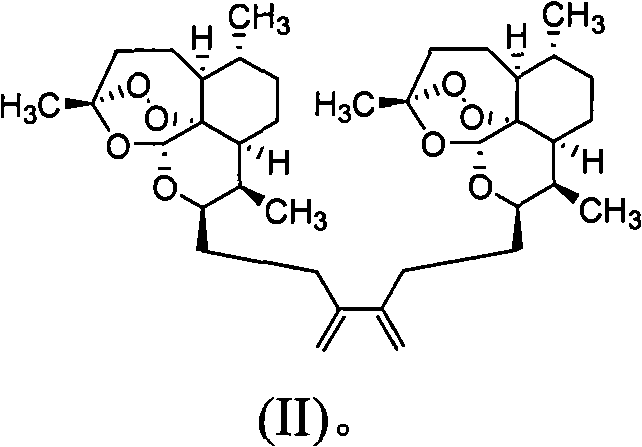
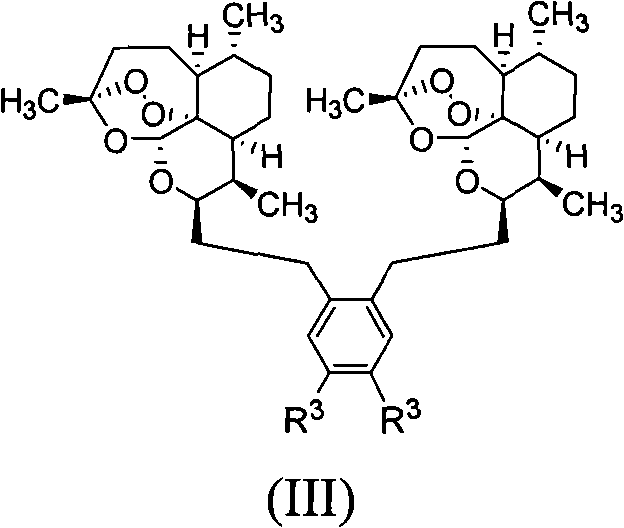
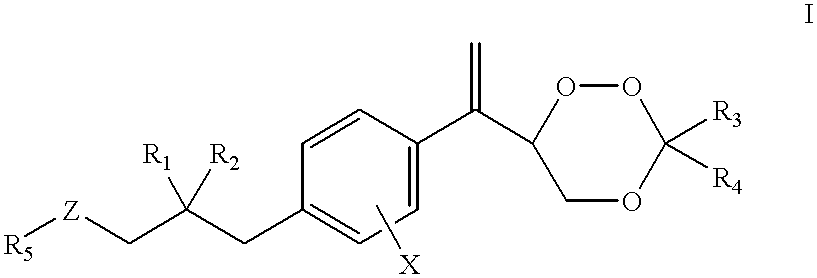
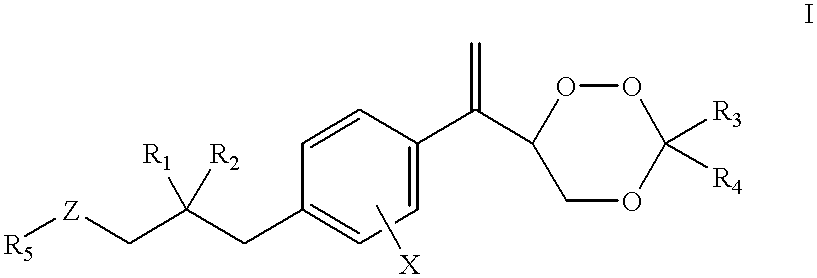
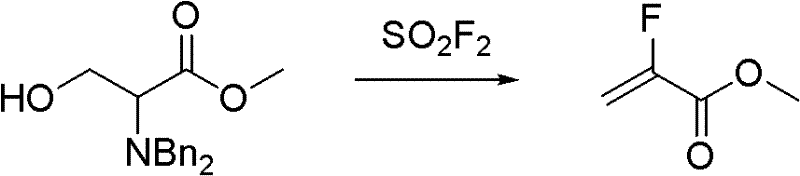Patents
Literature
155 results about "Trioxane" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
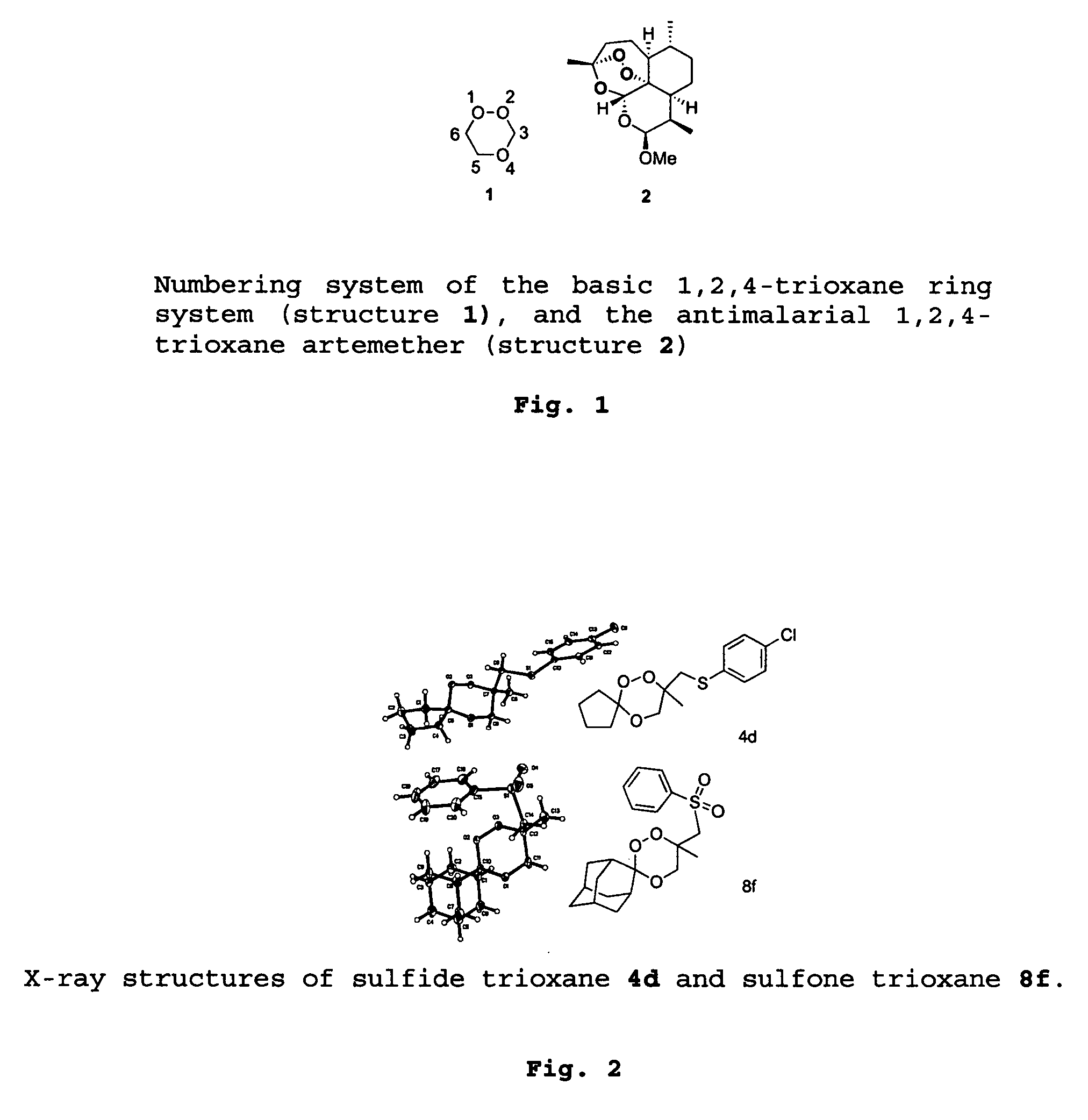
Trioxane refers to any of three isomeric organic compounds composed of a six-membered ring with three carbon atoms and three oxygen atoms, having the molecular formula C₃H₆O₃.
