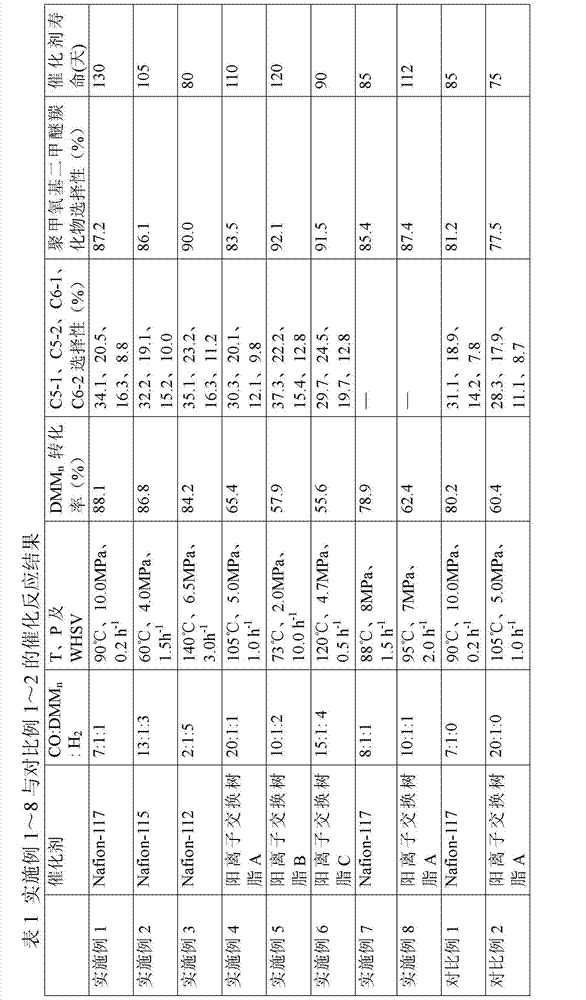
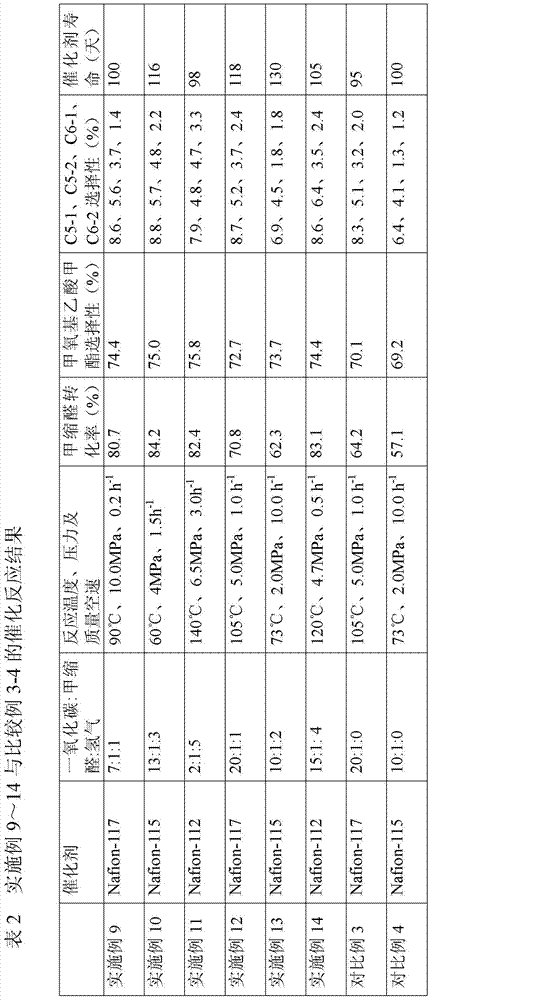
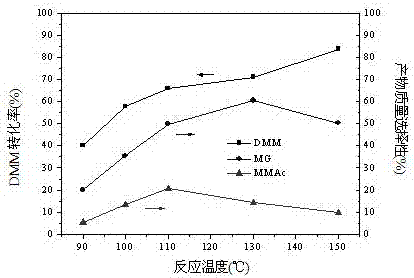
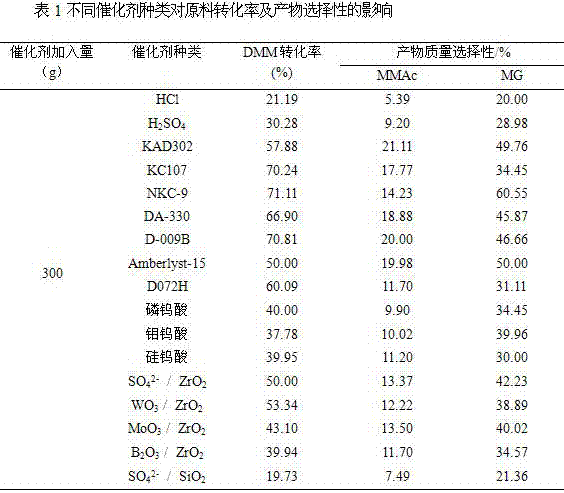
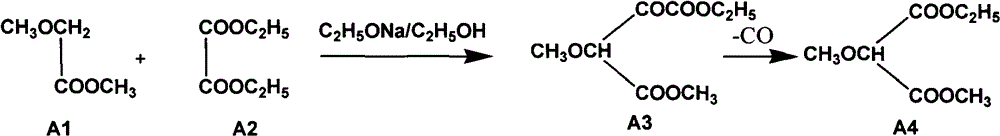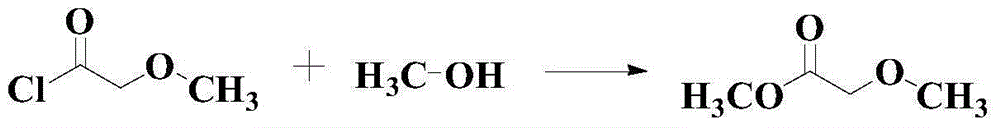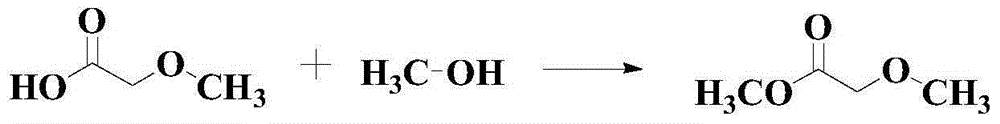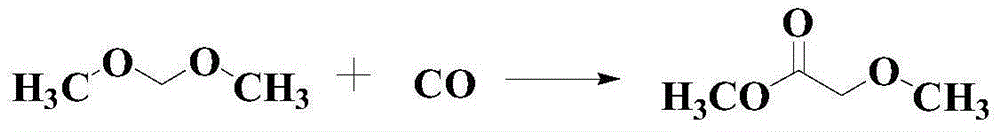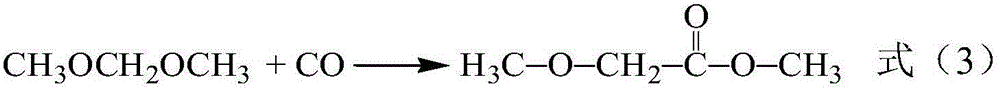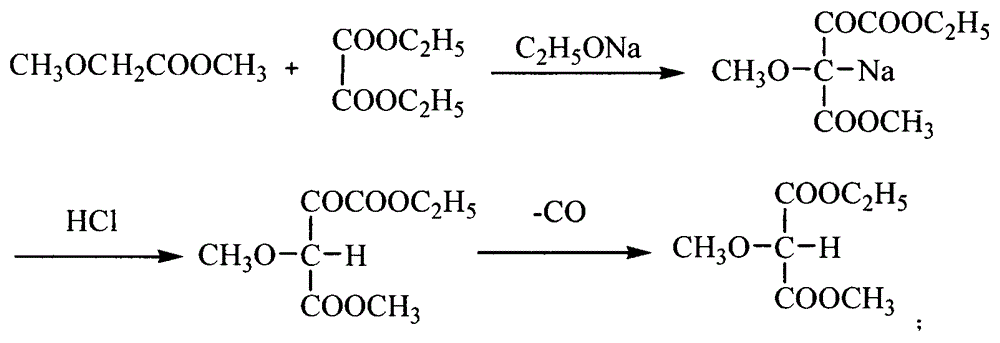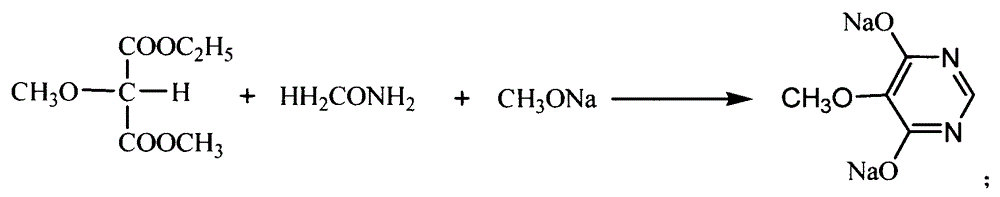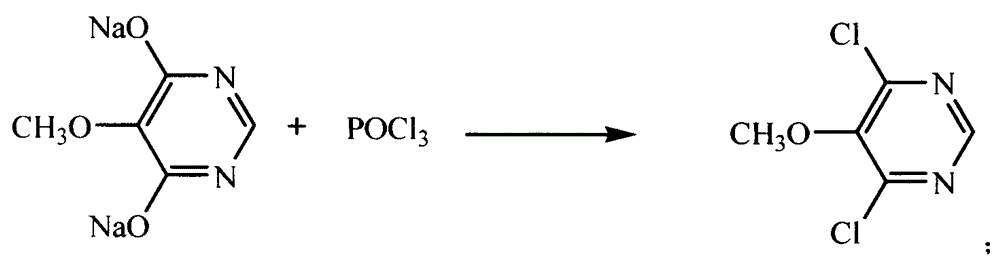Patents
Literature
89 results about "Methyl methoxyacetate" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
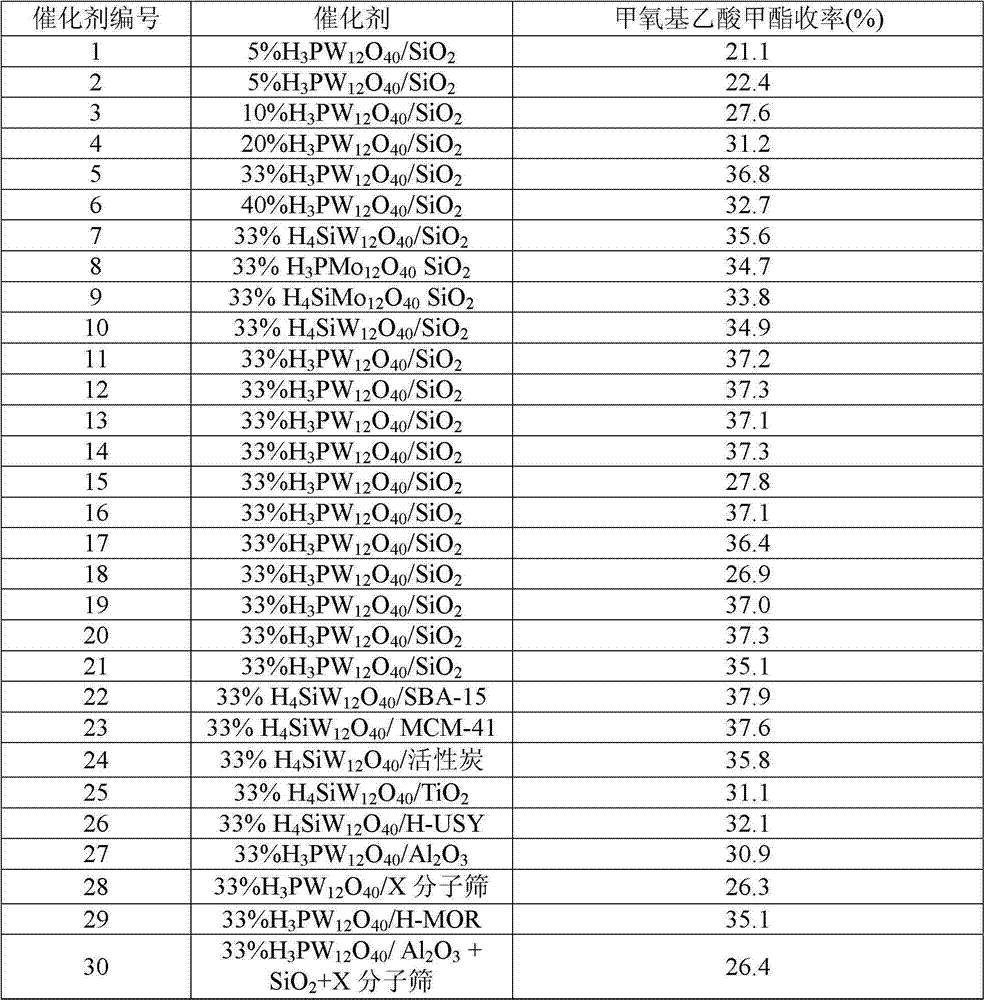
Methyl methoxyacetate (MMAC) synthesis method
InactiveCN104119228AEfficient synthesisMolecular sieve catalystsPreparation by carbon monoxide or formate reactionMolecular sieveHydrogen
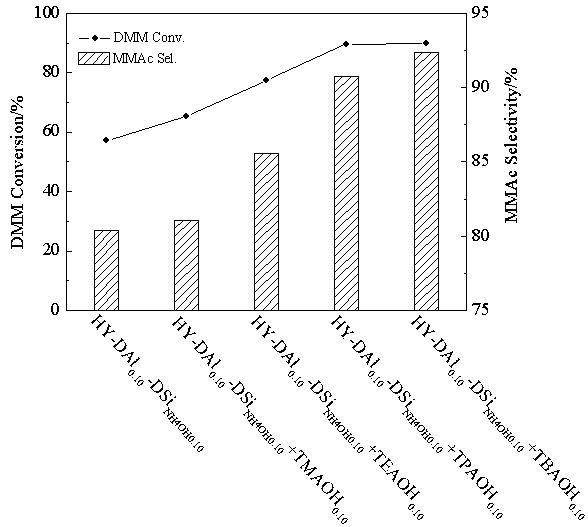
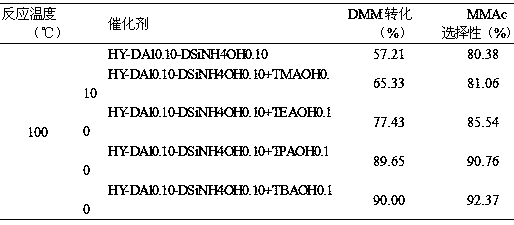
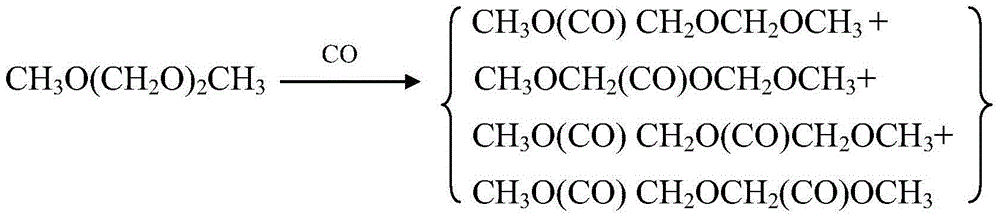
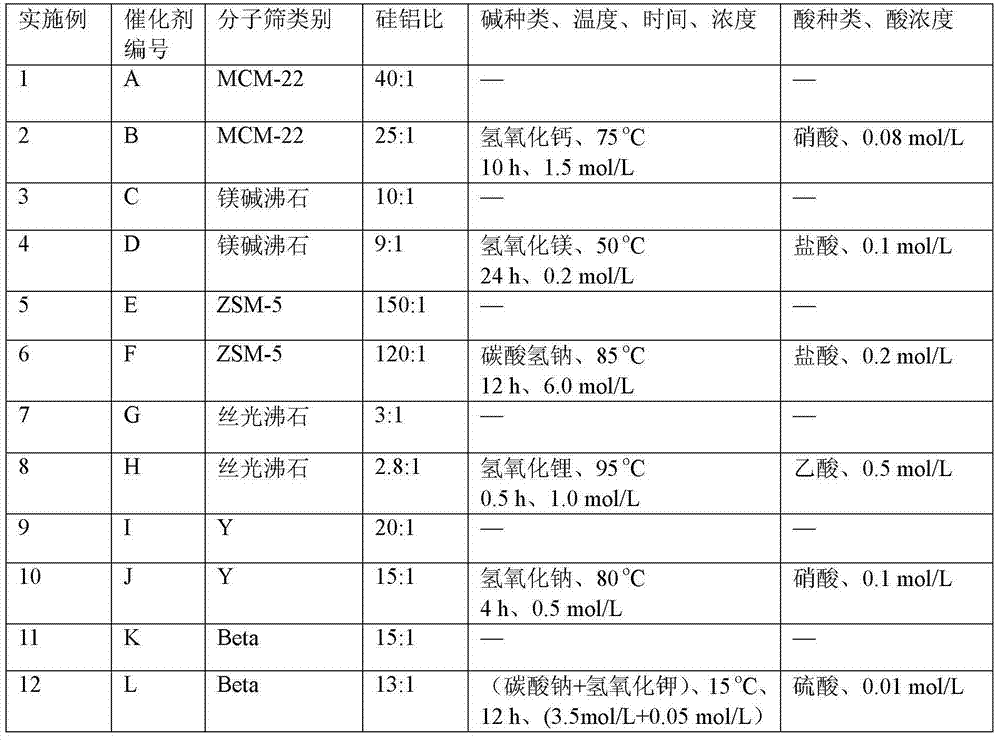
The invention discloses a catalyst for synthesis of methyl methoxyacetate (MMAC) by use of dimethoxymethane (DMM) and CO as raw materials, the catalyst is a molecular sieve with twelve membered ring cavity, ten membered ring channel and supercage structures, the International Zeolite Association (IZA) defines the topological structure of the catalyst as MWW type such as MCM-22 and MCM-49, the molecular formula is SixAlyOz, and the molecular sieve is hydrogen type. The molecular sieve SiO2 / Al2O3 ratio is 10-200. Carbonylation reaction is performed in a gas-solid phase fixed bed reactor, the reaction pressure is 1-50*105Pa, a reaction raw material gas comprises the dimethoxymethane with the partial pressure of 1-50kPa and balance of CO (1-49*105Pa) or CO / He (1-49* 105Pa), the reaction temperature is 60-180 DEG C, and the space velocity is 20-500L .g<-1>.h<-1>. Compared with a MFI type molecular sieve (HZSM-5) only having a crossed ten membered ring structure and a BEA type molecular sieve (Hbeta) only having a twelve membered ring structure, the MWW type molecular sieve has higher methyl methoxyacetate selectivity.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Continuous synthesis method for methyl methoxyacetate
InactiveCN102701977ANo emissionsEasy to operatePreparation by carbon monoxide or formate reactionChemical recyclingMethyl methoxyacetateWastewater
The invention relates to a synthesis method for methyl methoxyacetate, in particular to a continuous synthesis method for the methyl methoxyacetate. The continuous synthesis method is characterized in that: methylal and CO are used as raw materials; carbonylation reaction is performed in a carbonylation reactor in which a catalyst is filled to obtain a methyl-glycolate-containing methyl methoxyacetate rough product; then a series of separation is performed to obtain a pure product of the methyl methoxyacetate; and the methylal is recovered from a separated light component byproduct and is returned to the carbonylation reactor to realize a continuous synthesis process, wherein the purity of the methylal and the molar ratio of the methylal to the CO are any values, and the reaction pressure in the carbonylation reactor is greater than or equal to normal pressure. According to the method, the production efficiency can be effectively increased, the production process can be simplified, the emission of waste residue and wastewater in a production process can bee reduced, and the aims of continuous production and reduction of the production cost are achieved.
