Preparation method of 2-amino-5-phenylthio-(2-methoxy)acetanilide
A technology of phenylthioaniline and acetanilide, which is applied in the field of preparation of 2-amino-5-phenylthio-acetanilide, can solve problems such as inconvenient production and operation, large environmental pollution, and lack of economic value, and reduce equipment and The requirements of operating conditions, the reduction of waste disposal costs, and the effect of improving safety and stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
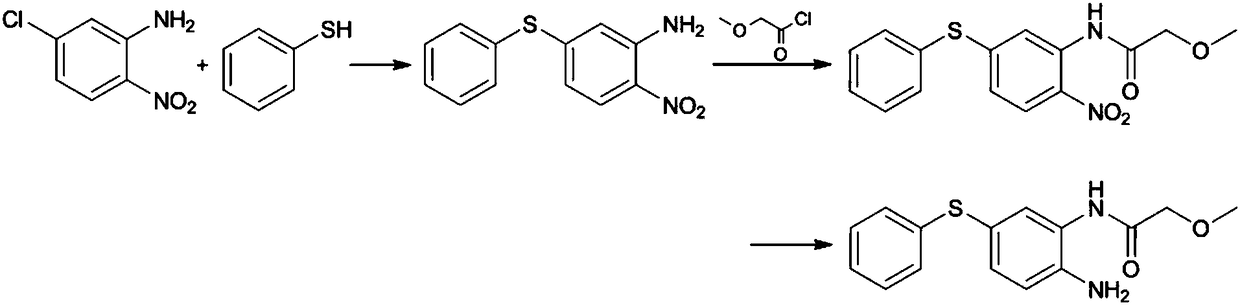
[0037] (1) Preparation of 2-nitro-5-phenylthioaniline: add 2-nitro-5-mercaptoaniline (17.0g) in the 250ml reaction flask equipped with thermometer, water separator, condenser and stirring device , Potassium Carbonate (13.8g) and Chlorobenzene (85.0g), nitrogen replacement three times, heated to 130-135°C, stirred and refluxed for 3-5 hours; filtered while hot, stirred and cooled the filtrate to 0-5°C, and then continued Stir for 1 hour, filter and collect the precipitated crystals, wash and dry to obtain 2-nitro-5-phenylthioaniline with a yield of 95.4%;
[0038] (2) Preparation of 2-nitro-5-phenylthio-(2-methoxy)acetanilide: add 2-nitro-5-benzene to a 500ml reaction bottle equipped with a thermometer, condenser and stirring device Thioaniline (24.7g) and methyl 2-methoxyacetate (123.0g) were replaced by nitrogen three times, stirred and heated to 120-130°C for 5-7 hours; the temperature was lowered to below 70°C, and n-hexane ( 200ml) and cooled to 0-5°C, continued to stir f...
Embodiment 2
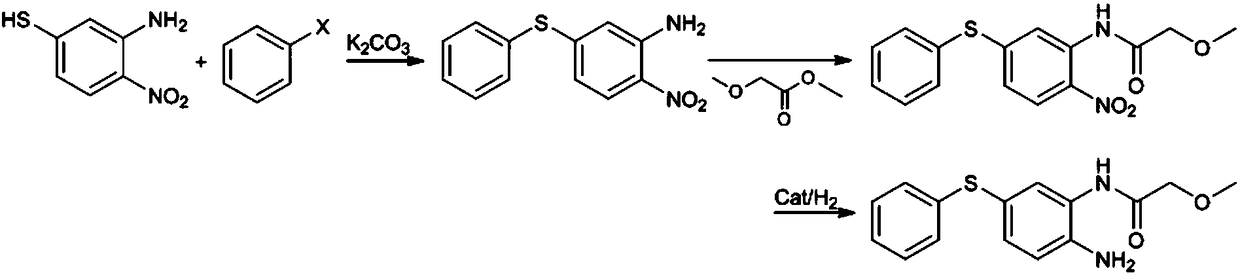
[0041] (1) Preparation of 2-nitro-5-phenylthioaniline: add 2-nitro-5-mercaptoaniline (85.0g) in the 1000ml reaction flask equipped with thermometer, water separator, condenser and stirring device , Potassium carbonate (69.1g) and bromobenzene (340.0g), replaced by nitrogen three times, stirred and refluxed at 150-160°C for 5-6 hours; filtered while hot, stirred and cooled the filtrate to -5-0°C, continued Stir for 1 hour, filter and collect the precipitated crystals, wash and dry to obtain 2-nitro-5-phenylthioaniline with a yield of 94.3%.
[0042](2) Preparation of 2-nitro-5-phenylthio-(2-methoxy)acetanilide: add 2-nitro-5-benzene to a 2000ml reaction flask equipped with a thermometer, condenser and stirring device Thioaniline (123.1g) and methyl 2-methoxyacetate (500.0g), replaced by nitrogen three times, stirred and heated to 110-120°C for 8-10 hours; cooled to below 70°C, added n-heptane dropwise (1000ml) after cooling down to 0~5°C, continue to stir for 1 hour, filter, c...
Embodiment 3
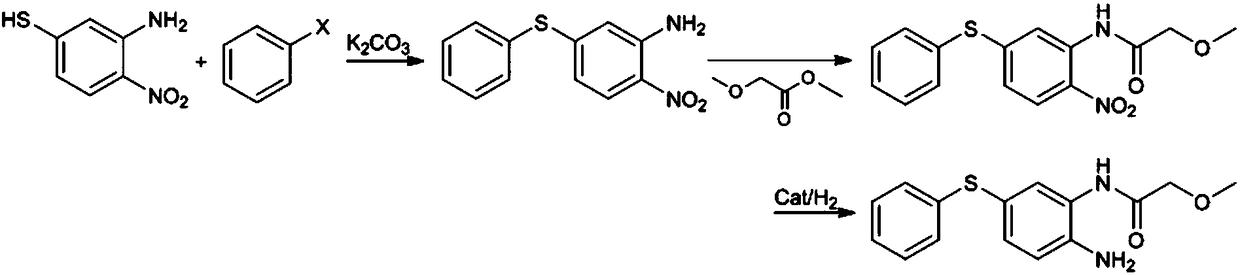
[0045] (1) Preparation of 2-nitro-5-phenylthioaniline: add 2-nitro-5-mercaptoaniline (17.0g) in the 500ml reaction flask equipped with thermometer, water separator, condenser and stirring device , Potassium carbonate (27.5g) and chlorobenzene (170.0g), replaced by nitrogen three times, heated to 130-135°C, stirred and refluxed for 3-5 hours; filtered while hot, stirred and cooled the filtrate to -20--10°C, Stirring was continued for another 1 hour, the precipitated crystals were collected by filtration, washed and dried to obtain 2-nitro-5-phenylthioaniline with a yield of 89.5%;
[0046] (2) Preparation of 2-nitro-5-phenylthio-(2-methoxy)acetanilide: add 2-nitro-5-benzene to a 250ml reaction bottle equipped with a thermometer, condenser and stirring device Thioaniline (24.7g) and methyl 2-methoxyacetate (50.0g) were replaced by nitrogen three times, stirred and heated to 120-130°C for 5-7 hours; the temperature was lowered to below 70°C, and n-hexane ( 50ml) and cooled to 0-...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com