Fluorinated short carbon atom side chain and polar group containing polymer, and flow, or leveling, or wetting agents thereof
InactiveUS6660828B2Little propensity to cause foamingEnsure efficient flowPolishing compositions with abrasivesPolyesterWax
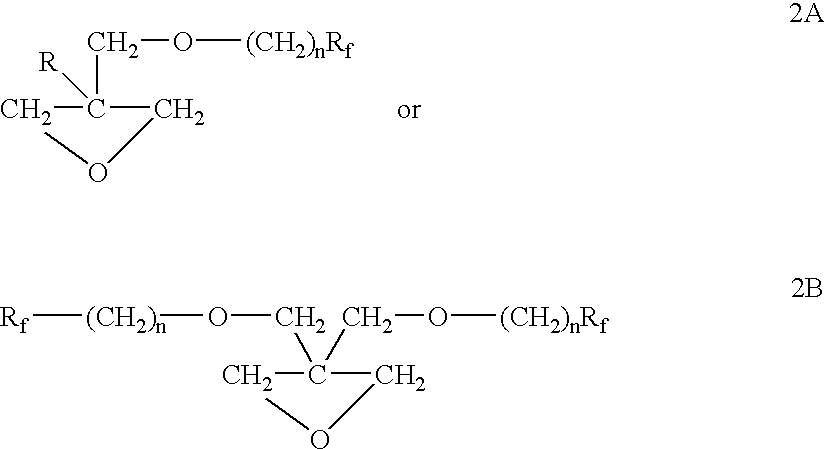
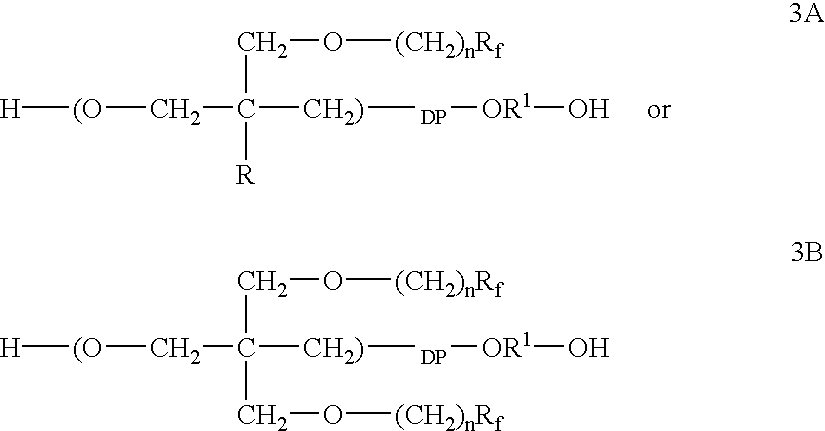
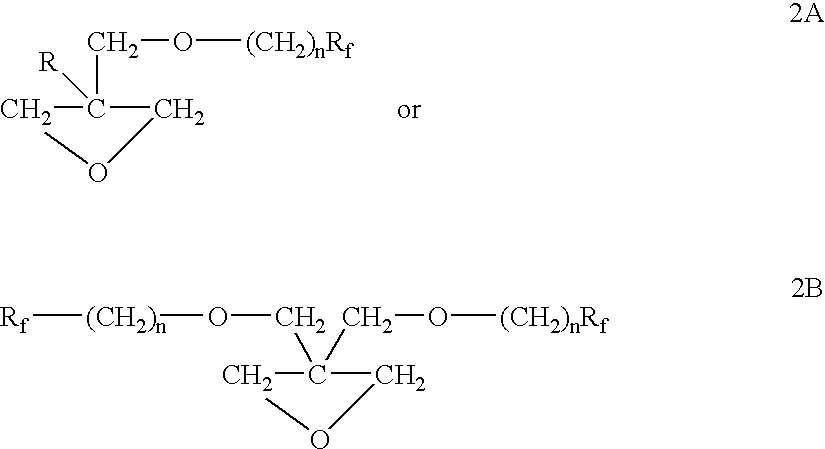
The polymer of the present invention is derived from cyclic ether monomers, dioxane, dioxalane or trioxane and contains both pendant Rf fluoro groups as well as polar groups. These polymers have good wetting, or flow, or leveling properties. They can be reacted with other monomers, such as in the preparation of polyesters, and can be used in waxes, polishes and coatings.
Owner:OMNOVA SOLUTIONS INC
Polyacetal resin composition and molded article thereof
A polyacetal resin composition comprises a polyacetal resin having a trioxane content of not more than 100 ppm (preferably not more than 50 ppm, and more preferably not more than 10 ppm) and at least one stabilizer selected from the group consisting of an antioxidant, a formaldehyde emission inhibitor, a processing stabilizer and a heat stabilizer. The polyacetal resin may be a polyacetal resin (particularly a polyacetal copolymer) in which the trioxane amount is reduced by a solvent treatment and / or a heat treatment. Moreover, the polyacetal resin composition may further contain at least one additive selected from the group consisting of a weather (light)-resistant stabilizer, an impact resistance improver, a gloss control agent, a sliding improver, a coloring agent, and a filler. Such a polyacetal resin composition can reduce the amount of trioxane elution and / or the amount of a volatile organic compound from a molded product thereof.
