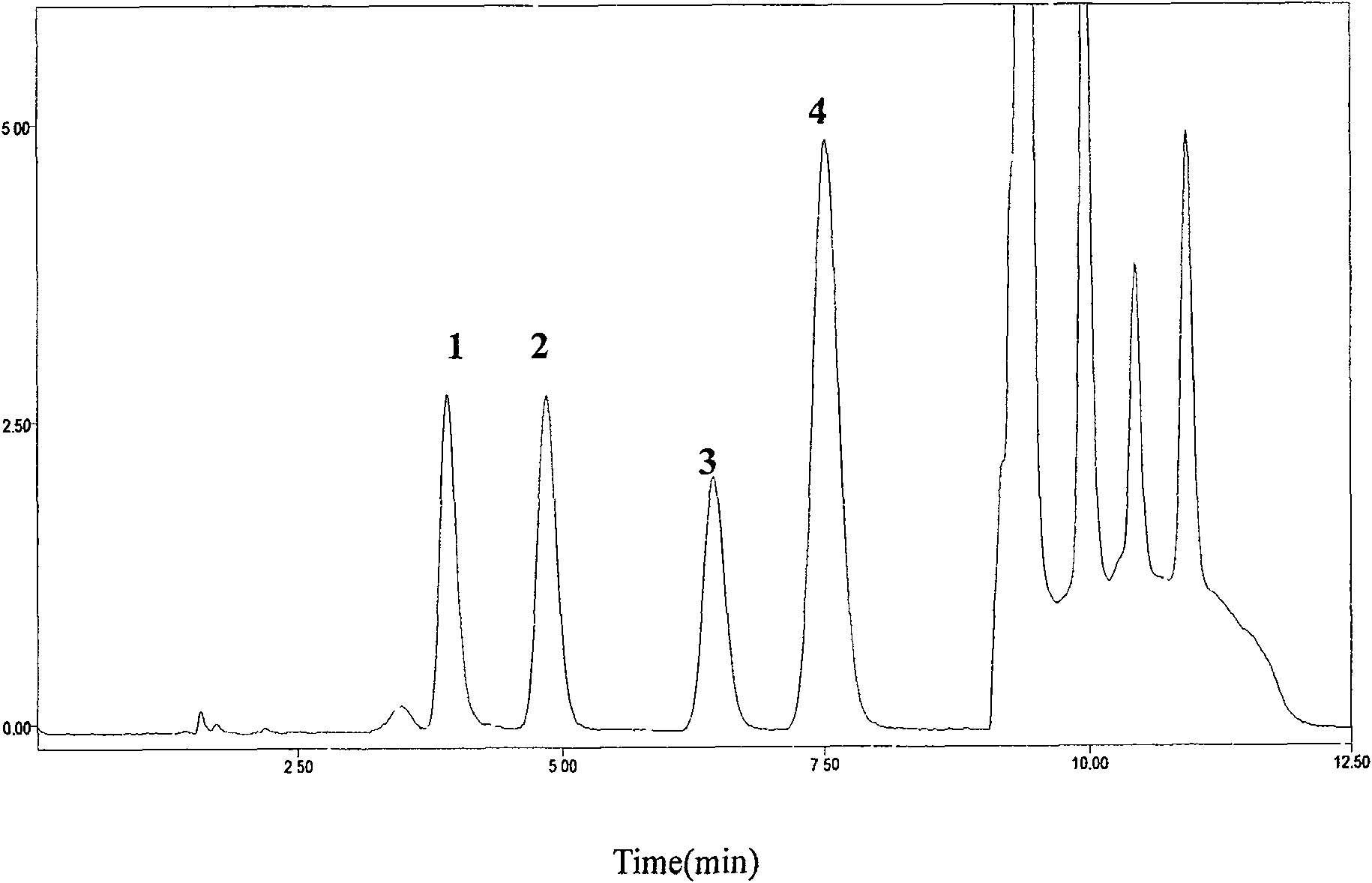
[0029] Chromatographic conditions:
[0030] HPLC system: Diamonsil C column 18 (200mm×4.6mm, 5μm), the mobile phase adopts gradient elution, 0~7min is 0.08%TFA-methanol (96:4, V / V), 7.01~10.0min is 0.08%TFA-methanol (40:60, V / V), 0.08% TFA-methanol (96:4, V / V) for 10.01~12.5min, flow rate 1.5mL·min -1 Column temperature 25℃; fluorescence excitation wavelength 260±1nm, emission wavelength 380±1nm.
[0031] Pretreatment of plasma samples:
[0032] Precisely draw 200μL of plasma into a 1.5mL centrifuge tube, add 50μL of 7% perchloric acid solution containing internal standard guanylic acid, vortex and shake for 30s, centrifuge for 15min (10000×g, 4℃), take 40μL of supernatant into The internal standard method is quantified by peak area.