Owner:东莞市同舟化工有限公司
Methyl methoxyacetate preparation method
InactiveCN103172517AEasy to separateMild reaction conditionsPreparation by carbon monoxide or formate reactionGas phaseMethyl methoxyacetate
The invention discloses a methyl methoxyacetate preparation method by gaseous carbonylation. The method comprises the step of reacting methylal and a CO-containing gas phase medium under the action of heteropolyacid and supported heteropolyacid catalyst to generate the methyl methoxyacetate, wherein the reaction temperature is 50-300 DEG C and the reaction pressure is 0.1-4MPa. Compared with the traditional ethylene glycol precursor preparation method by liquid phase carbonylation reaction synthesis, the method provided by the invention has the advantages of mild reaction conditions and high catalytic efficiency. Furthermore, the product is easily separated from the catalyst as no solvent is used in the reaction, which is conductive to large-scale industrial production.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Method for producing methyl methoxyacetate by methylal vapor-phase carbonylation by using supported heteropoly acid catalyst
InactiveCN103172516AEasy to separateMild reaction conditionsPreparation by carbon monoxide or formate reactionActivated carbonHeteropoly acid
The invention relates to application of a supported heteropoly acid catalyst in production of methyl methoxyacetate by methylal vapor-phase carbonylation. The catalyst is prepared by an isometric impregnation method, and uses at least one of activated carbon, TiO2, Al2O3, SiO2, SBA-15, MCM-41 and zeolite molecular sieve as a supporter, wherein the heteropoly acid accounts for 0.1-50 wt% of the supported heteropoly acid catalyst. When being used for the reaction for producing methyl methoxyacetate by methylal vapor-phase carbonylation, the catalyst has the characteristics of mild reaction conditions and high catalytic efficiency; and since no solvent is used in the reaction, the product and catalyst can be easily separated, thereby being beneficial to large-scale industrial production.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
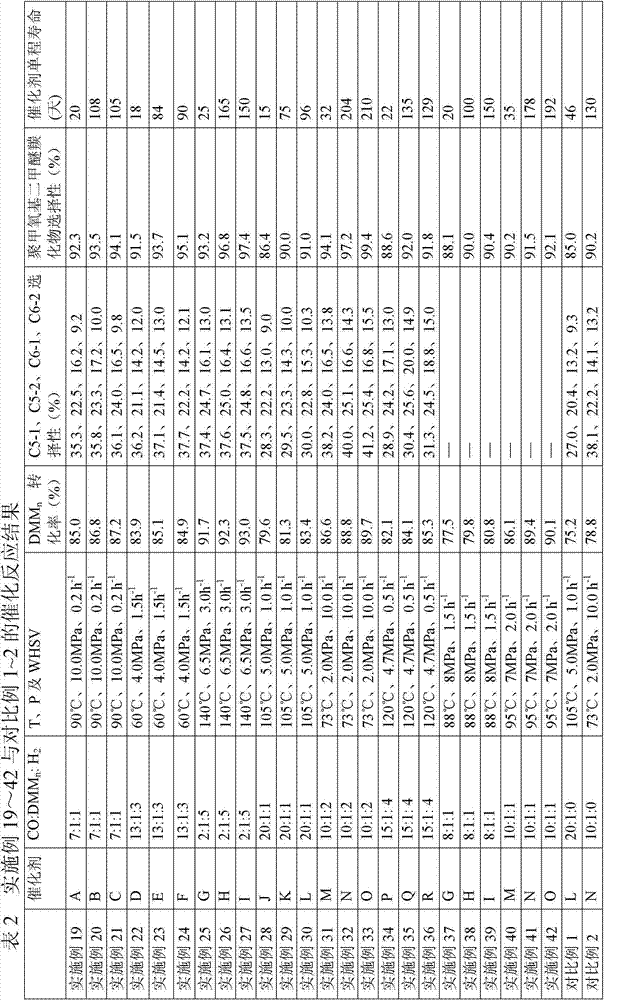
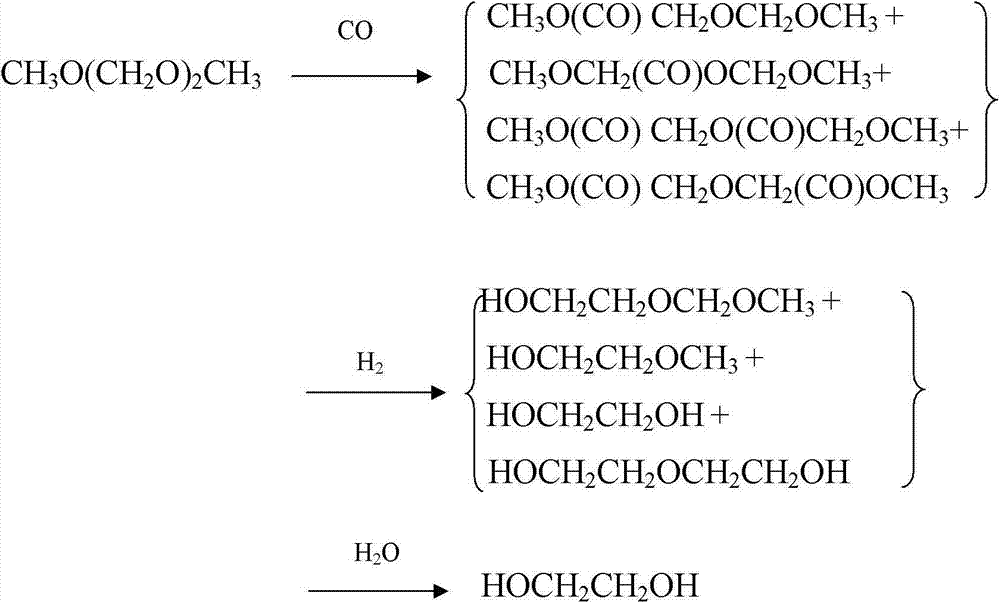
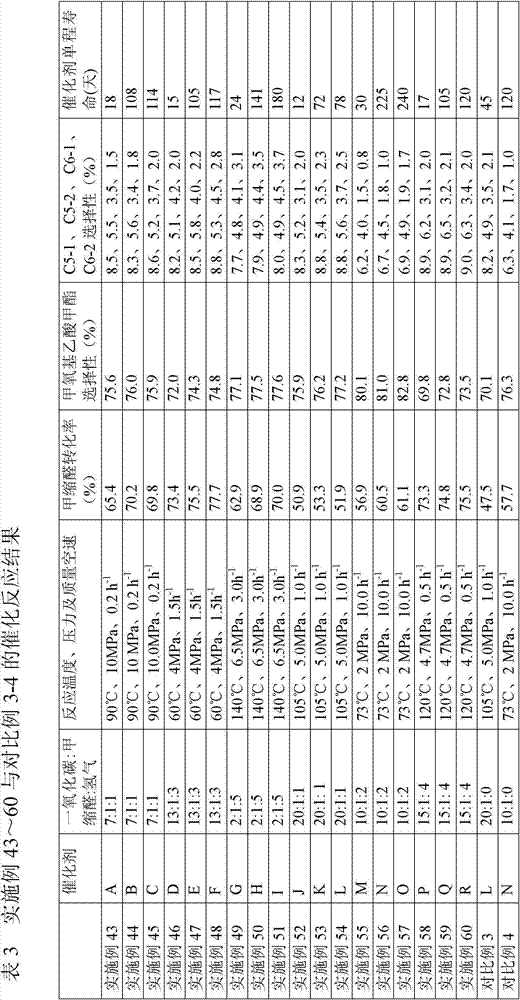
Method for preparing polyoxymethylene dimethyl ether carboxylate and methyl methoxy acetate
ActiveCN104725229AImprove conversion rateHigh selectivityOrganic compound preparationPreparation by carbon monoxide or formate reactionPolymer sciencePtru catalyst
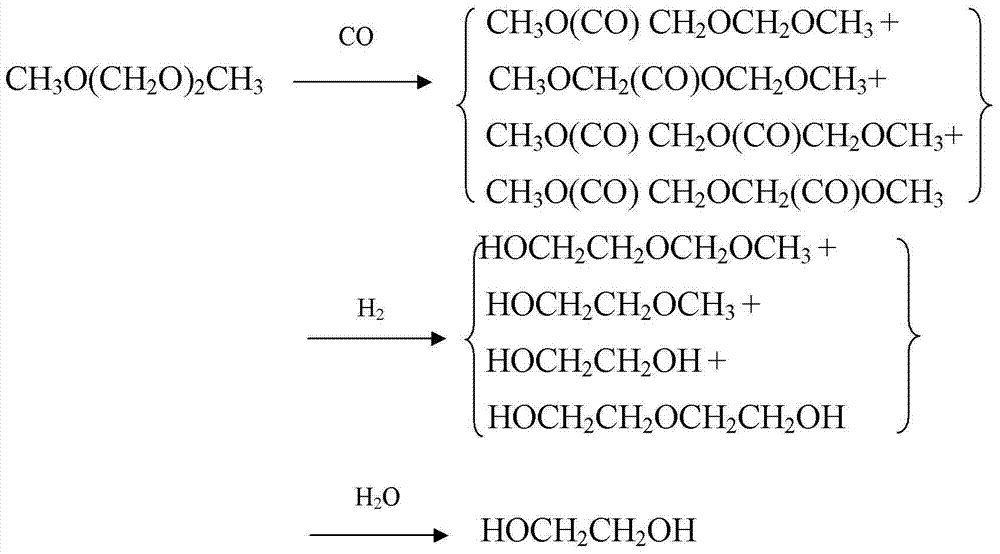
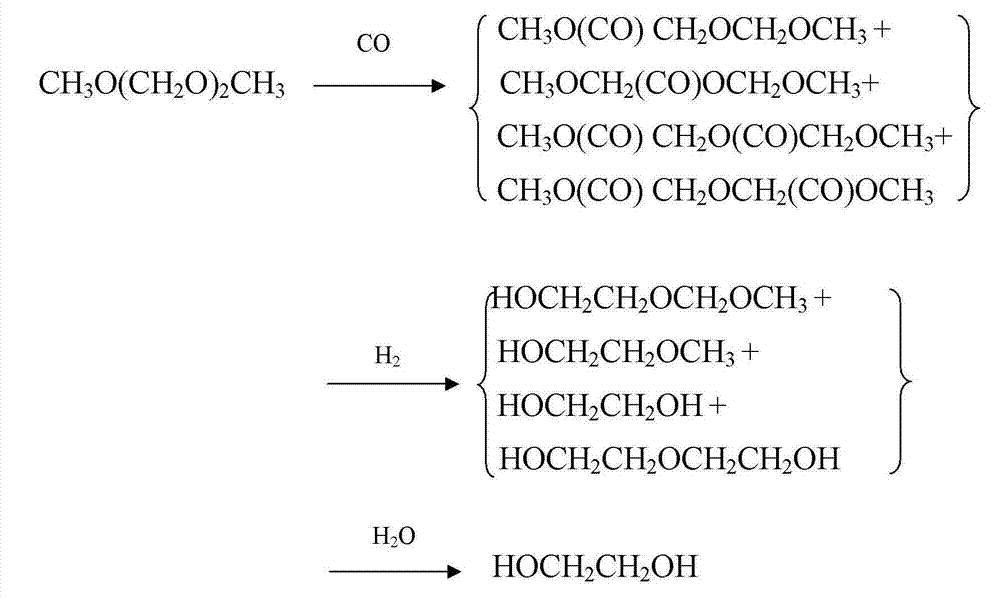
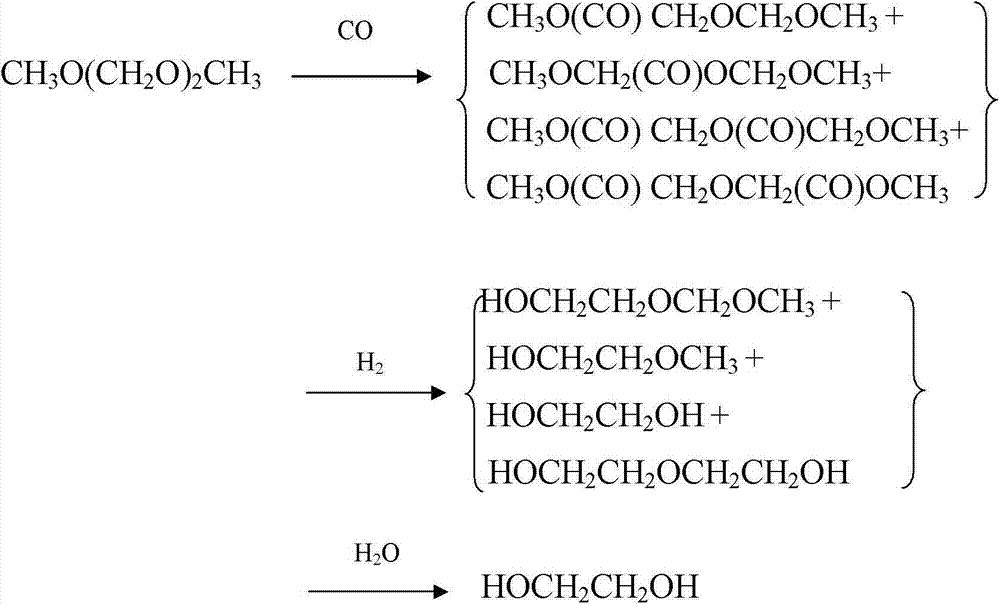