Owner:POLYPLASTICS CO LTD
Organic fuel cell methods and apparatus
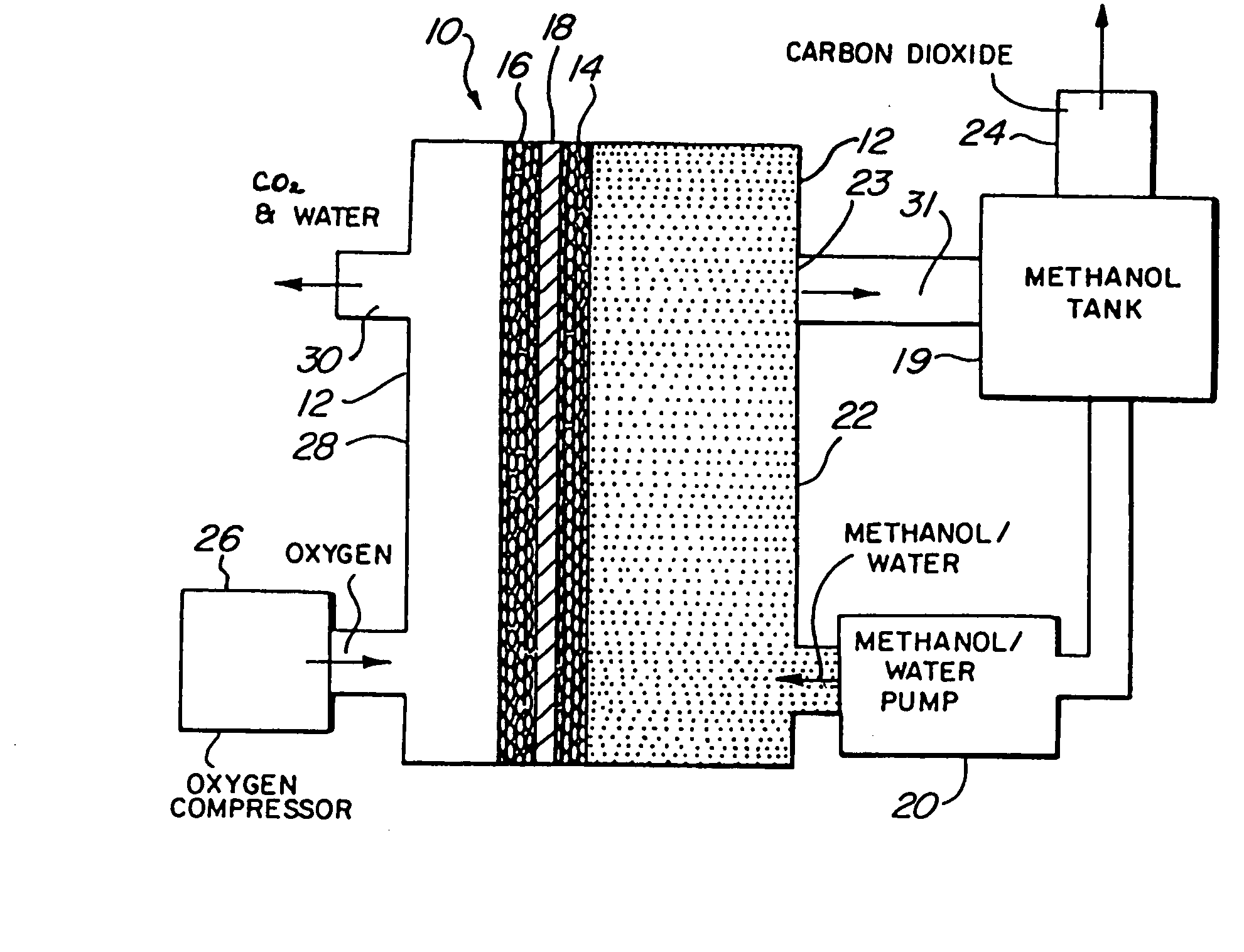
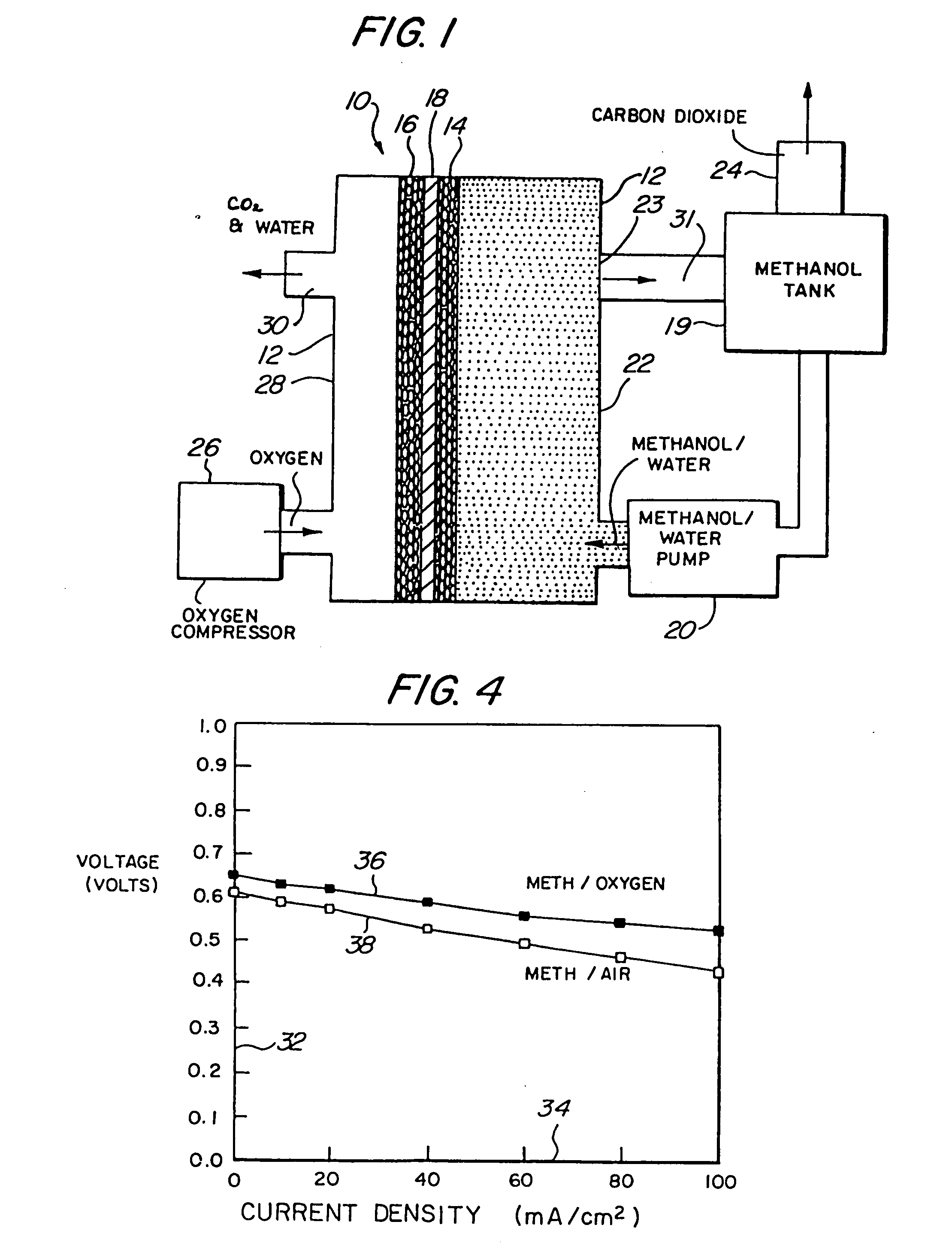
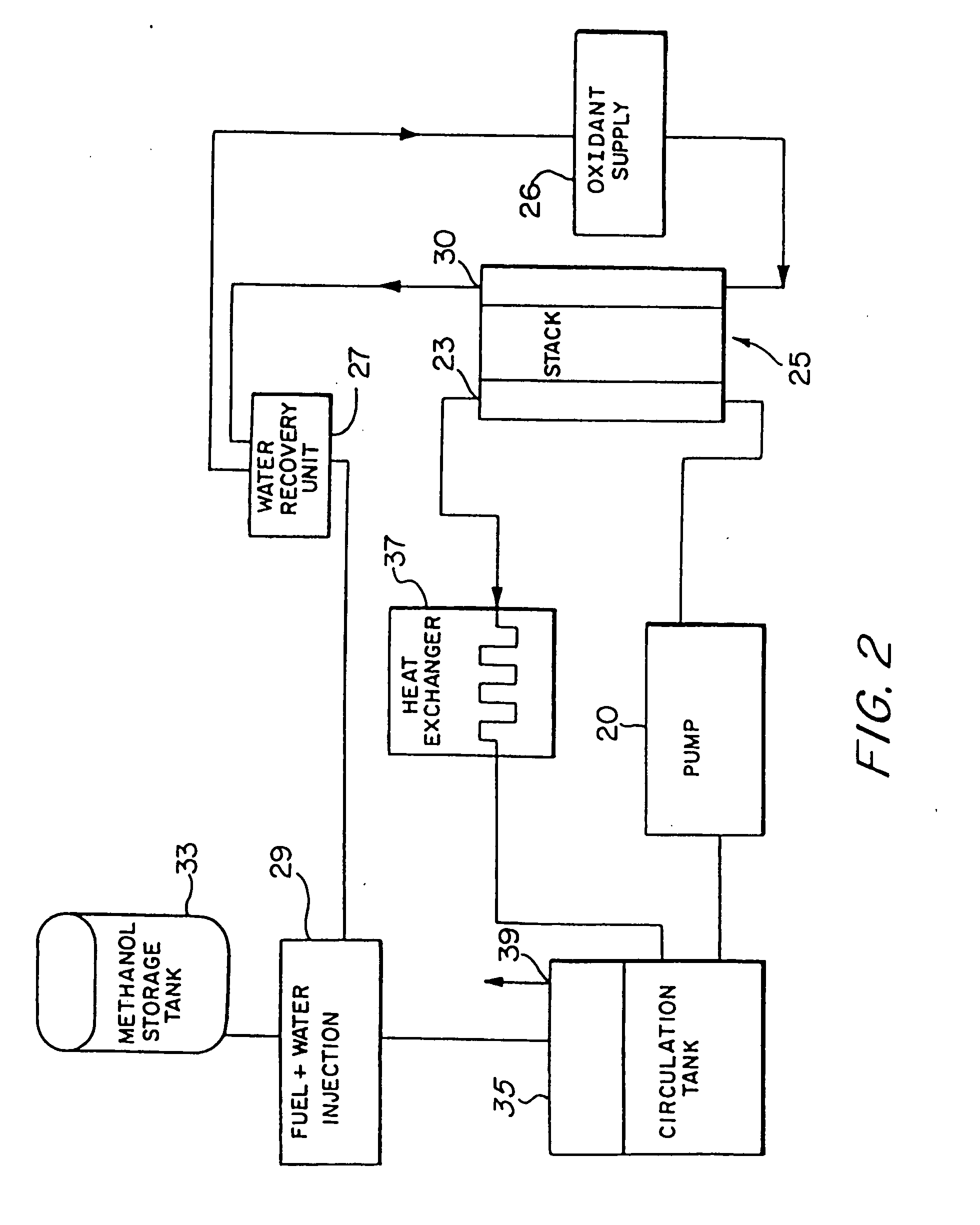
InactiveUS20060204810A1Poor fuel wetting propertyImprove wettabilitySolid electrolytesFuel cells groupingElectrical batteryOrganic fuel
A liquid organic, fuel cell is provided which employs a solid electrolyte membrane. An organic fuel, such as a methanol / water mixture, is circulated past an anode of a cell while oxygen or air is circulated past a cathode of the cell. The cell solid electrolyte membrane is preferably fabricated from Nafion™. Additionally, a method for improving the performance of carbon electrode structures for use in organic fuel cells is provided wherein a high surface-area carbon particle / Teflon™-binder structure is immersed within a Nafion™ / methanol bath to impregnate the electrode with Nafion™. A method for fabricating an anode for use in a organic fuel cell is described wherein metal alloys are deposited onto the electrode in an electro-deposition solution containing perfluorooctanesulfonic acid. A fuel additive containing perfluorooctanesulfonic acid for use with fuel cells employing a sulfuric acid electrolyte is also disclosed. New organic fuels, namely, trimethoxymethane, dimethoxymethane, and trioxane are also described for use with either conventional or improved fuel cells.
