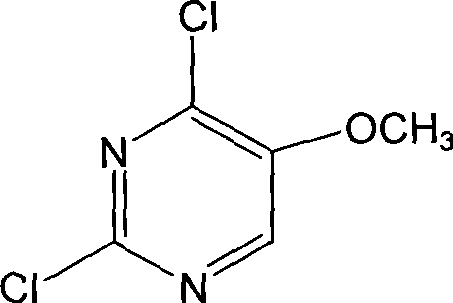
Preparation of 2,4-dichloro-5-methoxy pyrimidine
A technology of methoxypyrimidine and methoxy, which is applied in the field of preparation of pesticide intermediates, can solve the problems of high price, non-seizure and high DAT price, and achieve the effect of simple synthesis and less harsh process conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
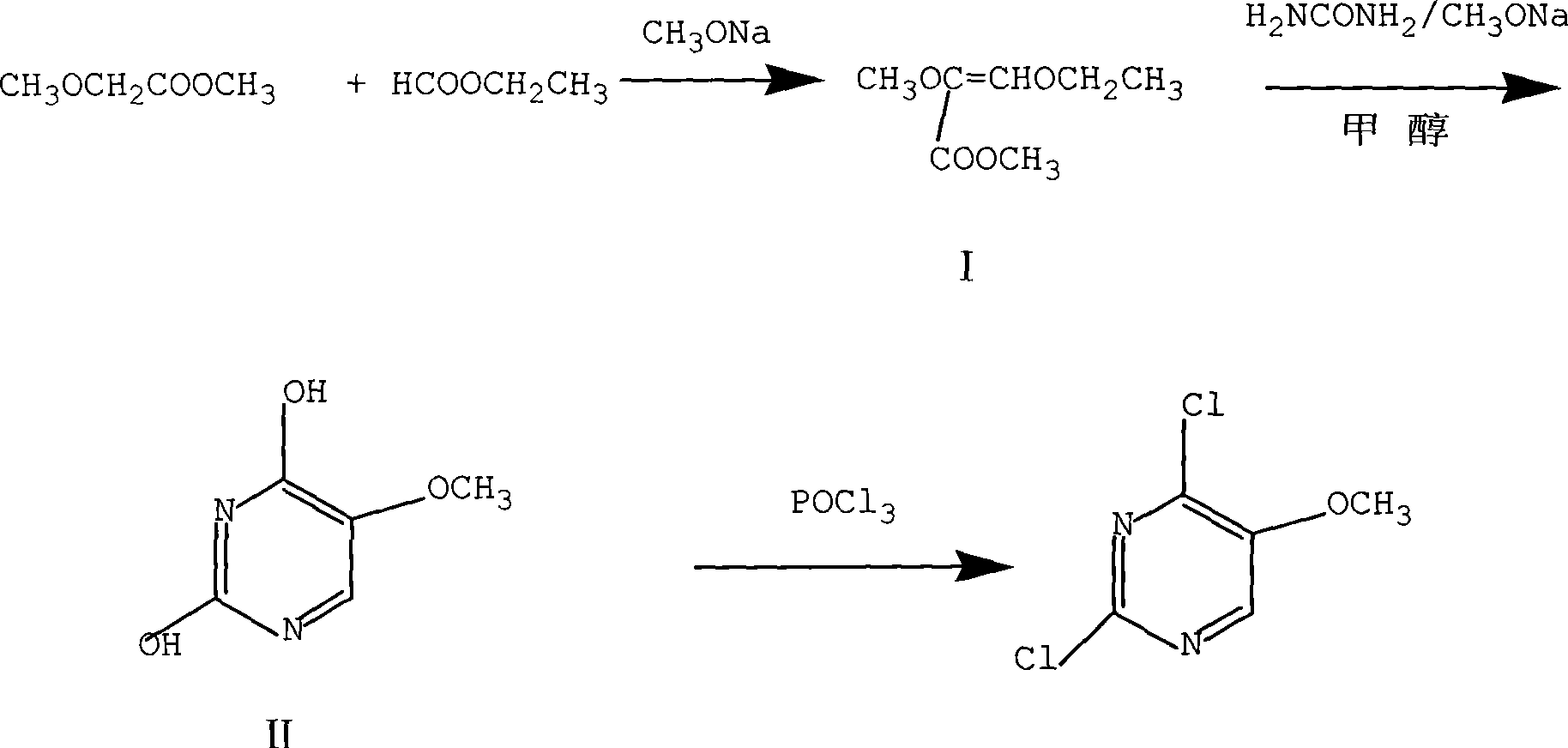
[0022] A To prepare 2,4-dihydroxy-5-methoxypyrimidine, put 222g (3.0mol) of ethyl formate and 81.0g (1.5mol) of solid sodium methylate into a thermometer, reflux condenser, dropping funnel and mechanical In a stirring 1000ml three-neck flask, stir at 30°C for 60 minutes, after cooling down to 20°C, add 104g (1mol) of methyl methoxyacetate to carry out condensation reaction at 20°C, and react for 240min after the dropwise addition to obtain the compound I, then add 300ml of methanol and 90g (1.5mol) of urea to compound I, reflux for 300min, concentrate to 1 / 2 volume, add 300ml of water to dissolve, cool to 15°C, neutralize with 6N hydrochloric acid to pH 3 , filtered, washed the filter cake twice with 100ml of water, and dried at 100°C for 8 hours to obtain 96.5g of compound II with a yield of 68% and a HPLC purity of 99.7%;
[0023] B To prepare 2,4-dichloro-5-methoxypyrimidine, add N, N-dimethyl 90.8g (0.75mol) of aniline and 230.2g (1.5mol) of phosphorus oxychloride, heated...
Embodiment 2
[0025] A Prepare 2,4-dihydroxy-5-methoxypyrimidine, put 444g (6.0mol) of ethyl formate and 56.7g (1.05mol) of solid sodium methylate into a thermometer, reflux condenser, dropping funnel and mechanical In a stirred 1000ml three-neck flask, stir at 30°C for 60 minutes, after cooling down to 20°C, add 