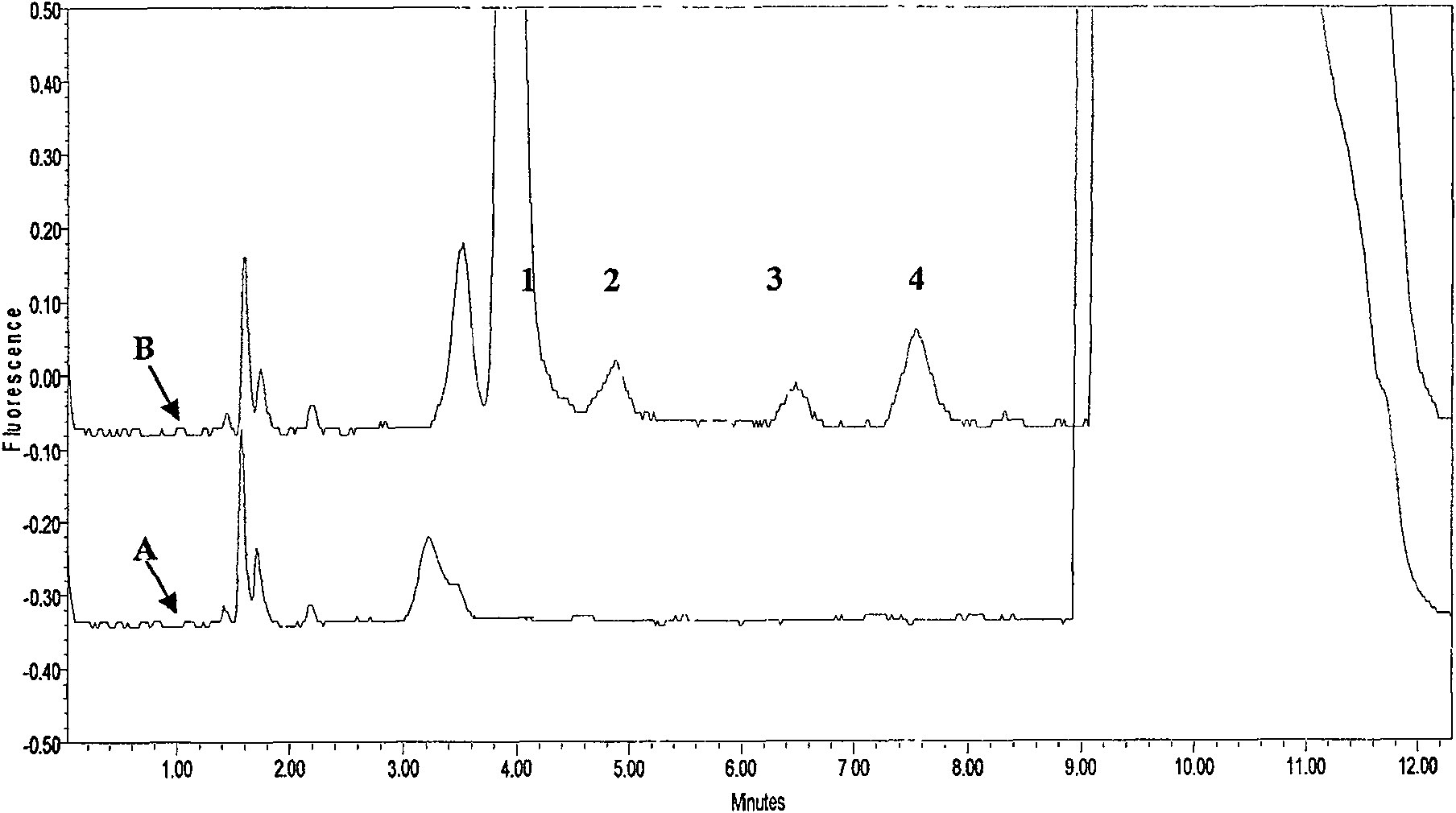
[0033] Specificity:
[0034] Take blank plasma from 10 subjects who have not taken ACV, GCV, and PCV from different sources, and measure them according to the above-mentioned sample pretreatment and measurement methods. The results show that no endogeno...
