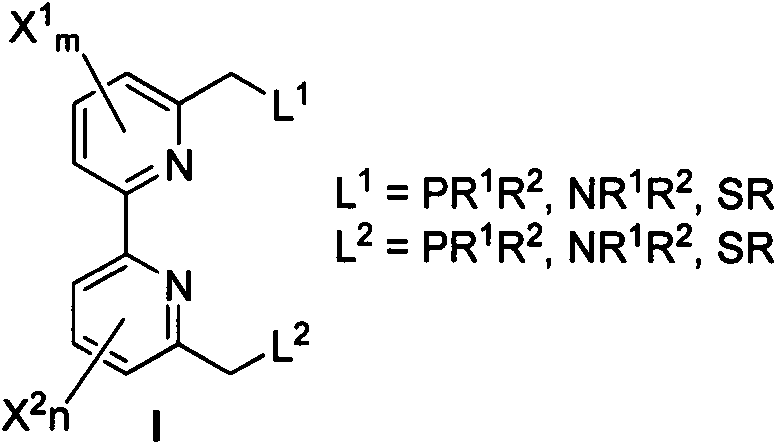
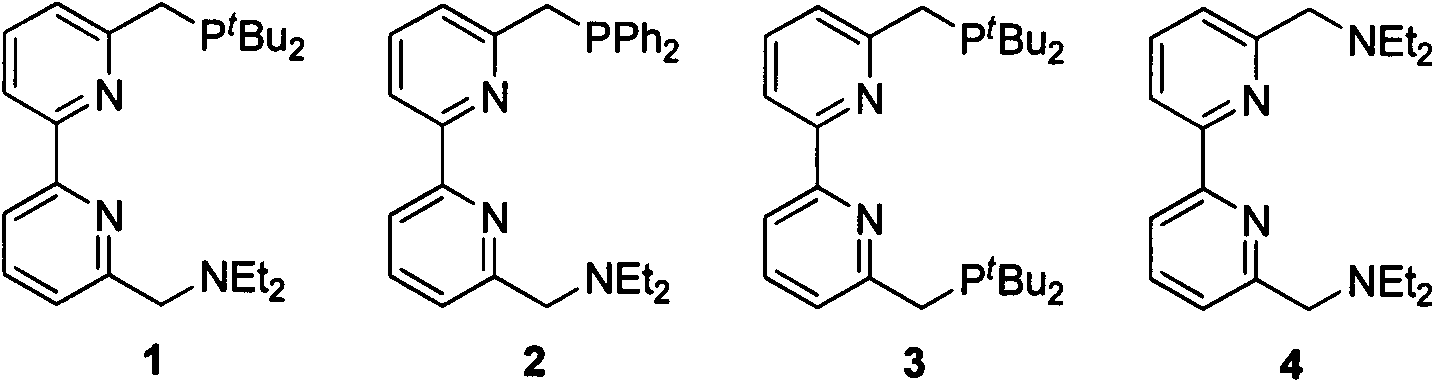
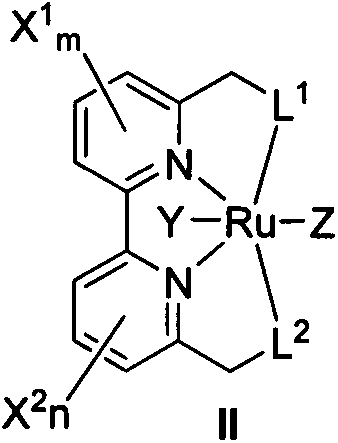
Dipyridyl tetradentate ligand ruthenium complex as well as preparation method and application thereof
The technology of a tetradentate ligand and a ruthenium complex is applied to the bipyridine tetradentate ligand ruthenium complex and the fields of preparation and application thereof, and can solve the problems of large amount of catalyst, increased production cost, unfavorable industrialized production and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] Preparation of Ligand 1
[0045]
[0046] 2-methyl-6-(tri-n-butyltin base)pyridine (8): add 2-methyl- 6-bromopyridine (20.0 g, 116 mmol), the system was replaced by an argon atmosphere, and anhydrous tetrahydrofuran (200 mL) was added. The temperature of the system was controlled to -78°C with a liquid nitrogen-acetone cooling bath, and a solution of n-butyllithium in n-hexane (2.4M, 56.0 mL, 134 mmol) was added dropwise for 30 minutes. After dropping, continue to stir at -78°C for 2 hours. Tri-n-butyltin chloride (45.4 g, 140 mmol) was added dropwise into the system with a syringe, and the dropping time was 30 minutes. After dropping, continue to stir at -78°C for 30 minutes, then return to room temperature and react overnight (14 hours). The system was desolvated with a rotary evaporator, the residue was diluted with ether (300 mL), washed with water (200 mL) and saturated brine (200 mL) successively, the organic phase was dried over anhydrous sodium sulfate, an...
Embodiment 2
[0053] Preparation of Ligand 2
[0054]
[0055] 2-[(tert-butyldimethylsilyl)methyl]-6-bromopyridine (12): Add 2-hydroxymethyl- 6-bromopyridine (10.6g, 56.4mmol) and imidazole (15.4g, 226mmol), the system was replaced by an argon atmosphere, anhydrous DMF (50mL) was added, and tert-butyldimethyl Chlorosilane (10.2 g, 67.7 mmol). After the addition was complete, the reaction was stirred at room temperature for 1 hour. TLC monitored the reaction to be complete. Water (50 mL) was added to quench the reaction, and the product was extracted with diethyl ether (3×100 mL). The organic phase was washed with water (100 mL) and saturated brine (100 mL) successively. Dry over anhydrous sodium sulfate and let stand. The desiccant was removed by suction filtration, the filtrate was desolvated by a rotary evaporator, the residue was distilled under reduced pressure, and the 92°C / 0.1mmHg fraction was collected to obtain 15.3 g of a colorless oily liquid product, yield: 90%. 1 H NMR (40...
Embodiment 3
[0061] Preparation of Ligand 3
[0062]
[0063] 6,6'-dimethylol-2,2'-bipyridine (16): Add 12 (8.49g, 28.1mmol), 13( 16.9g, 33.0mmol), tetrakis(triphenylphosphine) palladium (1.14g, 0.99mmol) and anhydrous lithium chloride (3.60g, 84.9mmol), the system was degassed three times by freezing and thawing with liquid nitrogen, and finally replaced with Argon atmosphere. The system was heated to 120° C. with an oil bath, and the reaction was stirred for 12 hours until a large amount of palladium black was produced in the system. At this time, GC showed that 12 had been completely converted. The system was cooled to room temperature, diluted with ethyl acetate (100 mL), filtered through celite to remove insoluble matter, and 6N hydrochloric acid was added to make the system acidic (pH=3), and the layers were separated. The aqueous phase was adjusted to alkaline (pH=13) with 6N aqueous sodium hydroxide solution, extracted with diethyl ether (3×100 mL), the organic phase was washe...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com