The invention provides a method for preparing polyoxymethylene dimethyl ether carboxylate and / or methyl methoxy acetate which serves as an intermediate for producing ethylene glycol. The method comprises the step of enabling a raw material, namely polyoxymethylene dimethyl ether or methylal, together with carbon monoxide and hydrogen gas to react in a dealuminzation modified acidic molecular sieve catalyst loaded reactor under appropriate reaction conditions without adding other solvents, so as to prepare corresponding products, wherein a reaction process is of gas-liquid-solid three-phase reaction. According to the method provided by the invention, the conversion ratio of the raw material polyoxymethylene dimethyl ether or methylal is high, the selectivity of each product is high, the service life of a catalyst is long, external solvents are not required to be used, reaction conditions are relatively mild, and continuous production can be carried out, so that the method has industrial application potential. Furthermore, the obtained products can be used for producing ethylene glycol through hydrolyzing after hydrogenating or hydrogenating after hydrolyzing.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Method for preparing polyoxymethylene dimethyl ether carboxylate and methyl methoxy acetate
ActiveCN104725230AImprove conversion rateHigh selectivityMolecular sieve catalystsPreparation by carbon monoxide or formate reactionMethyl methoxyacetateSolvent
The invention provides a method for preparing polyoxymethylene dimethyl ether carboxylate and / or methyl methoxy acetate which serves as an intermediate for producing ethylene glycol. The method comprises the step of enabling a raw material, namely polyoxymethylene dimethyl ether or methylal, together with carbon monoxide and hydrogen gas to react in a desiliconisation modified acidic molecular sieve catalyst loaded reactor under appropriate reaction conditions without adding other solvents, so as to prepare corresponding products, wherein a reaction process is of gas-liquid-solid three-phase reaction. According to the method provided by the invention, the conversion ratio of the raw material polyoxymethylene dimethyl ether or methylal is high, the selectivity of each product is high, the service life of a catalyst is long, external solvents are not required to be used, reaction conditions are relatively mild, and continuous production can be carried out, so that the method has industrial application potential. Furthermore, the obtained products can be used for producing ethylene glycol through hydrolyzing after hydrogenating or hydrogenating after hydrolyzing.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
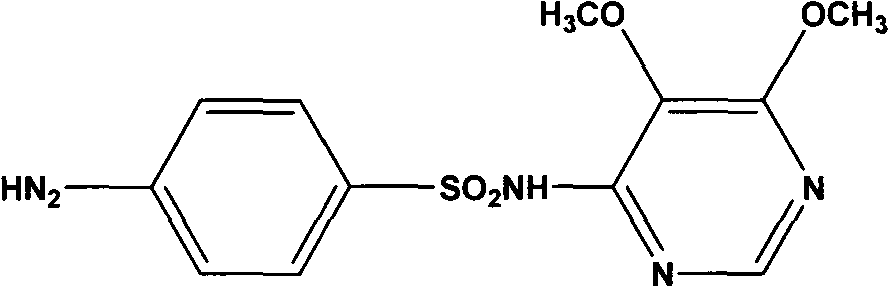
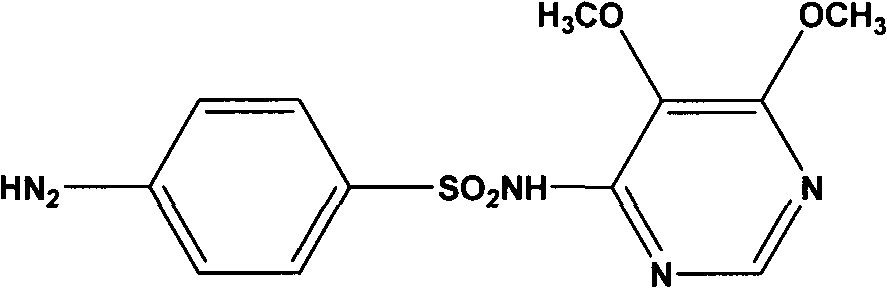
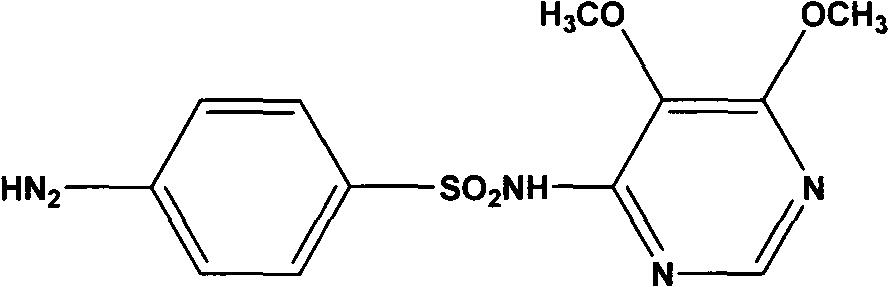
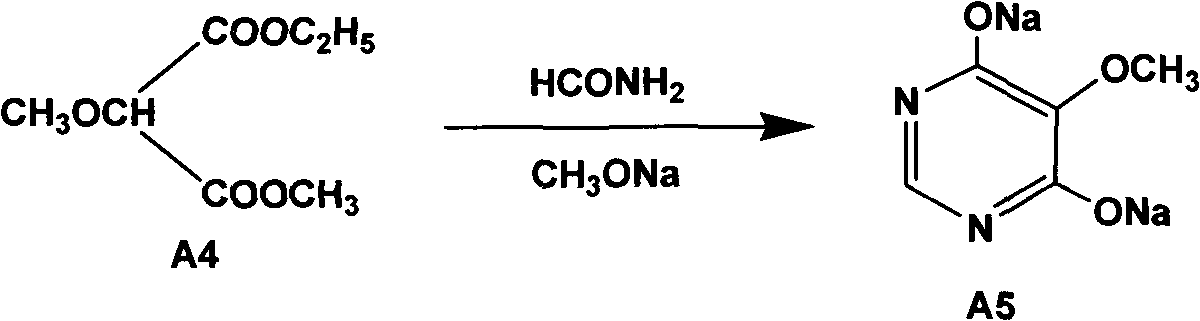
Preparation method of sulfadoxine and intermediate thereof
InactiveCN102304094AReduce manufacturing costHigh purityOrganic chemistryMethyl methoxyacetateMalonate
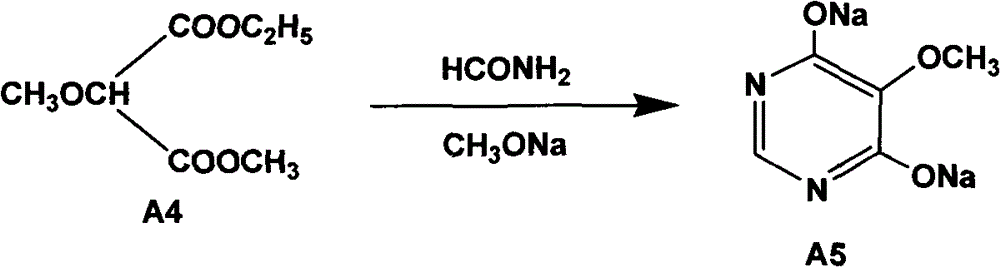
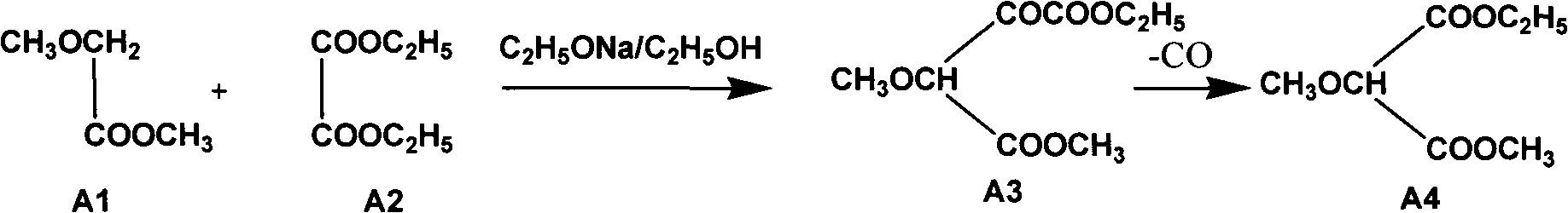
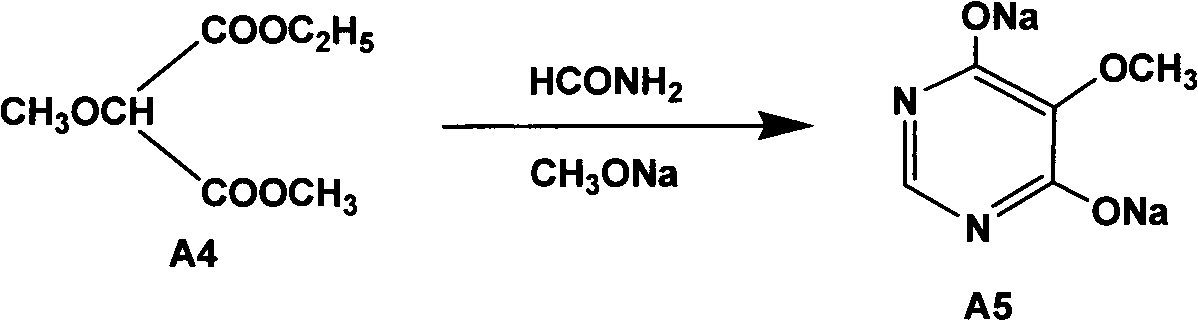