Embodiment 2
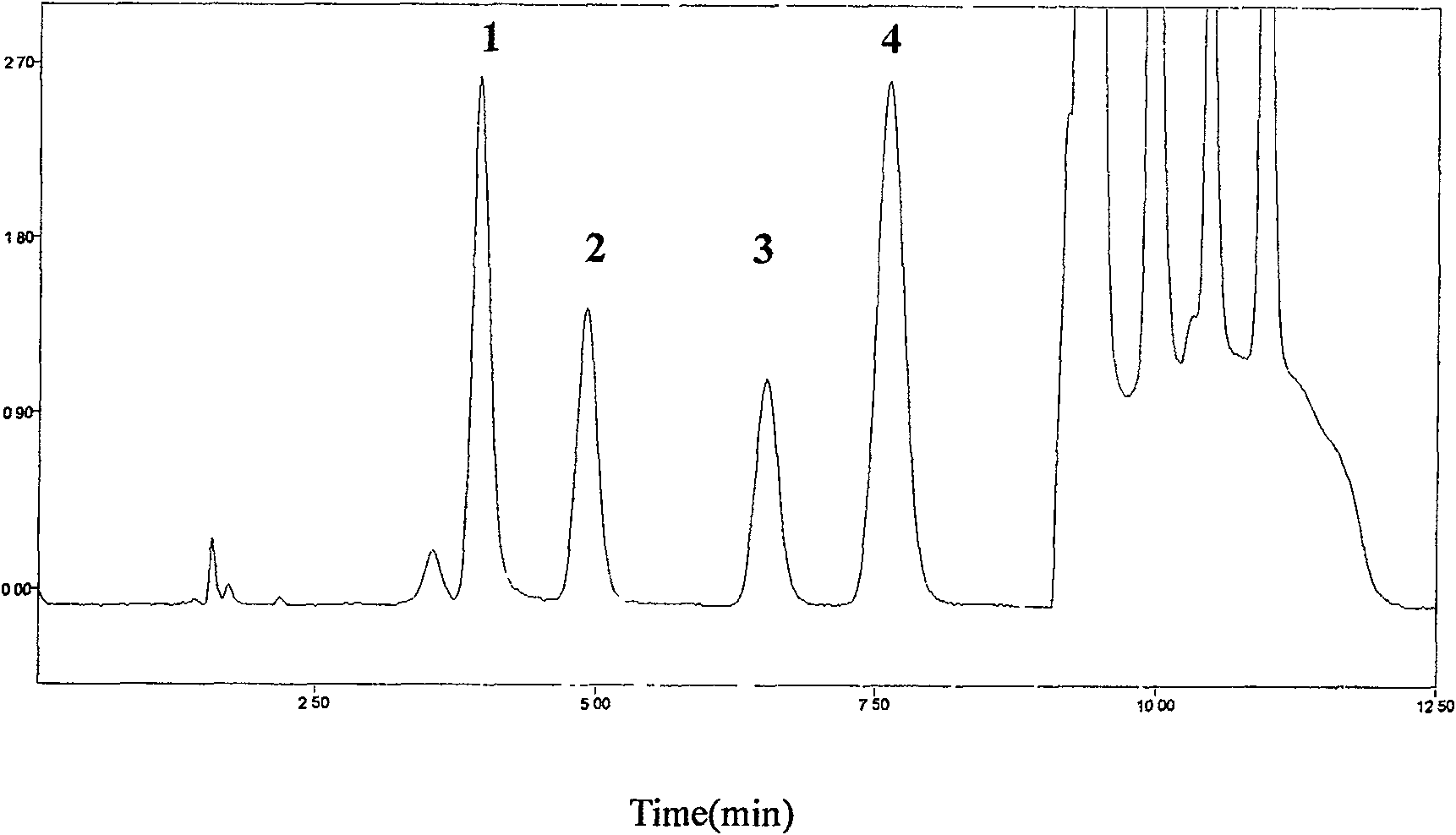
[0042] Chromatographic conditions:
[0043] HPLC system: Diamonsil C column 18 (200mm×4.6mm, 5μm), the mobile phase adopts gradient elution, 0~7min is 0.1%TFA-methanol (96:4, V / V), 7.01~10.0min is 0.1%TFA-methanol (40:60, V / V), 0.1% TFA-methanol (96:4, V / V) for 10.01~12.5min, flow rate 1.5mL·min -1 Column temperature 25℃; fluorescence excitation wavelength 260±1nm, emission wavelength 380±1nm.
[0044] Pretreatment of plasma samples:
[0045] Precisely draw 200μL of plasma into a 1.5mL centrifuge tube, add 25μL of 20% perchloric acid solution containing internal standard guanylic acid, vortex for 30s, centrifuge for 10min (12000×g, 4℃), take 40μL of supernatant for injection , The internal standard method is quantified by peak area.
[0046] Specificity:
[0047] The blank plasma of 10 subjects who did not take ACV, GCV and PCV were collected from different sources and measured according to the above-mentioned sample pretreatment and measurement methods. No plasma endogenous substance...
PUM
| Property | Measurement | Unit |
|---|---|---|
| linear range | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com