Owner:CALIFORNIA INST OF TECH +1
Polyacetal resin composition and molded article thereof
InactiveCN1875066AImprove the surrounding environmentImprove impact resistanceAntioxidantAcetal copolymer
A polyacetal resin composition which comprises a polyacetal resin having a trioxane content of 100 ppm or lower (desirably 50 ppm or lower, preferably 10 ppm or lower) and at least one stabilizer selected among an antioxidant, formaldehyde depressant, processing stabilizer, and heat stabilizer. The polyacetal resin may be a polyacetal resin (especially a copolyacetal) whose trioxane content has been reduced by a solvent treatment and / or heat treatment. The polyacetl resin composition may further contain at least one additive selected among a weathering (light) stabilizer, impact modifier, gloss control agent, sliding modifier, colorant, and filler. This polyacetal resin composition can give a molded article significantly reduced in trioxane extraction and / or volatile-organic amount.
Owner:POLYPLASTICS CO LTD
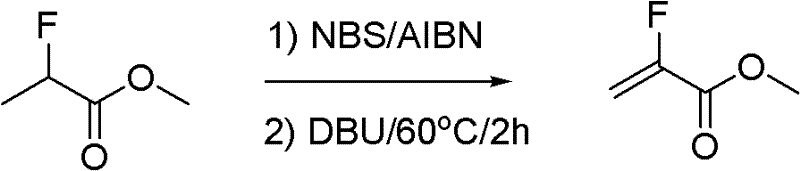
Synthesis method of methyl-alpha-fluoroacrylate and analogues thereof
InactiveCN102211998ALow costReduce manufacturing costOrganic compound preparationCarboxylic acid esters preparationSynthesis methodsSolvent
The invention relates to a synthesis method of methyl-alpha-fluoroacrylate and analogues thereof. The method comprises the following steps: step 1, based on methyl fluoroacrylate and dimethyl oxalate serving as raw materials, dissolving the raw materials in a solvent, and adding base for carrying out condensation reaction so as to generate sodium enolate