The invention relates to a preparation method of sulfadoxine and an intermediate thereof. The preparation method of sulfadoxine comprises the following steps: (1) reacting methyl methoxyacetate and excessive diethyl oxalate to generate 3-methoxy-2-oxo-methylethyl succinate, and decarbonylating to obtain 2-methoxy-methylethyl malonate; (2) reacting the 2-methoxy-methylethyl malonate and formamide to generate a cyclocompound; (3) reacting the cyclocompound and phosphorus oxychloride to generate chloride; (4) carrying out condensation reaction; and (5) carrying out methoxylation reaction. The obtained cyclocompound in the step (2) is controlled to exist in the form of anhydrous hydroxy sodium salt, so that N,N-dimethylaniline is not needed as a catalyst in the step (3), thereby lowering the cost, enhancing the quality of chloride and finally enhancing the quality of sulfadoxine.
Owner:CHANGSHU NANHU INDAL CHEM
Method for preparing polyoxymethylene dimethyl ether carboxylate and methyl methoxy acetate
ActiveCN104725224AImprove conversion rateHigh selectivityPreparation by carbon monoxide or formate reactionMethyl methoxyacetateCarboxylic salt
The invention provides a method for preparing polyoxymethylene dimethyl ether carboxylate and / or methyl methoxy acetate which serves as an intermediate for producing ethylene glycol. The method comprises the step of enabling a raw material, namely polyoxymethylene dimethyl ether or methylal, together with carbon monoxide and hydrogen gas to react in an acidic molecular sieve catalyst loaded reactor under appropriate reaction conditions without adding other solvents, so as to prepare corresponding products, wherein a reaction process is of gas-liquid-solid three-phase reaction. According to the method provided by the invention, the conversion ratio of the raw material polyoxymethylene dimethyl ether or methylal is high, the selectivity of each product is high, the service life of a catalyst is long, external solvents are not required to be used, reaction conditions are relatively mild, and continuous production can be carried out, so that the method has industrial application potential. Furthermore, the obtained products can be used for producing ethylene glycol through hydrolyzing after hydrogenating or hydrogenating after hydrolyzing.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
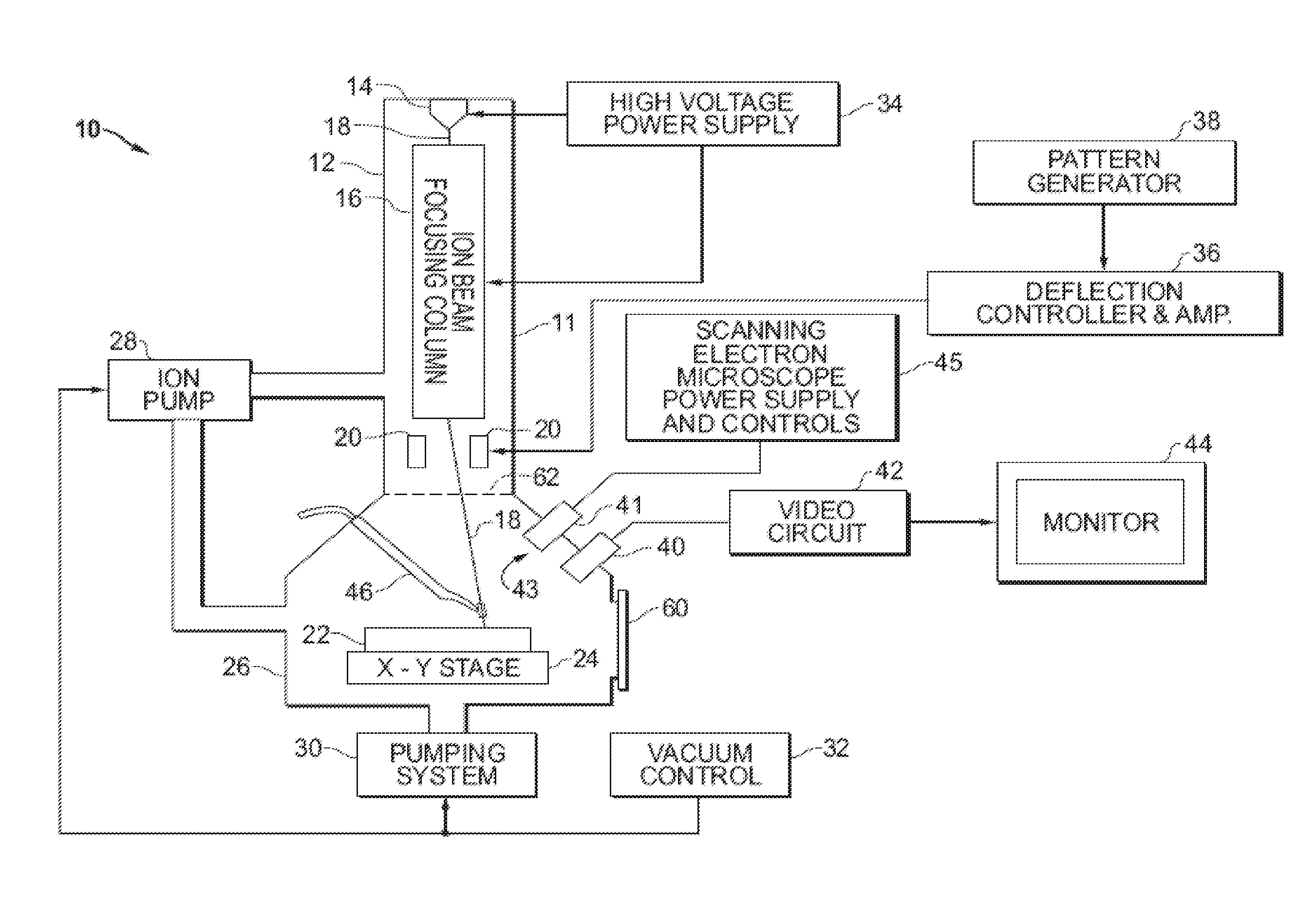
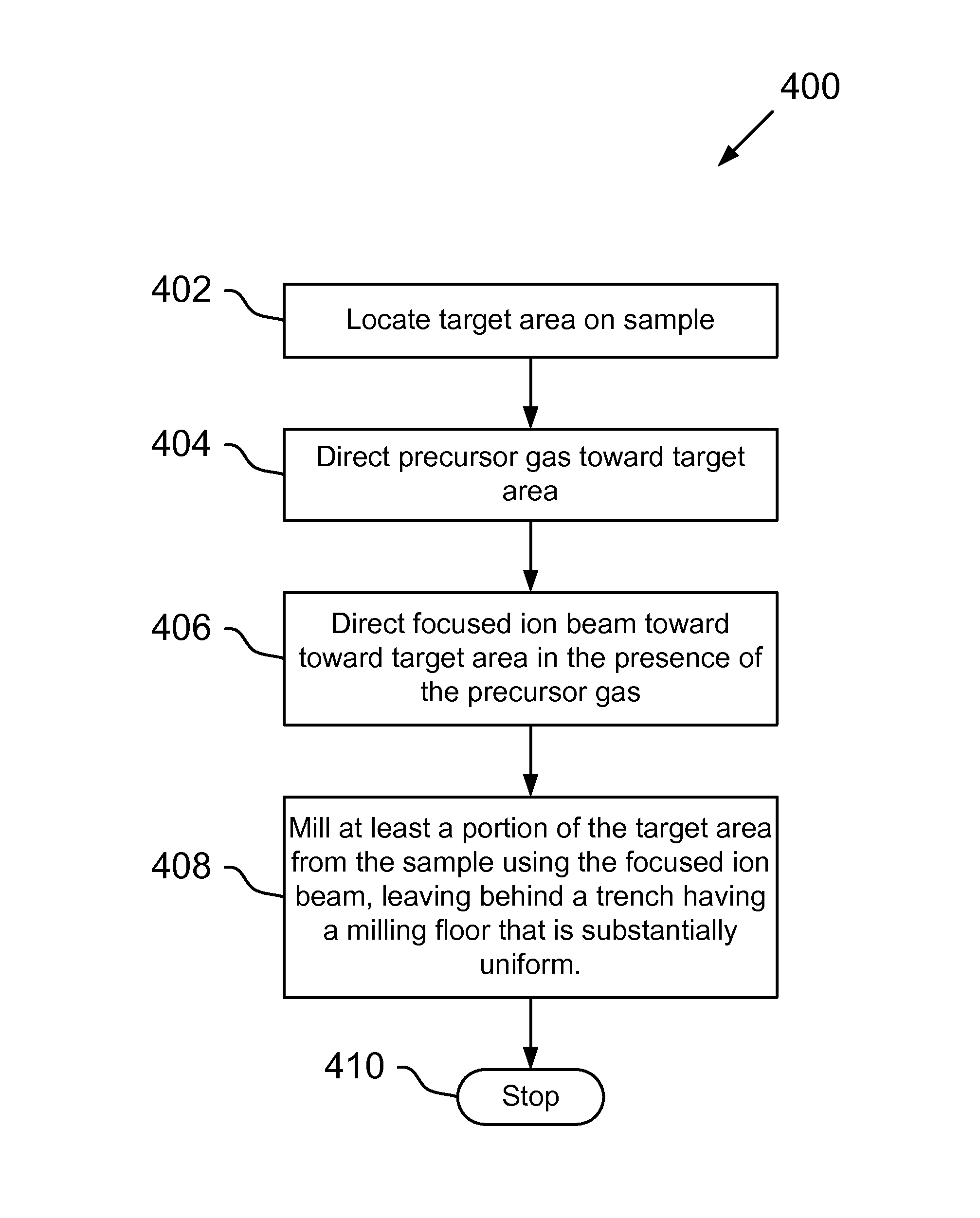
Precursor for planar deprocessing of semiconductor devices using a focused ion beam
ActiveUS9064811B2Easy to useElectric discharge tubesDecorative surface effectsMethyl methoxyacetateIon beam
A method and system for improved planar deprocessing of semiconductor devices using a focused ion beam system. The method comprises defining a target area to be removed, the target area including at least a portion of a mixed copper and dielectric layer of a semiconductor device; directing a precursor gas toward the target area; and directing a focused ion beam toward the target area in the presence of the precursor gas, thereby removing at least a portion of a first mixed copper and dielectric layer and producing a uniformly smooth floor in the milled target area. The precursor gas causes the focused ion beam to mill the copper at substantially the same rate as the dielectric. In a preferred embodiment, the precursor gas comprises methyl nitroacetate. In alternative embodiments, the precursor gas is methyl acetate, ethyl acetate, ethyl nitroacetate, propyl acetate, propyl nitroacetate, nitro ethyl acetate, methyl methoxyacetate, or methoxy acetylchloride.