104g (1mol) of methyl methoxyacetate to carry out condensation reaction at 0°C, and react for 400min after the dropwise addition to obtain the compound I, then add 300ml of methanol and 120g (2mol) of urea to compound I, reflux for 100min, concentrate to 1 / 2 volume, add 300ml of water to dissolve, cool to 15°C, neutralize with 2N sulfuric acid to a pH value of 3, Filter, wash the filter cake twice with 100ml of water, and dry at 100°C for 8 hours to obtain 93.6g of compound II with a yield of 66% and a purity of 99.7% by HPLC;
[0026] B prepares 2,4-dichloro-5-methoxypyrimidine, in the 500ml three-neck bottle that is equipped with thermometer, reflux condenser and mechanical stirr...
Embodiment 3
[0028] A To prepare 2,4-dihydroxy-5-methoxypyrimidine, put 296g (4.0mol) of ethyl formate and 108g (2mol) of solid sodium methylate into a tank equipped with a thermometer, reflux condenser, dropping funnel and mechanical stirring In a 1000ml three-neck flask, stir at 30°C for 60 minutes, cool down to 20°C, add 104g (1mol) of methyl methoxyacetate to carry out condensation reaction at 10°C, and react for 600min after the dropwise addition to obtain compound I. Then add 300ml of methanol and 240g (4mol) of urea to compound I, reflux for 500min, concentrate to 1 / 2 volume, add 300ml of water to dissolve, cool to 15°C, neutralize with acetic acid to pH 8, filter, and use The filter cake was washed twice with 100 ml of water, and dried at 100° C. for 8 hours to obtain 90.8 g of compound II with a yield of 64% and a purity of 99.7% by HPLC;
[0029] B To prepare 2,4-dichloro-5-methoxypyrimidine, add N, N-dimethyl Aniline 90.8g (0.75mol) and phosphorus oxychloride 153.5g (1mol), hea...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com