or potassium enolate intermediate; and step 2, reacting sodium enolate or potassium enolate intermediate with paraformaldehyde or trioxane in the solvent so as to generate methyl-alpha-fluoroacrylate. According to the invention, cheap methyl fluoroacrylate and dimethyl oxalate are used as the raw materials, and the low-cost solvent and other reactants are selected, thus the method has the advantages of low preparation cost and high yield and can be suitable for large-scale industrial production.
Owner:RADIANT PHARMA & TECH
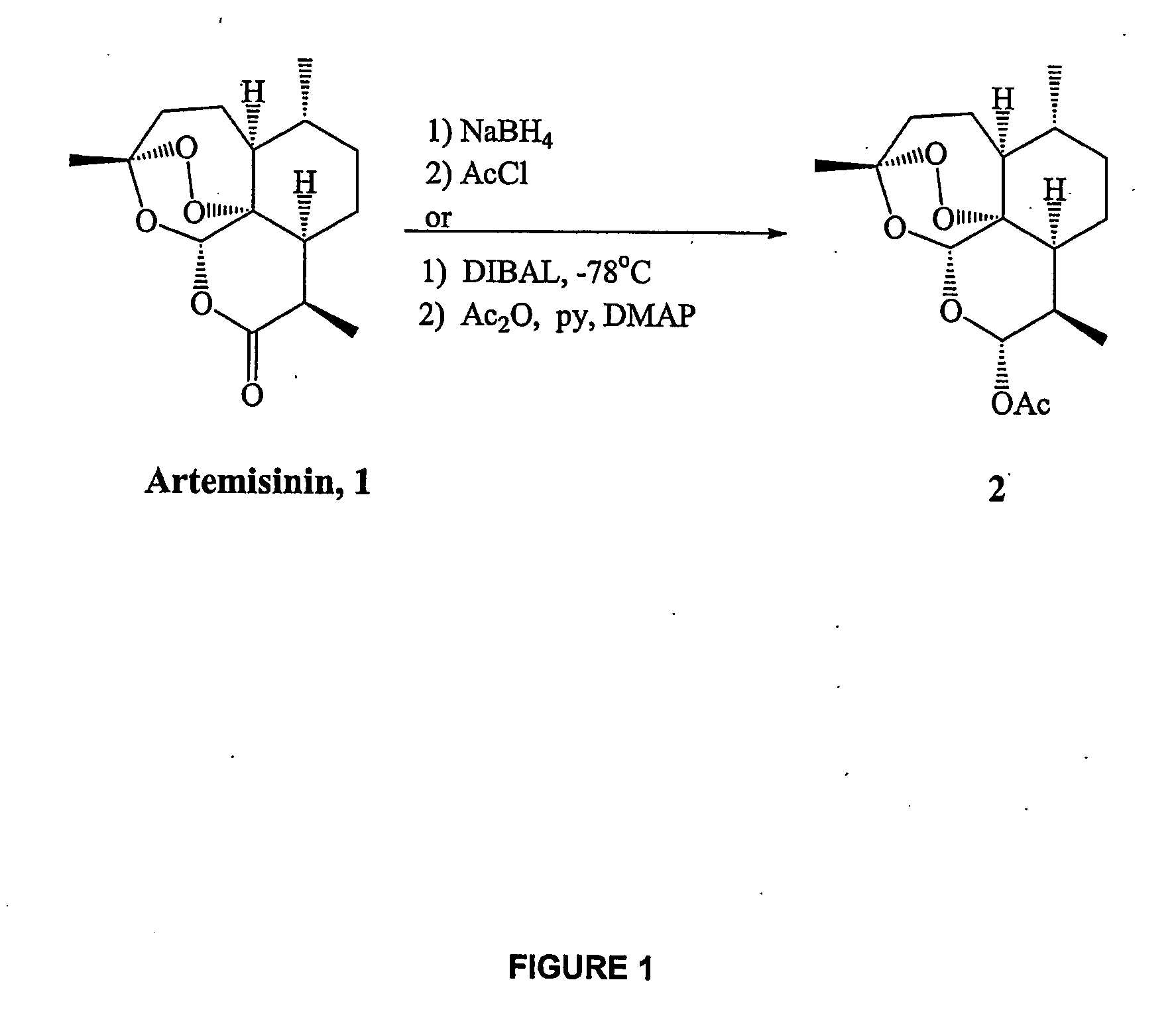
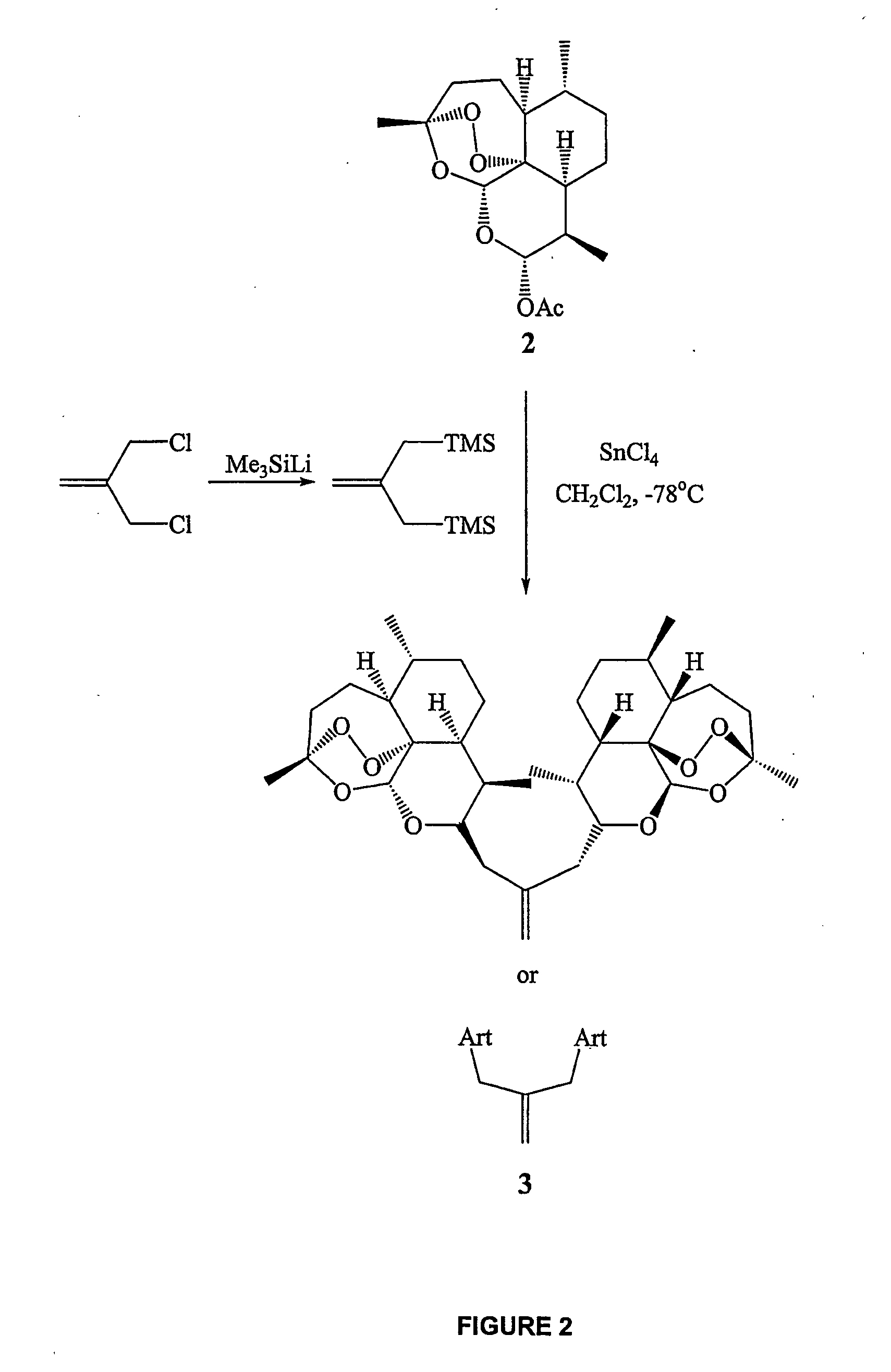
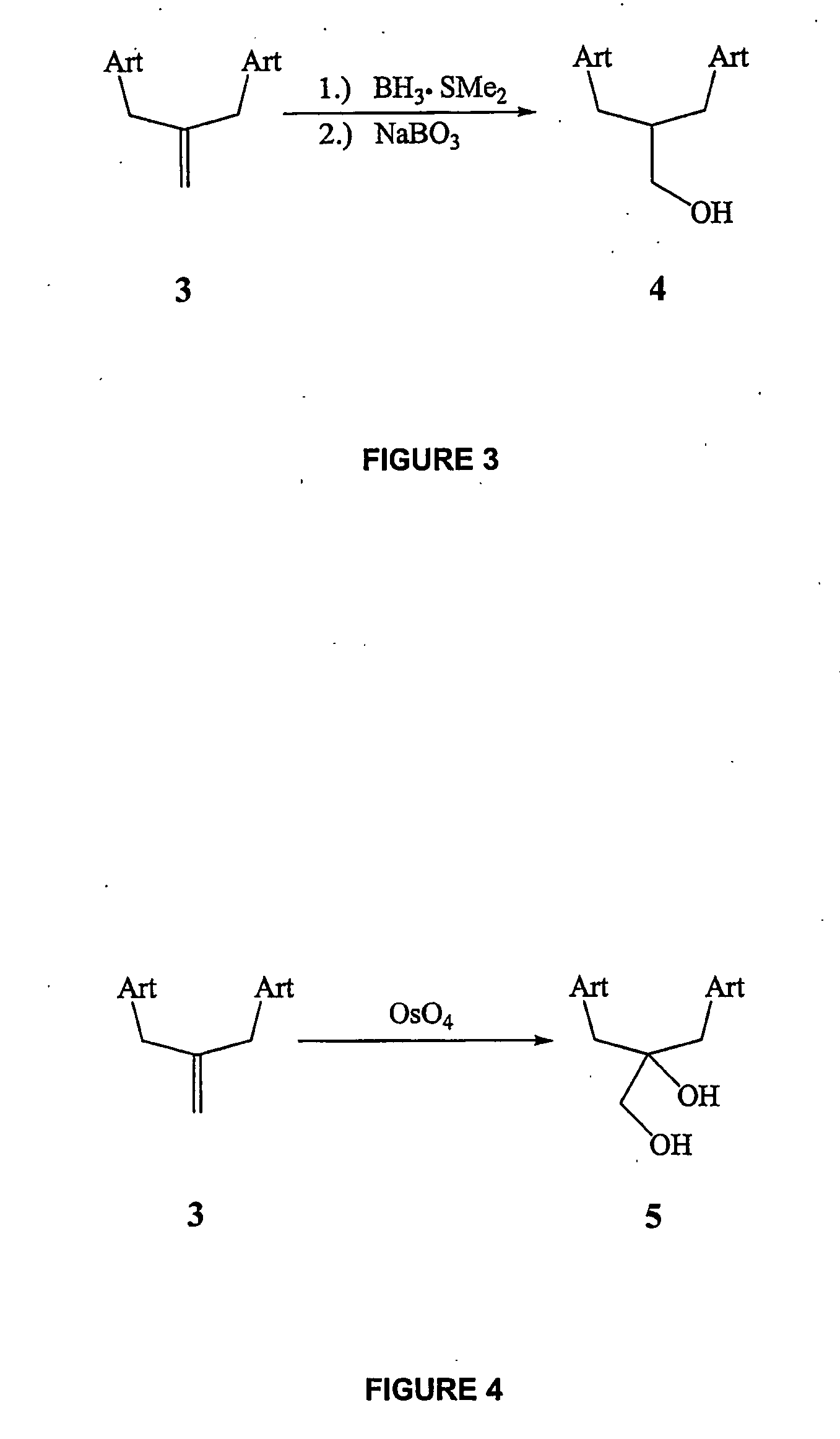
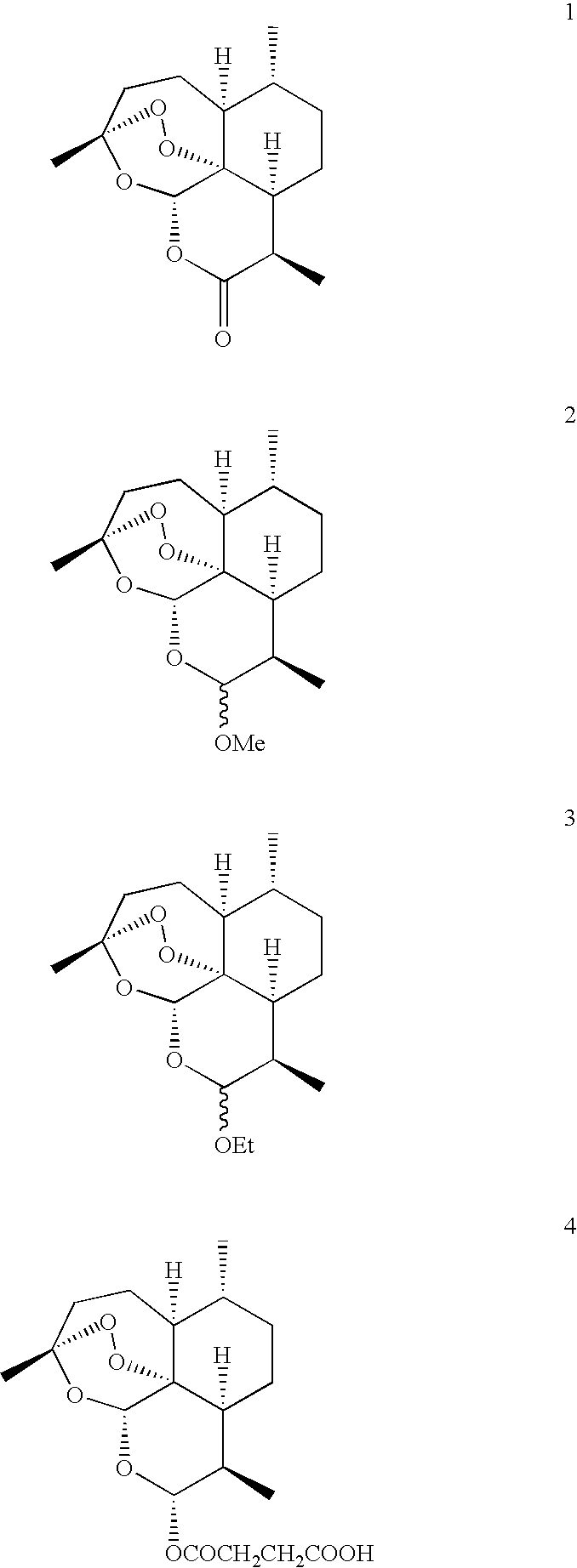
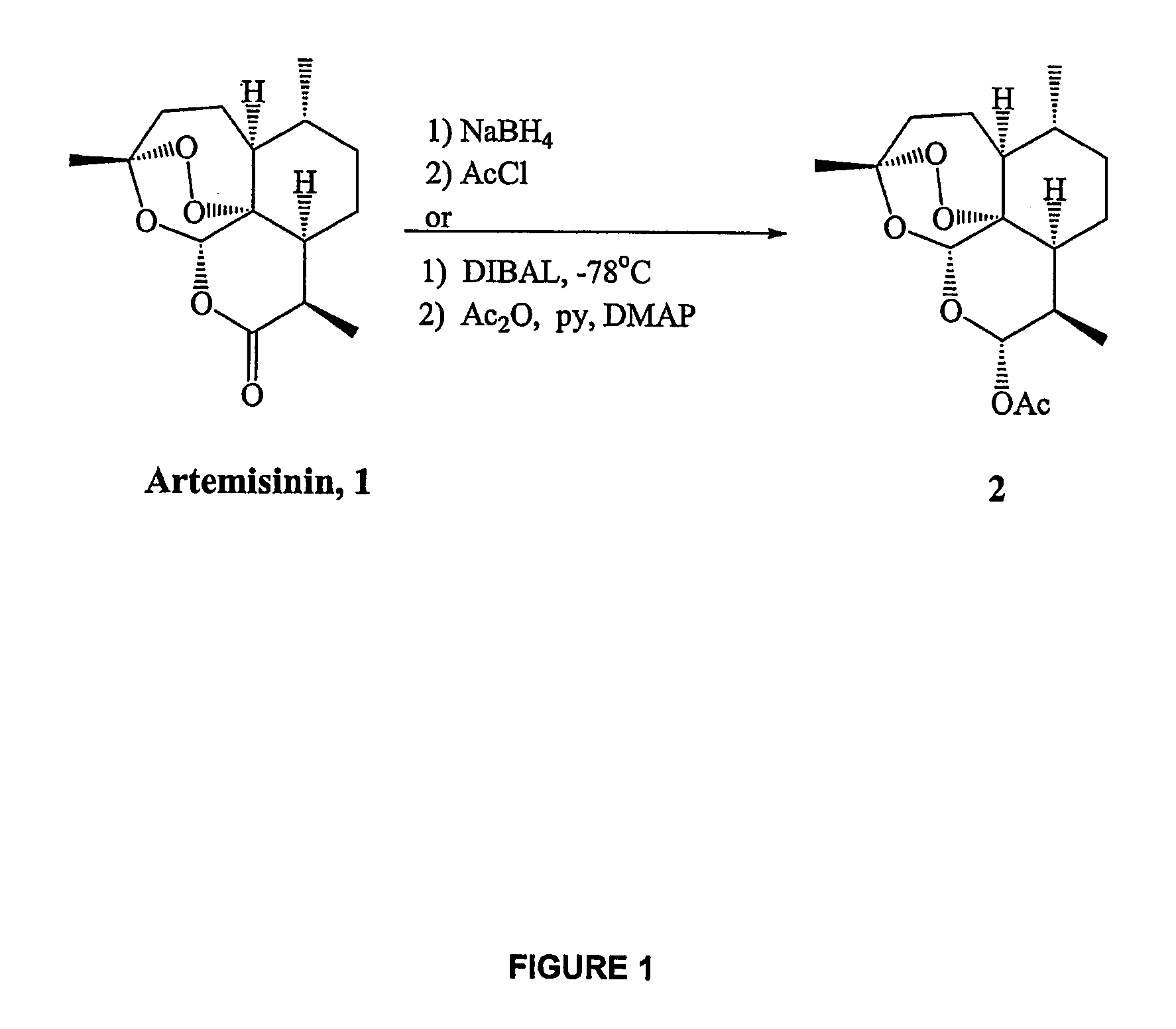
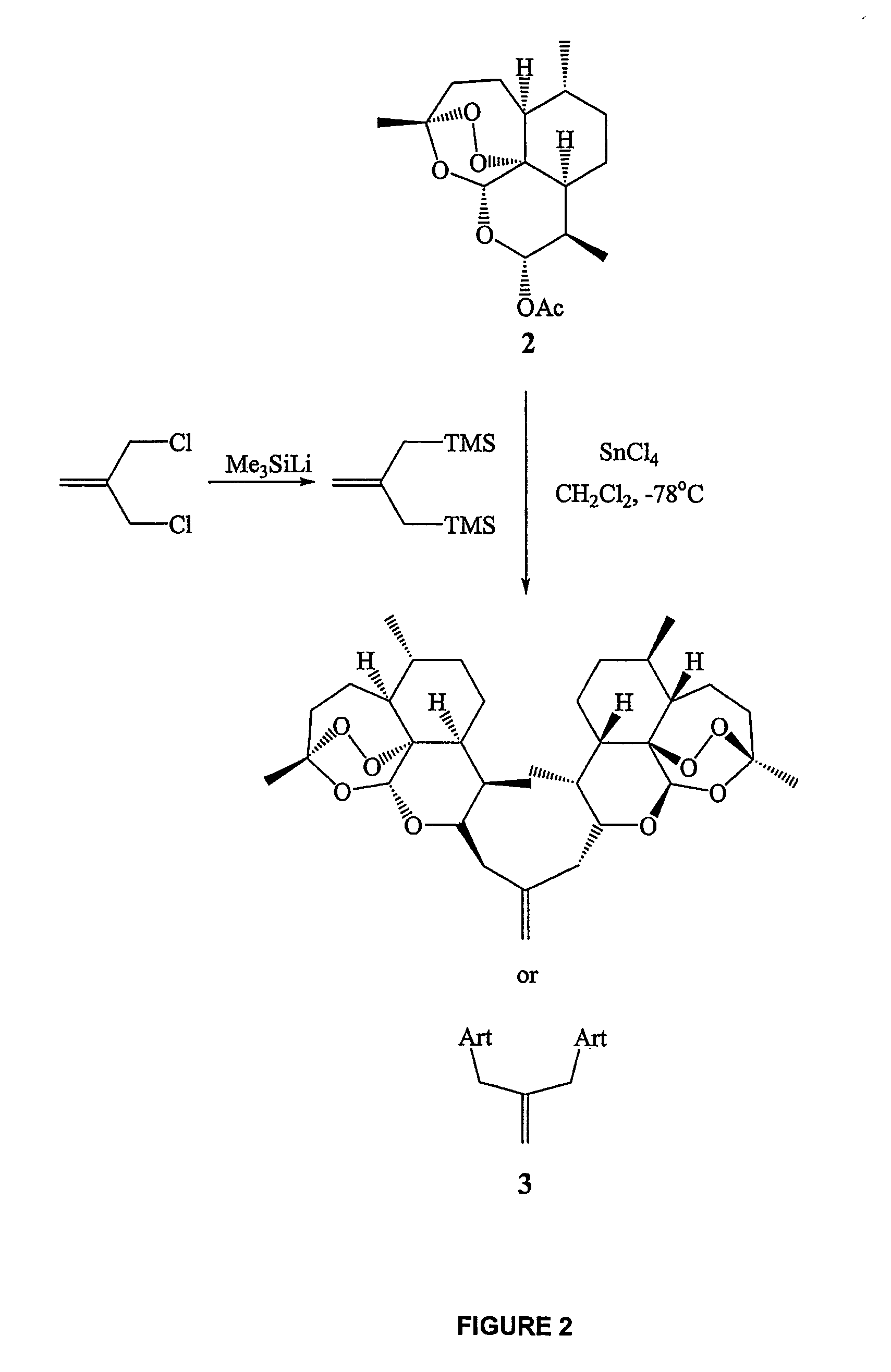
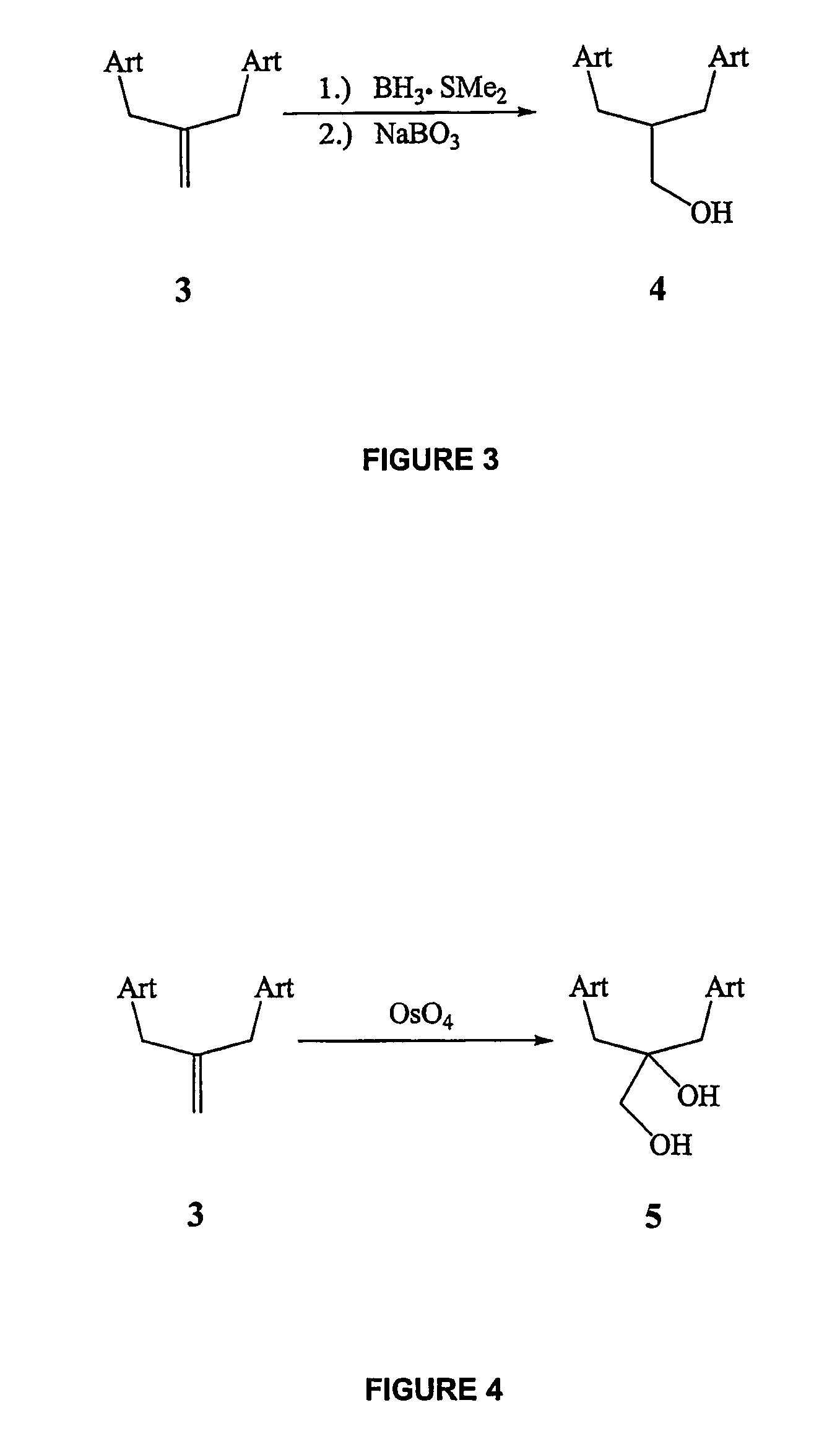
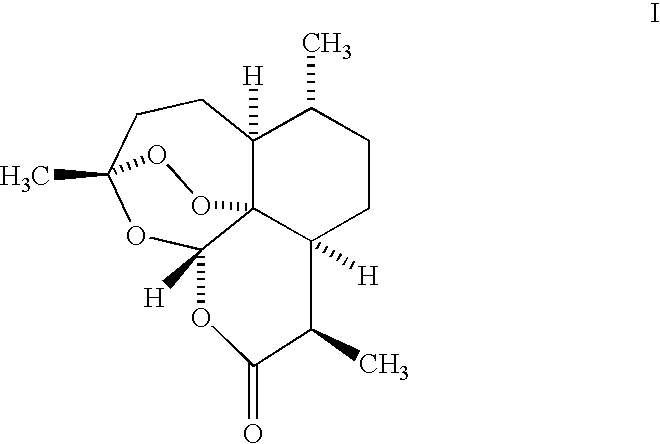
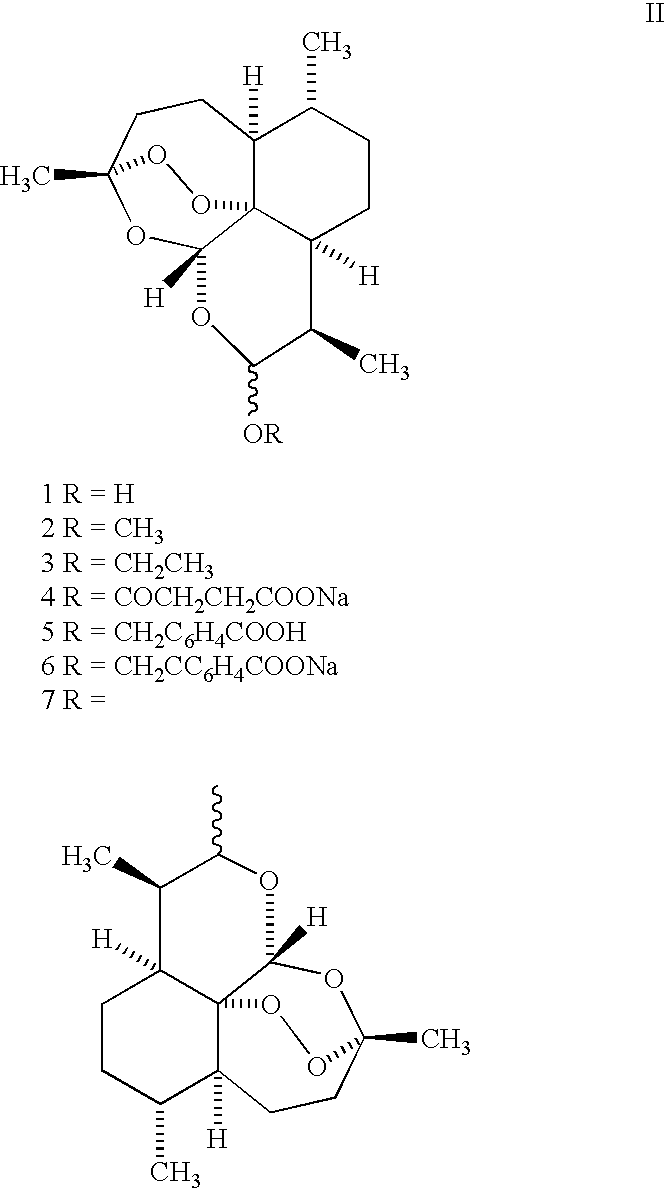
Orally active, antimalarial, anticancer, artemisinin-derived trioxane dimers with high selectively, stability and efficacy and methods of making the same
ActiveUS20060142377A1Easy to transformPotent and selective anticancer activityOrganic active ingredientsBiocideO-Phosphoric AcidCancer cell
In only two steps and in 65% overall yield, natural trioxane artemisinin (I) was converted on gram scale into C-10-carba trioxane dimer (3). This new, very stable dimer was then transformed easily in one additional step into four different dimers (4-7). Alcohol and diol dimers (4 and 5) and ketone dimer (7) are 10 times more antimalarially potent in vitro than artemisinin (1), and alcohol and diol dimers (4 and 5) are strongly