Owner:FEI CO
Preparation method of sulfadoxine
InactiveCN102304095AReduce usageQuality improvementOrganic chemistrySodium methoxideDimethylaniline N-oxide
The invention relates to a preparation method of sulfadoxine, which comprises the following steps: (1) reacting methyl methoxyacetate and excessive diethyl oxalate to generate 3-methoxy-2-oxo-methylethyl succinate, and decarbonylating to obtain 2-methoxy-methylethyl malonate; (2) reacting the 2-methoxy-methylethyl malonate and formamide to generate a cyclocompound; (3) carrying out chlorination reaction; (4) carrying out condensation reaction; and (5) carrying out methoxylation reaction. The purity of the 2-methoxy-methylethyl malonate obtained in the step (1) is strictly controlled, and the cyclocompound in the step (2) is controlled to exist in the form of anhydrous hydroxy sodium salt; in the step (3), no catalyst, including N,N-dimethylaniline, is used; and in the step (5), solid sodium hydroxide is substituted for sodium methoxide solution. The invention is easy to operate, has the advantage of high product quality, greatly lowers the production cost, and has obvious economic benefit and environmental benefit.
Owner:CHANGSHU NANHU INDAL CHEM
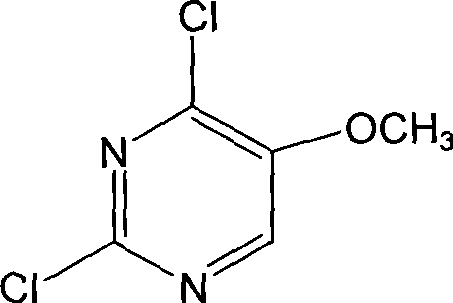
Preparation of 2,4-dichloro-5-methoxy pyrimidine
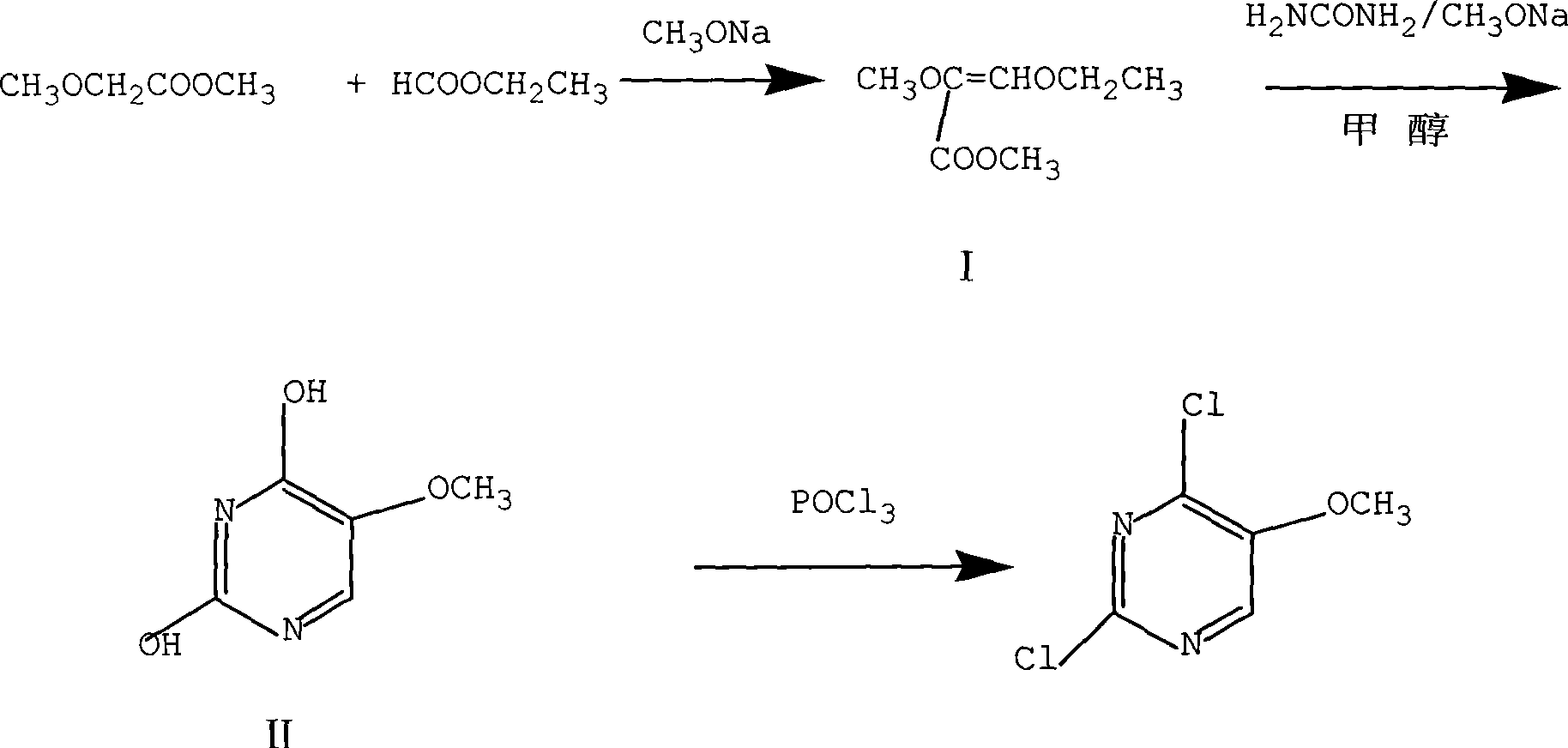
InactiveCN101486684ARaw materials are easy to getEasy to synthesizeOrganic chemistrySodium methoxideMethyl methoxyacetate
The invention discloses a preparation method of 2, 4-dichloro-5-methoxypyrimidine, pertaining to the technical field of pesticide intermediate preparation. The method includes steps as follows: 2, 4-dihydroxy-5-methoxypyrimidine is prepared, and ethyl formate and solid sodium methoxide are added into a reaction device and stirred. After the temperature is lowered, methyl methoxyacetate is added for carrying out a condensation reaction to obtain a compound I, and then methanol and carbamide are added into the compound I and a refluxing reaction is carried out. A compound II is obtained after condensation, dissolution with water, cooling, neutralization, filtration and drying; the 2, 4-dichloro-5-methoxypyrimidine is prepared, and a chlorinating agent and an acid-binding agent are added into the compound II; and then a temperature reaction, dilution and filtration are carried out in sequence to obtain a crude product of the 2, 4-dichloro-5-methoxypyrimidine. The crude product is refined to obtain a pure product of the 2, 4-dichloro-5-methoxypyrimidine. The method has the advantages of easy availability of all raw materials, convenient synthesis, not exacting technological conditions, overall yield up to 57 percent to 67 percent, purity over 99.6 percent and applicability to industrialized production.
Owner:JIANGSU HUAYI TECH
Precursor for Planar Deprocessing of Semiconductor Devices using a Focused Ion Beam
ActiveUS20140357088A1Uniform removalEasy to useElectric discharge tubesSemiconductor/solid-state device manufacturingMethyl methoxyacetateIon beam
A method and system for improved planar deprocessing of semiconductor devices using a focused ion beam system. The method comprises defining a target area to be removed, the target area including at least a portion of a mixed copper and dielectric layer of a semiconductor device; directing a precursor gas toward the target area; and directing a focused ion beam toward the target area in the presence of the precursor gas, thereby removing at least a portion of a first mixed copper and dielectric layer and producing a uniformly smooth floor in the milled target area. The precursor gas causes the focused ion beam to mill the copper at substantially the same rate as the dielectric. In a preferred embodiment, the precursor gas comprises methyl nitroacetate. In alternative embodiments, the precursor gas is methyl acetate, ethyl acetate, ethyl nitroacetate, propyl acetate, propyl nitroacetate, nitro ethyl acetate, methyl methoxyacetate, or methoxy acetylchloride.
Owner:FEI CO
Process for the production of alkyl alkoxyacetates
InactiveUS7772423B2Organic compound preparationPreparation by carbon monoxide or formate reactionGas phaseMethyl methoxyacetate
A process for the production of lower alkyl alkoxyacetates, preferably methyl methoxyacetate, by reaction of a di-(lower alkoxy)methane, preferably dimethoxymethane, with the acid form of a medium-pore or large-pore zeolite catalyst, preferably the acid form of faujasite, ZSM-5, mordenite, or beta, in the gas phase at atmospheric or near-atmospheric pressures.
Owner:RGT UNIV OF CALIFORNIA
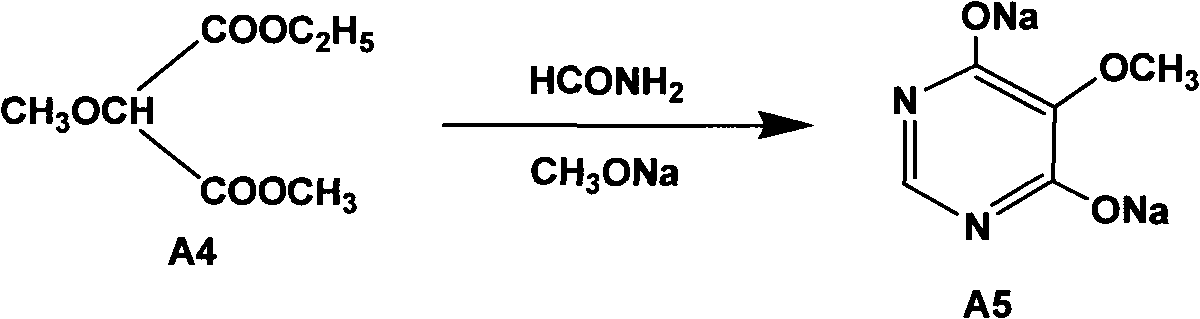
Synthesizing method of 2-amino-5,8-dimethoxy[1,2,4]-triazolo[1,5-c]-pyrimidine
The invention relates to a synthesizing method of 2-amino-5,8-dimethoxy[1,2,4]-triazolo[1,5-c]-pyrimidine. The invention solves the problems such as low yield, high pollution, complicated operation, and the like of current preparation methods. The method provided by the invention comprises the steps that: methoxy methyl acetate and methyl formate are condensed under a strong-alkali condition; the condensation product is subjected to cyclization with thiourea; methylation is carried out by using chloromethane; chlorination is carried out by using phosphorus oxychloride; hydrazination is carried out by using hydrazine hydrate; the product is subjected to cyclization with cyano bromine; and under the effect of strong alkali and acrylate, a final product is obtained. With the method provided by the invention, the yield of each step is higher than 80%, and a total yield reaches 39%. The method is suitable for industrialized productions. The method provided by the invention belongs to the field of paddy rice herbicide penoxsulam intermediate preparation.
