Preparation method of penciclovir
The technology of penciclovir and amino group is applied in the preparation field of antiviral drug penciclovir, which can solve the problems of increasing reaction steps, three wastes, unfavorable industrialized production and the like, and achieves the advantages of less reaction steps, good product quality and easy operation. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
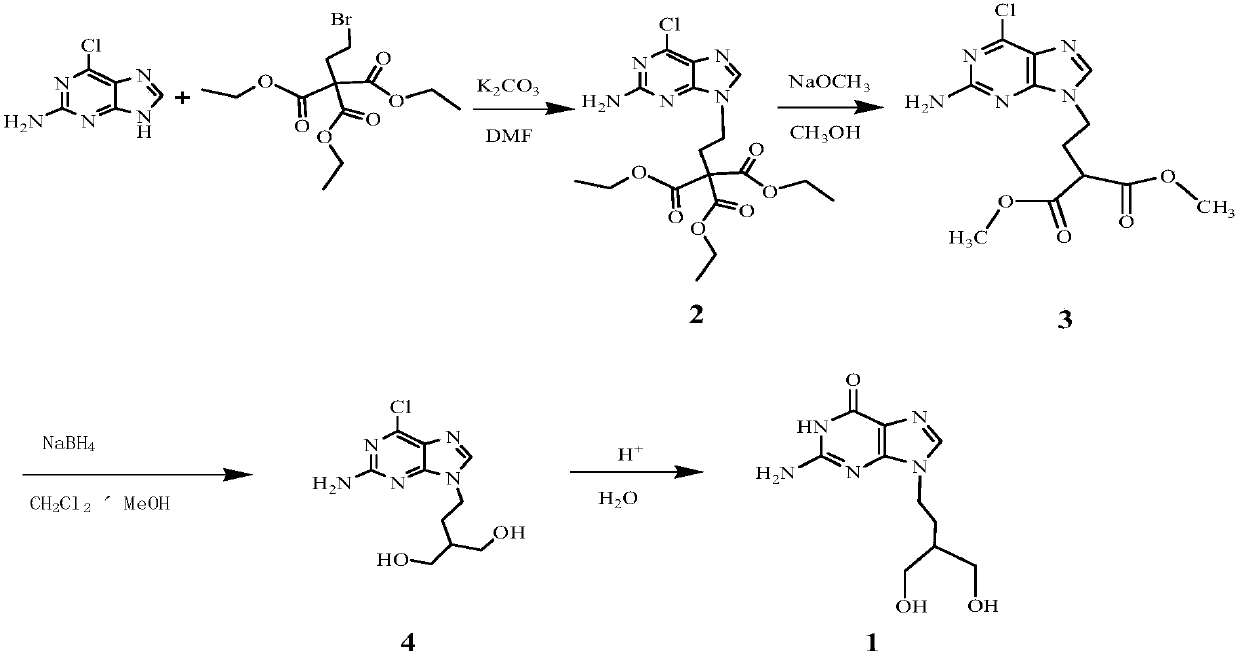
[0021] 1) Preparation of 2-amino-6-chloro-9-(3,3-dimethoxycarbonyl-1-propyl)purine
[0022] N,N-dimethylformamide 250ml (DMF, 3.24mol), potassium carbonate 15.6g (0.113mol), 2-amino-6-chloropurine 17.0g (0.1mol), bromopropane triethyl ester 36.6g ( 0.108mol) into the reaction flask, stirred, heated to 80°C, reacted overnight, filtered, washed the filter cake with DMF, combined the filtrates, and recovered DMF under reduced pressure. Add 375ml (9.3mol) of methanol and 10.7g of 21% sodium methoxide methanol solution to the remaining liquid, react at RT for 1-2h, filter, wash with methanol, and dry the wet product in vacuum at 50°C to obtain off-white solid 2-amino-6-chloro -9-(3,3-dimethoxycarbonyl-1-propyl)purine 24.8g, yield 75.6%.
[0023] 2) Preparation of 2-amino-6-chloro-9-(3-hydroxymethyl-4-hydroxyl-1-butyl)purine
[0024] Add 92ml (1.43mol) of dichloromethane, 41ml (1.01mol) of methanol, and 20g (0.06mol) of 2-amino-6-chloro-9-(3-hydroxymethyl-4-hydroxyl-1-butyl)purine...
Embodiment 2
[0028] 1) Preparation of 2-amino-6-chloro-9-(3,3-dimethoxycarbonyl-1-propyl)purine
[0029] See Example 1
[0030] 2) Preparation of 2-amino-6-chloro-9-(3-hydroxymethyl-4-hydroxyl-1-butyl)purine
[0031] See Example 1
[0032] 3) Preparation of Penciclovir
[0033] Put 8ml (0.09mol) of 36% hydrochloric acid, 80ml (4.4mol) of water, and 12g (0.044mol) of 2-amino-6-chloro-9-(3-hydroxymethyl-4-hydroxyl-1-butyl) into the reaction bottle, stirred, and heated to reflux for 4h-6h. Cool to 20°C, add ammonia water dropwise at ≤30°C to adjust the pH value to 6.5-7.5, solidify, filter, and wash the filter cake with purified water. Vacuum drying at 50°C gave 10.18 g of penciclovir with a yield of 91.1%.
Embodiment 3
[0035] 1) Preparation of 2-amino-6-chloro-9-(3,3-dimethoxycarbonyl-1-propyl)purine
[0036] See Example 1
[0037] 2) Preparation of 2-amino-6-chloro-9-(3-hydroxymethyl-4-hydroxyl-1-butyl)purine
[0038] See Example 1
[0039] 3) Preparation of Penciclovir
[0040] Add 90ml (5mol) of purified water, 3.6ml of 98% concentrated sulfuric acid and 12g (0.044mol) of 2-amino-6-chloro-9-(3-hydroxymethyl-4-hydroxyl-1-butyl) into the reaction bottle under stirring. ), heated to reflux for 3-5h. Cool to RT, add dropwise 25% sodium hydroxide aqueous solution to adjust the pH value to 6.5-7.5, precipitate solid, filter, and wash the filter cake with purified water. Vacuum drying at 50°C gave 10.3 g of penciclovir with a yield of 92.1%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com