inhibitory but not cytotoxic toward several human cancer cell lines. Water-soluble carboxylic acid derivatives (8a-10c and 12) were easily prepared from dimers (4-6); they are thermally stable even at 60° C. for 24 hours, are more orally efficacious as antimalarials than either artelinic acid or sodium artesunate, and have potent and selective anticancer activities. Further derivitization of the alcohol dimers (4 and 17), diol dimer (5) and ketone (7) has produced a number of analogs also antimalarially active in vitro at sub-nanomolar concentrations (most notably: pyridine N-oxides (13, 15, 18, 23, 24 and 25), phosphoric acid triesters (26 and 27), sulfonamide (40) and cyclic carbonate (41)). In addition, dimers (13 and 19) are more efficacious (when administered both orally and i.v.) and less toxic (when administered intraperitoneally to mice as a single dose) than clinically-used sodium artesunate, thereby giving them a better antimalarial therapeutic index than sodium artesunate.
Owner:THE JOHN HOPKINS UNIV SCHOOL OF MEDICINE
Process for continuous production of polyacetal resin
To provide a process for stable and continuous production of a polyacetal resin, mainly comprising a copolymer of 1,3-dioxolane and trioxane under the presence of a catalyst, in a high yield on an industrial scale for a long period of time. In the continuous production of a polyacetal resin by mixing 1,3-dioxolane with boron trifluoride and / or a coordination compound of boron trifluoride and then, mixing the obtained mixture with trioxane to conduct bulk polymerization in a polymerization machine, 1,3-dioxolane is