Owner:HEILONGJIANG UNIV
Preparation method of high-crystallinity hierarchical pore molecular sieve
InactiveCN109368655AImprove mass transfer efficiencyImprove the crystallinity of molecular sieveMolecular sieve catalystsCatalyst activation/preparationMolecular sieveMethyl methoxyacetate
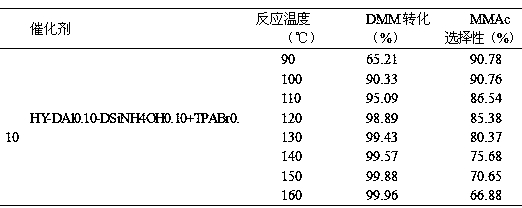
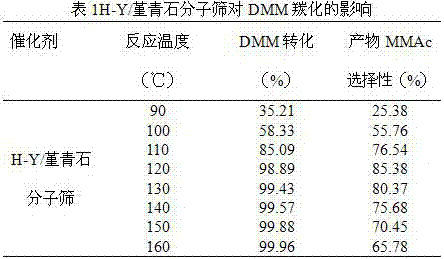
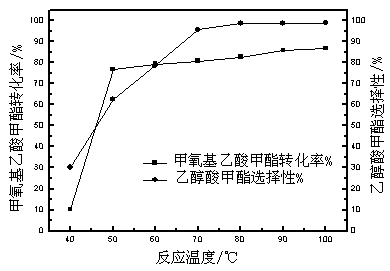
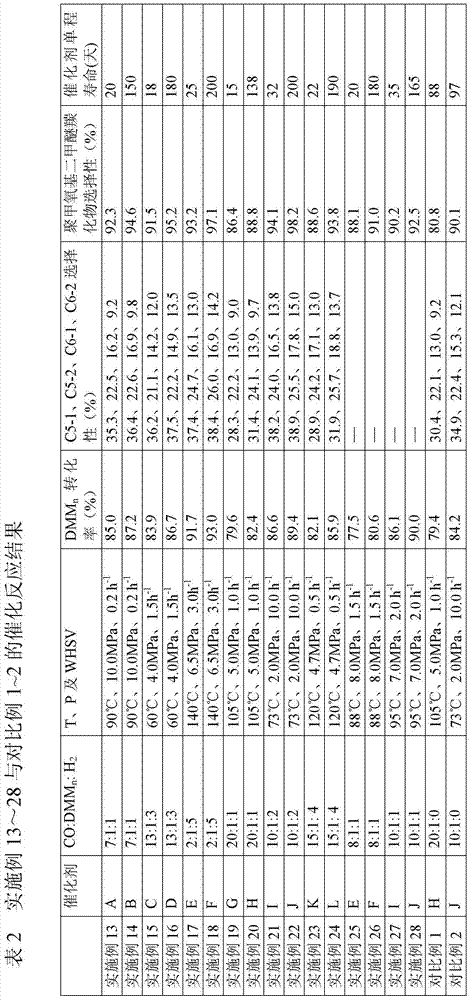
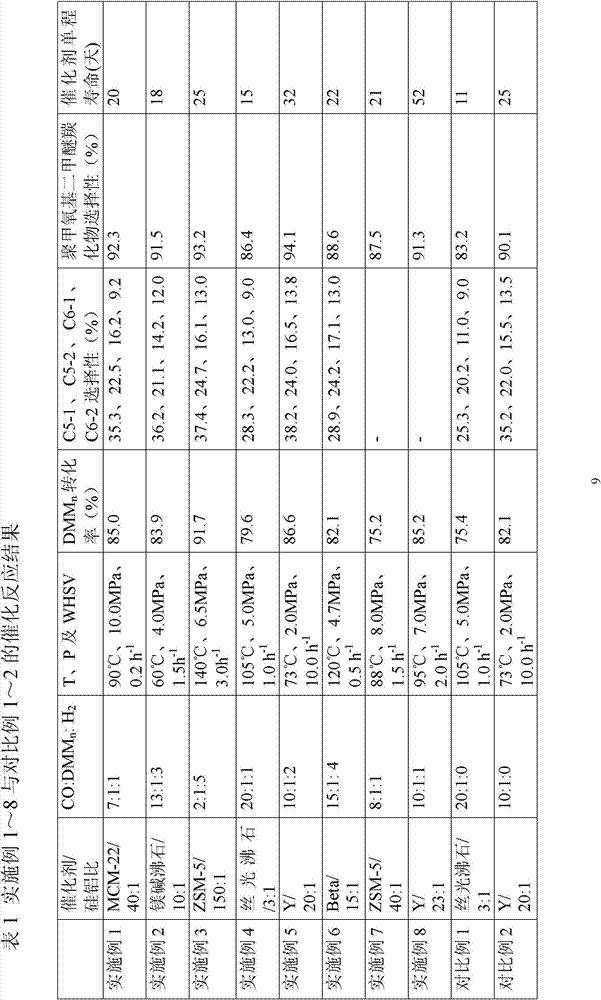
The invention discloses a preparation method of a high-crystallinity hierarchical pore molecular sieve and relates to a preparation method of a molecular sieve catalyst. According to the preparation method disclosed by the invention, an organic guide agent is added in the conventional acid and base desilicication and dealumination process and achieves the effects of molecular sieve pore channel supporting and porous structure optimizing, and acid strength and acid distribution in the molecular sieve pore channel are effectively optimized. The crystallinity and acid strength of the modified molecular sieve are obviously improved, and the molecular sieve has large pore diameter and mesoporous-microporous composite structure, and the structure obviously promotes the mass transfer efficiency of the heavy component product. The prepared high-crystallinity large-pore diameter hierarchical pore HY molecular sieve is used for methylal carbonylation reaction for preparing high-value-added methyl methoxyacetate, and when the reaction pressure is 5.0 MPa and the reaction temperature is 100 DEG C, the methylal conversion rate is close to 100%, the methyl methoxyacetate selectivity is higher than 95%, and the catalyst does not have obvious inactivation during stability evaluation of 1000 hours.
Owner:SHENYANG INSTITUTE OF CHEMICAL TECHNOLOGY
Preparation method of sulfadoxine
InactiveCN102304095BHigh melting pointReduce usageOrganic chemistrySodium methoxideDimethylaniline N-oxide
Owner:CHANGSHU NANHU INDAL CHEM
Method for preparing methyl methoxyacetate from methanol-containing methylal
ActiveCN106397201AConvenient sourceReduce pollutionOrganic compound preparationPreparation by carbon monoxide or formate reactionMethyl methoxyacetateReaction temperature
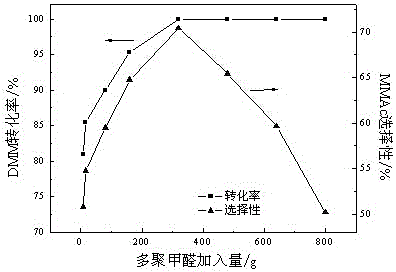
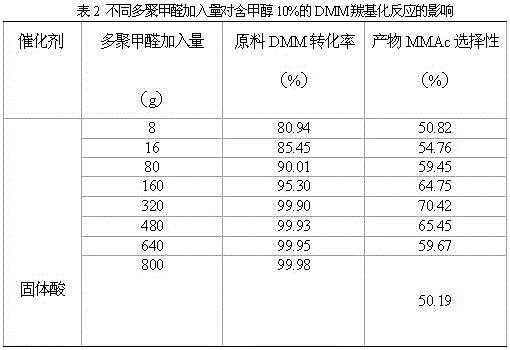
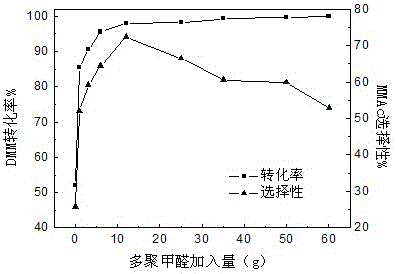
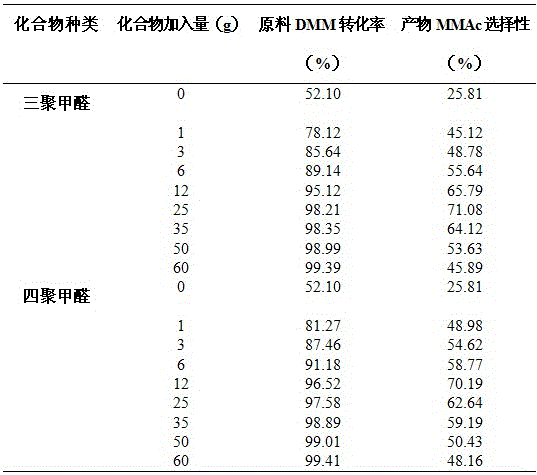
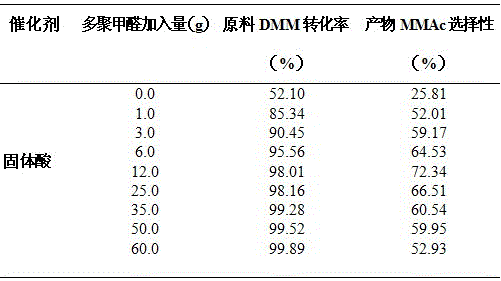
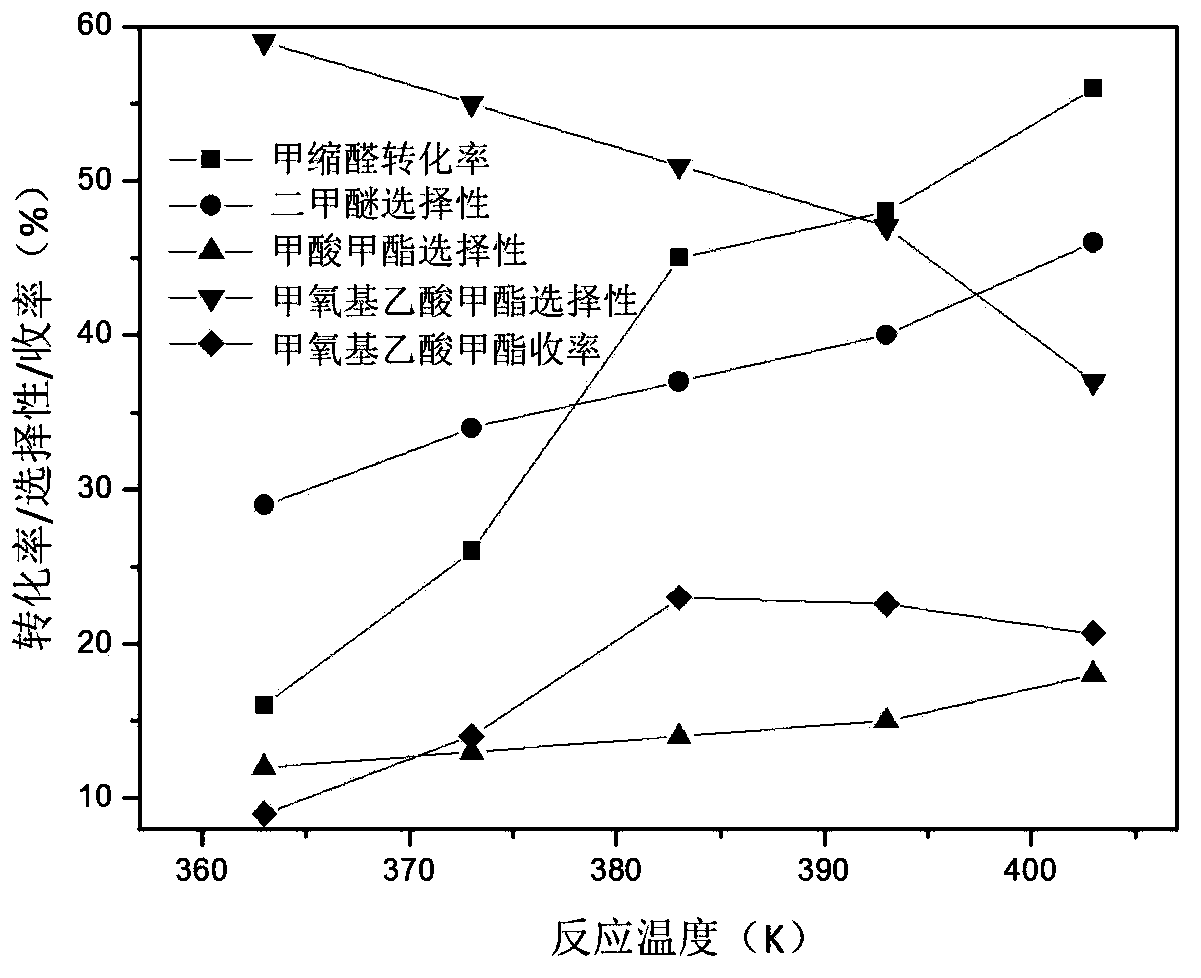
A method for preparing methyl methoxyacetate from methanol-containing methylal relates to a method for preparing the methyl methoxyacetate. According to the method, the influence of trace methanol in raw materials on carbonylation is eliminated by adding formaldehyde compounds into an industrial raw material, namely the methylal, so that the selectivity and the yield of the main product, namely the methyl methoxyacetate, are higher; the formaldehyde compounds are added during carbonylation of methanol-containing methylal; the formaldehyde substances are one or more paraformaldehyde mixtures of trioxymethylene, tetraoxymethylene or paraformaldehyde; the mass ratio of the added formaldehyde to the raw material methylal is 0.5% to 50%; the reaction temperature is 90 to 160 DEG C and the reaction pressure is 3.0 to 10.0 MPa. According to the method, the influence of the methanol on DMM carbonylation is eliminated by adding a small amount of formaldehyde compounds into the raw material methylal, so that treatment is simple and convenient after reaction, environmental pollution is low, and a new thought and a new method are provided for large-scale industrialized production.
