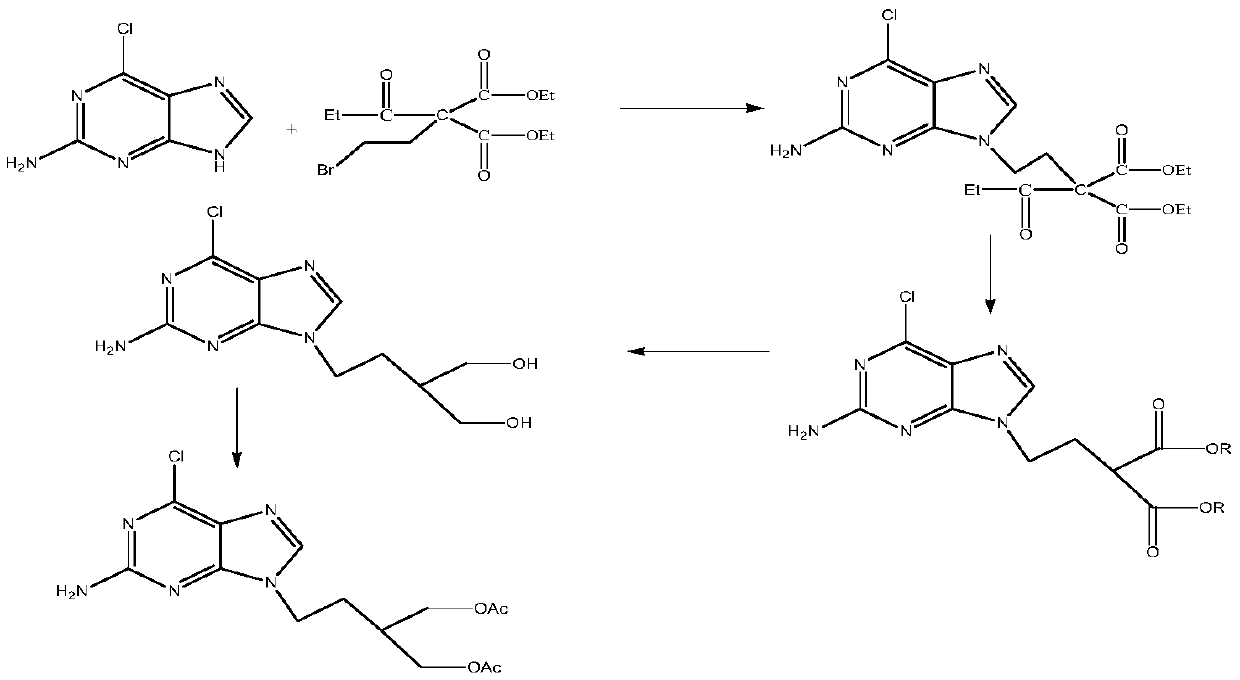
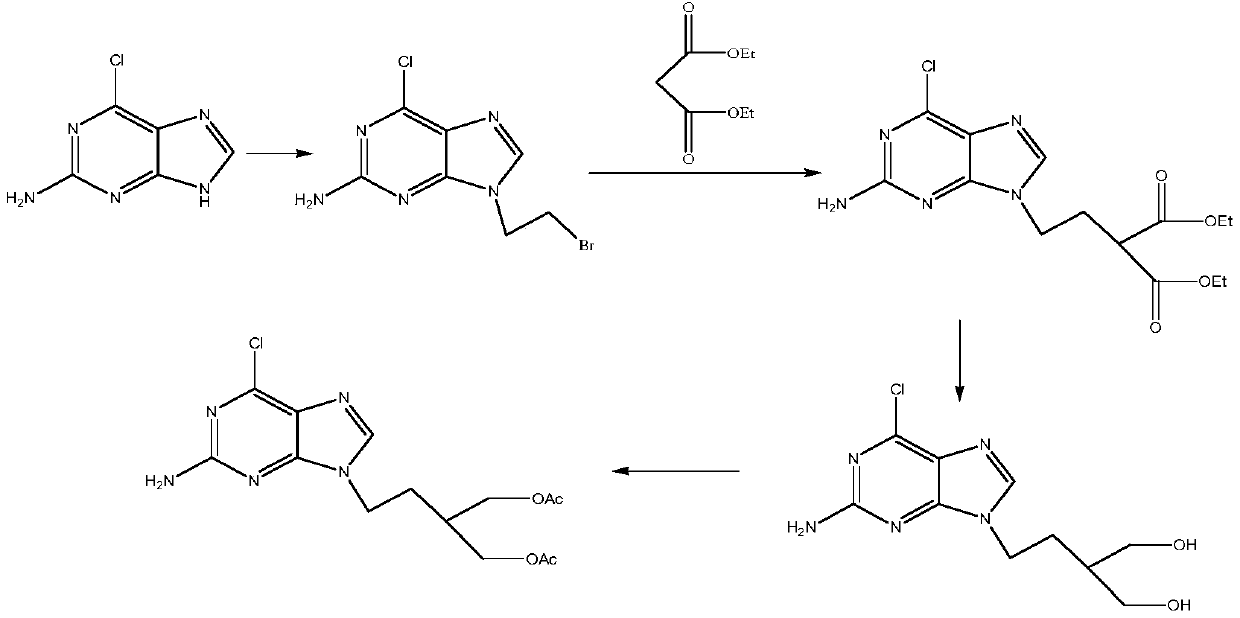
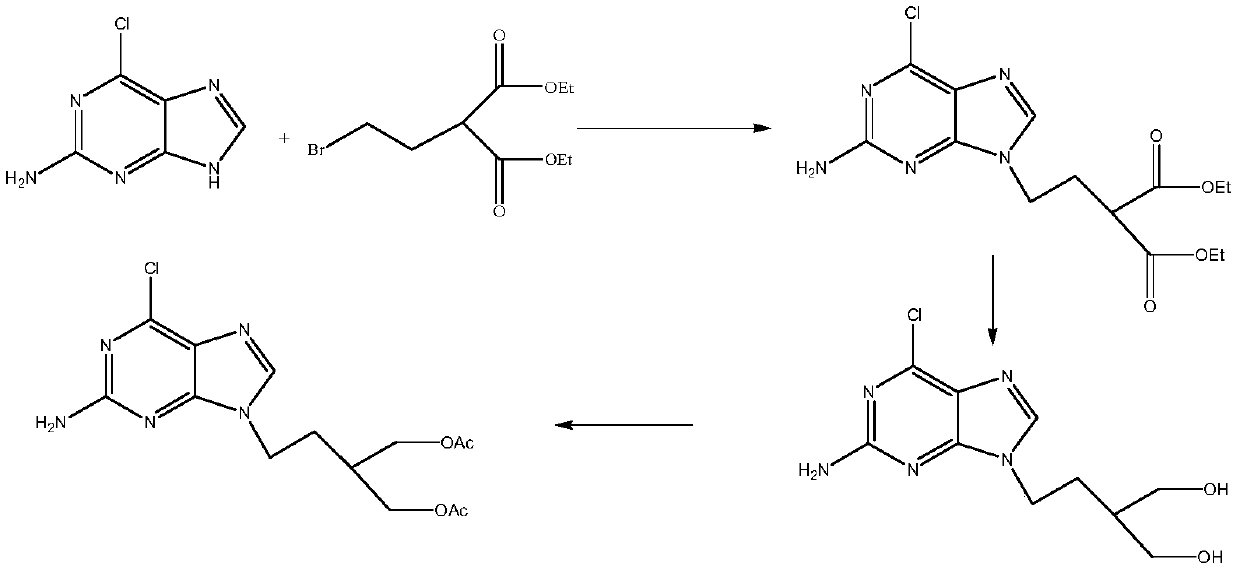
A kind of preparation method of 2-amino-6-chloro-9-(4-acetoxy-3-acetoxymethylbutyl)purine
A technique of acetoxymethylbutyl and acetoxy, which is applied in the field of preparation of 2-amino-6-chloro-9-purine, can solve the problem of low overall yield and achieve good process safety and reduced amount, to avoid the effect of esterification reaction process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] 129g of 1,3-dichloro-2-propanol, 1000ml of DMF, 300g of potassium acetate, and 53.5g of ammonium chloride were put into the reaction bottle, and the reaction was stirred and kept at 100-110°C for 10 hours. After heat preservation, cool to room temperature, filter out the solids in the system, concentrate the filtrate to remove DMF under reduced pressure, add ethyl acetate to dissolve the concentrated solution, wash with water twice, dry with anhydrous sodium sulfate, add triethylamine hydrobromic acid 270g of salt, heat up to 70-80°C, stir and keep warm for 5-6 hours, cool to room temperature, filter out the solids in the system, then wash twice with saturated sodium chloride solution, concentrate under reduced pressure to remove ethyl acetate, then carry out Rectification gave 110 g of 1,3-diacetoxy-2-bromopropane with a GC content of 90.4%.
[0037] Add 100ml of THF, 9g of anhydrous lithium bromide and 33g of zinc powder into the reaction flask, replace with nitrogen ...
Embodiment 2
[0042] Zinc reagent (1,3-diacetoxy-2-zinc bromide propane) and catalyst were prepared according to the method of Example 1, and were ready for use.
[0043] Add 300ml of THF and 50g of bromoethanol benzyl ether into the reaction bottle, add the newly prepared catalyst under nitrogen protection, heat to 50-60°C, slowly add the newly prepared zinc reagent dropwise, and react at 50-60°C for 10h after dropping. Cool down to 15-20°C, add 10ml of ethanol dropwise, and stir for 2h. Concentrate under reduced pressure to dryness, add 300ml ethyl acetate, 200ml 25% aqueous ammonium chloride solution, stir for 0.5h, let stand, separate layers, wash the organic layer twice with water, transfer to an autoclave, add palladium carbon (5% , containing 50% water) 2g, reacted at a temperature of 30-40°C and a pressure of 0.1-0.2MPa for 10 hours, filtered to remove palladium carbon, washed the filtrate twice with water, and concentrated to dryness under reduced pressure to obtain 1,3-diacetoxy ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com