Owner:SHENYANG INSTITUTE OF CHEMICAL TECHNOLOGY
Method of improving performance of methylal carbonylation reaction catalyst
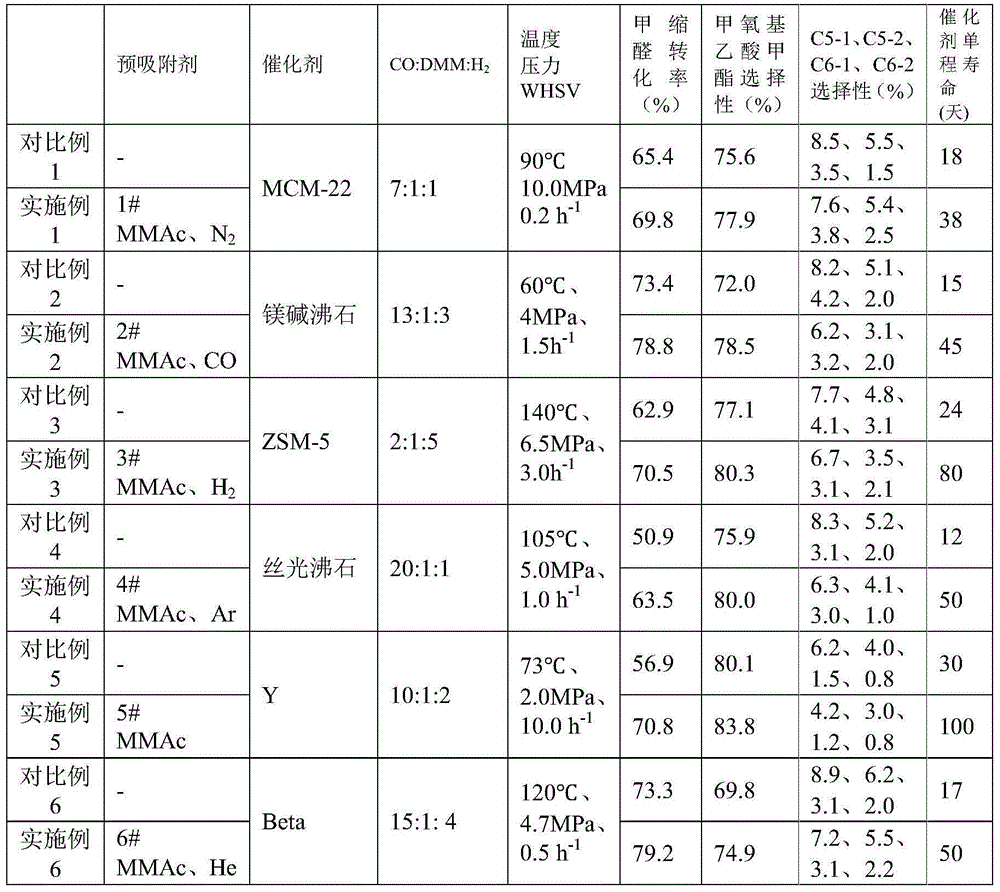
ActiveCN105585484AHigh reactivityHigh selectivityPreparation by carbon monoxide or formate reactionMolecular sieveMethyl methoxyacetate
The application relates to a method of improving performance of a methylal carbonylation reaction catalyst. The method comprises the following steps: pre-adsorbing an acidic molecular sieve catalyst through methyl methoxyacetate; and performing a reaction to a raw material, methylal, with CO and H2 through a reactor carrying the acidic molecular sieve catalyst under a proper reaction condition. The catalyst has long service life, is high in conversion rate of the methylal, is high in selectivity on the product, methyl methoxyacetate, and is free of any additional solvent. The method employs mild reaction conditions, allows continuous product and has industrial application potential. The product can be hydrolyzed through hydrogenation or hydrolyzed and then hydrogenated to produce ethylene glycol.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Method for preparing polyoxymethylene dimethyl ether carboxylate and methyl methoxy acetate
ActiveCN104725225AImprove conversion rateHigh selectivityPreparation by carbon monoxide or formate reactionHydrogenMethyl methoxyacetate
The invention provides a method for preparing polyoxymethylene dimethyl ether carboxylate and / or methyl methoxy acetate which serves as an intermediate for producing ethylene glycol. The method comprises the step of enabling a raw material, namely polyoxymethylene dimethyl ether or methylal, together with carbon monoxide and hydrogen gas to react in an acidic resin catalyst loaded reactor under appropriate reaction conditions without adding other solvents, so as to prepare corresponding products, wherein a reaction process is of gas-liquid-solid three-phase reaction. According to the method provided by the invention, the conversion ratio of the raw material polyoxymethylene dimethyl ether or methylal is high, the selectivity of each product is high, the service life of a catalyst is long, external solvents are not required to be used, reaction conditions are relatively mild, and continuous production can be carried out, so that the method has industrial application potential. Furthermore, the obtained products can be used for producing ethylene glycol through hydrolyzing after hydrogenating or hydrogenating after hydrolyzing.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Method for preparation of methyl glycolate and by-product methyl methoxyacetate with catalyst
ActiveCN107501091AImprove conversion rateRaise the ratioOrganic compound preparationPreparation by carbon monoxide or formate reactionMethoxyacetic acidMethyl methoxyacetate
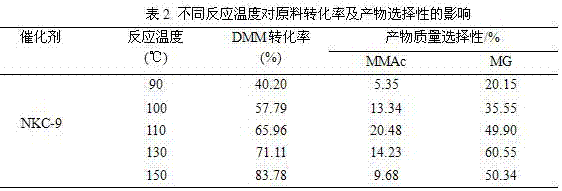
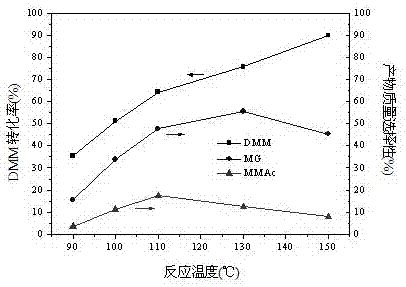
Relating to a preparation method of chemical raw materials, the invention provides a method for preparation of methyl glycolate and by-product methyl methoxyacetate with a catalyst. The method adopts methylal as the solvent, s-trioxane, tetrameric or paraformaldehyde and methylal are taken as the source of formaldehyde, H2SO4, HCl and other liquid acids or cation exchange resin, heteropoly acid, impregnation solid acid, solid super acid and other solid acids are employed as the catalyst, one-step synthesis with high conversion rate and high selectivity is carried out to obtain methyl glycolate and the by-product methyl methoxyacetate. In a fixed bed reactor, 300g of an NKC-9 sulfonic acid resin catalyst is used, the raw materials include: 20kg of methylal, 4kg of water and 2.9kg of s-trioxane, the mass space velocity of the raw materials is 30h<-1>, reaction is carried out under a reaction temperature of 130DEG C and a reaction pressure of 6.0MPa, the catalyst is stable without inactivation for 2000h, the methylal conversion rate is 89.83%, the product methyl glycolate has mass selectivity of 28.88%, the methyl methoxyacetate has mass selectivity of 67.68%. The method provided by the invention has the advantages of short synthetic route, high raw material conversion rate or high product selectivity, and the synthesis process has no pollution to the environment.
Owner:SHENYANG INSTITUTE OF CHEMICAL TECHNOLOGY
Gas injection system with precursor for planar deprocessing of semiconductor devices using a focused ion beam
ActiveUS20150294885A1Easy to useElectric discharge tubesSemiconductor/solid-state device manufacturingMethyl methoxyacetateSemiconductor device modeling
A method and system for improved planar deprocessing of semiconductor devices using a focused ion beam system. The method comprises defining a target area to be removed, the target area including at least a portion of a mixed copper and dielectric layer of a semiconductor device; directing a precursor gas toward the target area; and directing a focused ion beam toward the target area in the presence of the precursor gas, thereby removing at least a portion of a first mixed copper and dielectric layer and producing a uniformly smooth floor in the milled target area. The precursor gas causes the focused ion beam to mill the copper at substantially the same rate as the dielectric. In a preferred embodiment, the precursor gas comprises methyl nitroacetate. In alternative embodiments, the precursor gas is methyl acetate, ethyl acetate, ethyl nitroacetate, propyl acetate, propyl nitroacetate, nitro ethyl acetate, methyl methoxyacetate, or methoxy acetylchloride.
Owner:FEI CO
Catalytic synthesis method for methyl methoxyacetate
ActiveCN105037152AHigh yieldHuge potential for industrial productionOrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsAlcoholMethyl methoxyacetate
The invention relates to a catalytic synthesis method for methyl methoxyacetate with the formula (I) shown in the specification. The catalytic synthesis method comprises the steps that a compound with the formula (II) shown in the specification reacts with methyl alcohol under normal pressure in the presence of a solid carrier load catalyst and a bi-component accelerant, and therefore methyl methoxyacetate is obtained. According to the catalytic synthesis method, by means of the unique catalyst and accelerant, and particular load component selected and specific preparation method used in the catalyst preparing process, a target product can be obtained with the high yield, and the research value and application potential are high.
Owner:SHANDONG TONGCHENG MEDICINE TECH CO LTD
Preparation method of Y molecular sieve catalyst with cordierite as carrier
ActiveCN107497473AImprove heat transfer efficiencyImprove mass transfer efficiencyMolecular sieve catalystsPreparation by carbon monoxide or formate reactionMolecular sieveChemical reaction
The invention relates to a preparation method of a catalyst, in particular to a preparation method of a Y molecular sieve catalyst with cordierite as the carrier. According to the invention, cordierite is adopted as the carrier, a transition coating is coated on the carrier by SiO2 sol liquid dipping, and then the transition coating is coated with a Y molecular sieve to obtain the Y / cordierite catalyst. The method also provides the carrier with high thermal stability at the same time for loading of silicon source and aluminum source, wherein the high thermal stability can improve the heat transfer and mass transfer efficiency of chemical reaction, improve the conversion rate of reactants and the mass selectivity of the product. The loaded Y / cordierite catalyst catalyzes the reaction of methylal and carbon monoxide for synthesis of high purity methyl methoxyacetate, and has very high reaction activity. The invention provides a new method for large-scale industrial production.
Owner:SHENYANG INSTITUTE OF CHEMICAL TECHNOLOGY
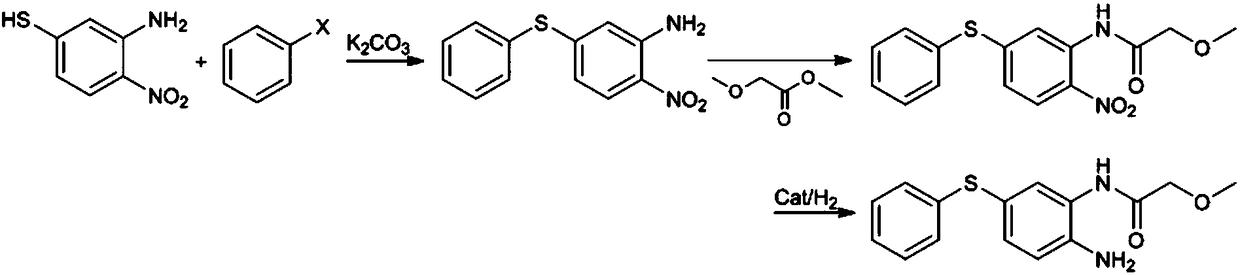
Preparation method of 2-amino-5-phenylthio-(2-methoxy)acetanilide
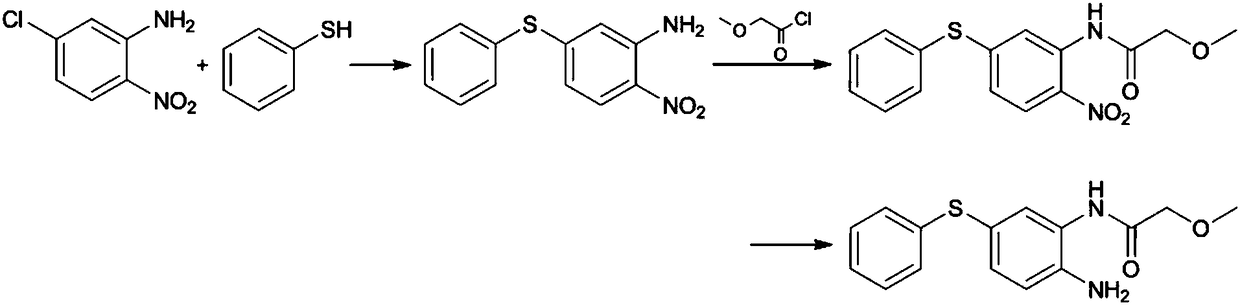
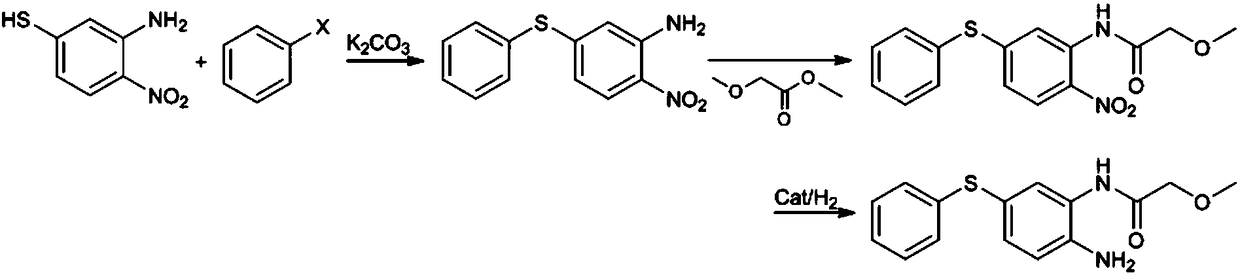
ActiveCN108299259AGood reaction selectivityReduce usageSulfide preparationMethyl methoxyacetateOrganic synthesis
The invention belongs to the technical field of organic synthesis and particularly relates to a preparation method of 2-amino-5-phenylthio-(2-methoxy)acetanilide. According to the method, 2-nitro-5-(phenylthio)aniline is prepared from 2-nitro-5-mercaptoaniline and benzene halide and then subjected to a reaction with methyl methoxyacetate, 2-nitro-5-phenylthio-(2-methoxy)acetanilide is generated and subjected to catalytic hydrogenation reduction finally, and the target product is obtained. The preparation method has good reaction selectivity, and the obtained target product is high in purity and yield; use of high-toxicity materials is avoided, requirements for equipment and operation conditions are reduced, and safety and stability of the preparation method are improved; doses of the reaction materials are optimized, and excess benzene halide and methyl methoxyacetate can be recycled after being recovered simply; little solid waste is produced, waste treatment cost is reduced greatly,and the requirement for environmental protection is met.
Owner:珠海优润医药科技有限公司
Methyl methoxyacetate production method
InactiveCN105481693ASimple processLess investmentPreparation by carbon monoxide or formate reactionMethyl methoxyacetateSolid acid
The invention relates to a methyl methoxyacetate production method, which comprises that methanol is sequentially subjected to methanol oxidation to generate formaldehyde, methanol and formaldehyde condensation reaction to synthesize methylal, and methylal carbonylation reaction to generate methyl methoxyacetate on an oxidation catalyst and a solid acid catalyst in a reactor. According to the method of the present invention, the three steps of the reactions are integrated into the one reactor, such that the process can be simplified, the investment can be saved, the operation cost can be reduced, and the effective way can be provided for the synthesis of the methyl methoxyacetate.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Methods for preparing sulfadoxine and intermediate of sulfadoxine
InactiveCN102432550AQuality improvementImprove qualityOrganic compound preparationCarboxylic acid esters preparationMethyl methoxyacetateDiethyl oxalate
The invention relates to methods for preparing sulfadoxine and an intermediate of the sulfadoxine. The method for preparing the sulfadoxine sequentially comprises the following steps of: (1), in the presence of sodium ethylate, reacting methyl methoxyacetate with excessive diethyl oxalate to generate 3-methoxyl-2-oxo-ethyl methyl succinate, decarbonylating the 3-methoxyl-2-oxo-ethyl methyl succinate to obtain 2-methoxyl-ethyl methyl malonate; (2) reacting the 2-methoxyl-ethyl methyl malonate with formamide to generate a cyclized compound; (3) reacting the cyclized compound with phosphorus oxychloride to generate chloride; (4) performing condensation reaction; and (5) performing methyl oxidation reaction, wherein the purity of the 2-methoxyl-ethyl methyl malonate which is obtained in the step (1) is controlled to be more than or equal to 95 weight percent. By adoption of the methods, the purity of the 2-methoxyl-ethyl methyl malonate is effectively controlled to be more than 95 weight percent, the quality of the cyclized compound for cyclization reaction in next step can be improved, operation is simplified, and production cost is reduced.
Owner:CHANGSHU NANHU INDAL CHEM
Method for preparing methyl methoxyacetate by industrial aqueous raw material methylal
ActiveCN106518676AConvenient sourceReduce pollutionPreparation by carbon monoxide or formate reactionAfter treatmentMethyl methoxyacetate
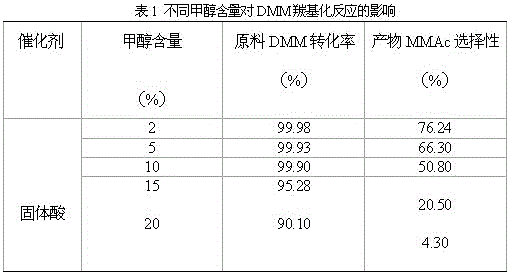
The invention discloses a method for preparing methyl methoxyacetate by an industrial aqueous raw material methylal, and relates to the method for preparing methyl methoxyacetate. According to the method, influence of water on a carbonylation reaction is alleviated by adding a formaldehyde compound to the raw material, and the selectivity of the main product methyl methoxyacetate is improved; the preparation method includes the following process: the formaldehyde compound is added in the methylal carbonylation reaction. Under a condition without use of a solvent, provided is a brand-new methyl methoxyacetate production technological route, by adding a small amount of the formaldehyde compound to the aqueous methylal raw material, influence of trace water is removed in the methylal carbonylation reaction process, and the conversion rate of the raw material and the selectivity of the main product methyl methoxyacetate are significantly improved. The process is simple, the conversion efficiency of a catalyst after treatment is high, sources of the raw material are convenient, the amount of by-products is low, reaction posttreatment is simple, the environmental pollution is small, and a new idea and method are provided for large-scale industrialized production.
Owner:SHENYANG INSTITUTE OF CHEMICAL TECHNOLOGY
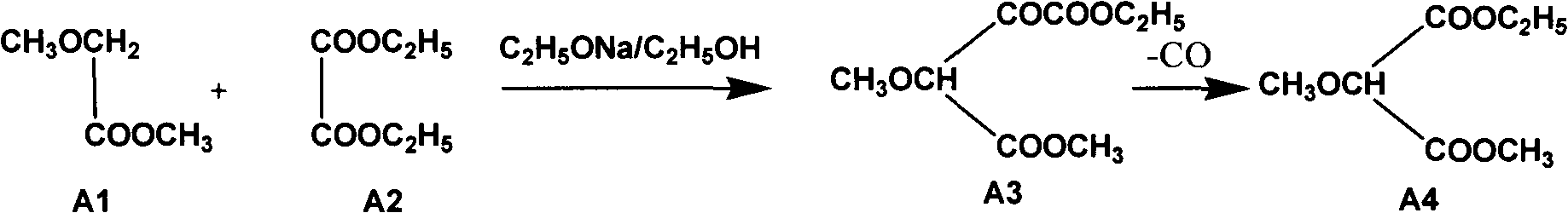
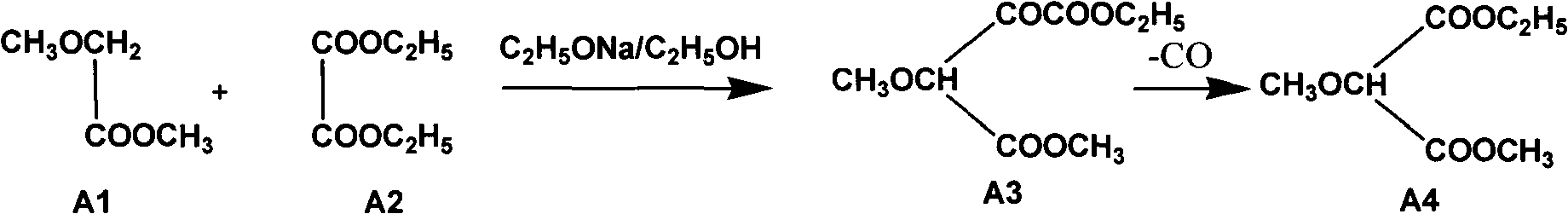
Method used for smooth and steady preparation of 2-methoxypropandioic acid ethyl methyl ester
InactiveCN103910629AHigh yieldSmooth responseOrganic compound preparationCarboxylic acid esters preparationOrganic solventMethyl methoxyacetate
The invention relates to a method used for smooth and steady preparation of 2-methoxypropandioic acid ethyl methyl ester. The method comprises following steps: (a) methyl methoxyacetate and diethyl oxalate are delivered into a reaction container, and a mixed solution is obtained via mixing; (b) solid sodium methylate is dispersed in diethyl oxalate, an obtained mixture is added into the mixed solution dropwise, and an obtained mixed material is reacted for 2 to 8h at a temperature of 50 to 60 DEG C so as to obtain a reaction liquid; (c) an organic solvent, water, and an acid solution are delivered into another reaction container for mixing, the reaction liquid is added, a temperature of an obtained material is controlled below 15 DEG C, pH value is adjusted to 1 to 2, and the material is allowed to stand so as to obtain a water layer and an organic solvent layer; (d) the organic solvent layer is collected and is subjected to distillation so as to remove the organic solvent, and an obtained product is subjected to decarbonylation at a temperature of 178 to 182 DEG C under a vacuum degree of -0.04 to -0.053MPa for 2 to 6h, and fractions obtained under a vacuum degree of -0.095MPa at a temperature above 170 DEG C are collected. According to the method, solid sodium methylate is dispersed in diethyl oxalate which is not too active, and then the obtained mixture is added into the mixed solution dropwise, so that reaction fierce degree of the whole system after adding of solid sodium methylate can be reduced effectively, and smoothness of the reaction is ensured.
Owner:CHANGSHU NANHU INDAL CHEM
Method for preparing methyl glycolate and haloalkane as byproduct
ActiveCN107602388AHigh selectivityNo pollution in the processOrganic compound preparationCarboxylic acid esters preparationMethoxyacetic acidMethyl methoxyacetate
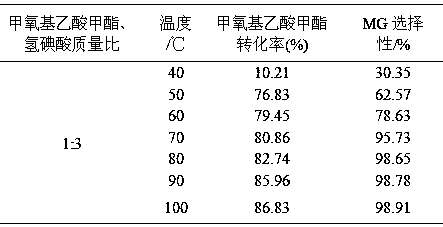
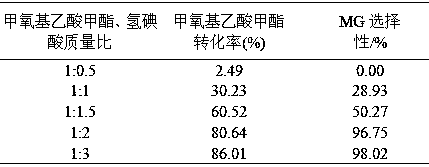
The invention relates to a method for preparing methyl glycolate and haloalkane as a byproduct, which relates to the method for preparing methyl glycolate. The method takes methoxyacetic acid methyl ester and halogen (or its hydride) as raw materials, and methyl glycolate and haloalkane as the byproduct are synthesized with high selectivity through an ether bond cracking reaction. During a synthesis route, halogen hydride is strong acid, and is taken as a reaction raw material and a reaction catalyst, compared with a traditional methyl glycolate synthesis technology, The reaction path of a brand new reaction route is short, reaction condition is mild, an extra catalyst is not required, separating problem of the catalyst is not existed, the byproduct is little, the raw material conversion rate and the product selectivity are high, no pollution is generated on environment during synthesis, and the method has the advantages of energy saving and environmental protection. When the mass ratio of methoxyacetic acid methyl ester to hydroiodic acid is 1:3, the reaction temperature is 80 DEG C, the reaction time is 5 h, the raw material methoxyacetic acid methyl ester conversion rate is 86.01%, and the product methyl glycolate selectivity can reach 98.02%.
Owner:SHENYANG INSTITUTE OF CHEMICAL TECHNOLOGY
Method for preparing methyl glycolate and by-producing methyl methoxyacetate
ActiveCN107337602AHigh selectivityRaise the ratioOrganic compound preparationPreparation by carbon monoxide or formate reactionSolubilityChemical industry
The invention provides a method for preparing methyl glycolate and by-producing methyl methoxyacetate, and relates to a chemical industry raw material preparation method. According to the method, by using methylal as a solvent, using trioxymethylene, formaldehyde tetramer or paraformaldehyde and methylal as a formaldehyde source, and using a solid acid or a liquid acid as a catalyst, in the presence of a right amount of water in a system, methyl glycolate is subjected to one-step high-conversion-rate and high-selectivity synthesis and methyl methoxyacetate is by-produced. According to the present invention, the reaction system can be performed under a low water condition, and the methyl methoxyacetate is the high added value pharmaceutical intermediate, and has excellent solubility, wherein organic matters can be dissolved in the methyl methoxyacetate, and the methyl methoxyacetate can be dissolved in water at any proportions, so the dissolving of the formaldehyde can be promoted, the formaldehyde can be dissolved in the methyl methoxyacetate, and a lot of CO in the gas phase can be dissolved in the methyl methoxyacetate during the reaction process so as to make the surface of the catalyst in the liquid phase contact more CO so as to significantly improve the ratio of CO to the aldehyde group in the liquid phase.
Owner:SHENYANG INSTITUTE OF CHEMICAL TECHNOLOGY
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
![Synthesizing method of 2-amino-5,8-dimethoxy[1,2,4]-triazolo[1,5-c]-pyrimidine Synthesizing method of 2-amino-5,8-dimethoxy[1,2,4]-triazolo[1,5-c]-pyrimidine](https://images-eureka.patsnap.com/patent_img/f9d04bb3-ee16-4c50-a3dd-18ac6fa044c3/BDA00003068755500031.PNG)
![Synthesizing method of 2-amino-5,8-dimethoxy[1,2,4]-triazolo[1,5-c]-pyrimidine Synthesizing method of 2-amino-5,8-dimethoxy[1,2,4]-triazolo[1,5-c]-pyrimidine](https://images-eureka.patsnap.com/patent_img/f9d04bb3-ee16-4c50-a3dd-18ac6fa044c3/BDA00003068755500071.PNG)