brought into contact with boron trifluoride and / or a coordination compound of boron trifluoride while feeding both at the same direction and they are mixed together for 0.1 to 30 seconds under the condition of at least 0.1 m / sec of the linear velocity of the mixture and subsequently mixed with trioxane.
Owner:POLYPLASTICS CO LTD
Substituted 1,2,4-trioxanes useful as antimalarial agents and a process for the preparation thereof
Owner:COUNCIL OF SCI & IND RES
Polyacetal resin composition
A polyacetal resin composition having high rigidity and also excellent in dimensional stability and creep characteristics is provided. A polyacetal resin composition prepared by blending (A) 99.9 to 90 parts by weight of a linear polyacetal resin having a melt index of 1 to 50 g / min obtained by copolymerizing (a) 99.5 to 97.5% by weight of trioxane and (b) 0.5 to 2.5% by weight of a compound selected from a mono-functional cyclic ether compound and a mono-functional cyclic formal compound, with (B) from 0.1 to 10 parts by weight of a branched or crosslinked polyacetal resin having a melt index of 0.1 to 10 g / min obtained by copolymerizing (a) 99.49 to 95.0% by weight of trioxane, (b) 0.5 to 4.0% by weight of a compound selected from a mono-functional cyclic ether compound and mono-functional cyclic formal compound and (c) 0.01 to 1.0% by weight of a poly-functional glycidyl ether compound with the number of functional groups of 3 to 4, in which (A) and (B) are selected so that the ratio between the melt index of (A) and the melt index of (B) can satisfy the relation of the following formula: 0.02≦MIB / MIA≦1.5 (1) (where MIA is a melt index of (A) and MIB is a melt index of (B))
Owner:POLYPLASTICS CO LTD
Primary concentration and purification method for trioxymethylene after synthesizing
InactiveCN101121709AShort operating timeIncrease productionOrganic chemistryLiquid wastePurification methods
The invention discloses a new method for preliminary concentration and purification of paraformaldehyde after synthesis, which belongs to the technical field of concentration and purification of crude paraformaldehyde. The mixture is sent to the rectification tower for rectification; the top product obtained after rectification enters the condenser, and enters the absorption tower after cooling; the rectification tower still liquid returns to the paraformaldehyde synthesis reactor; after being absorbed by the absorption tower, the absorption tower The top gas and the bottom product enter the pre-concentration tower together for rectification treatment, and the solution obtained at the bottom of the pre-concentration tower is used as the reflux of the synthesis rectification tower and the absorption liquid of the absorption tower; The top product is sent to the concentration tower for concentration, and formaldehyde solution is obtained at the bottom of the concentration tower; the top product obtained from the concentration tower enters the light component removal tower, and the top of the light component removal tower obtains combustible waste liquid, and the bottom of the tower obtains 30-90% aqueous solution of paraformaldehyde. The invention has large output and low operation cost.
Owner:ZHEJIANG SANPO POLYMER
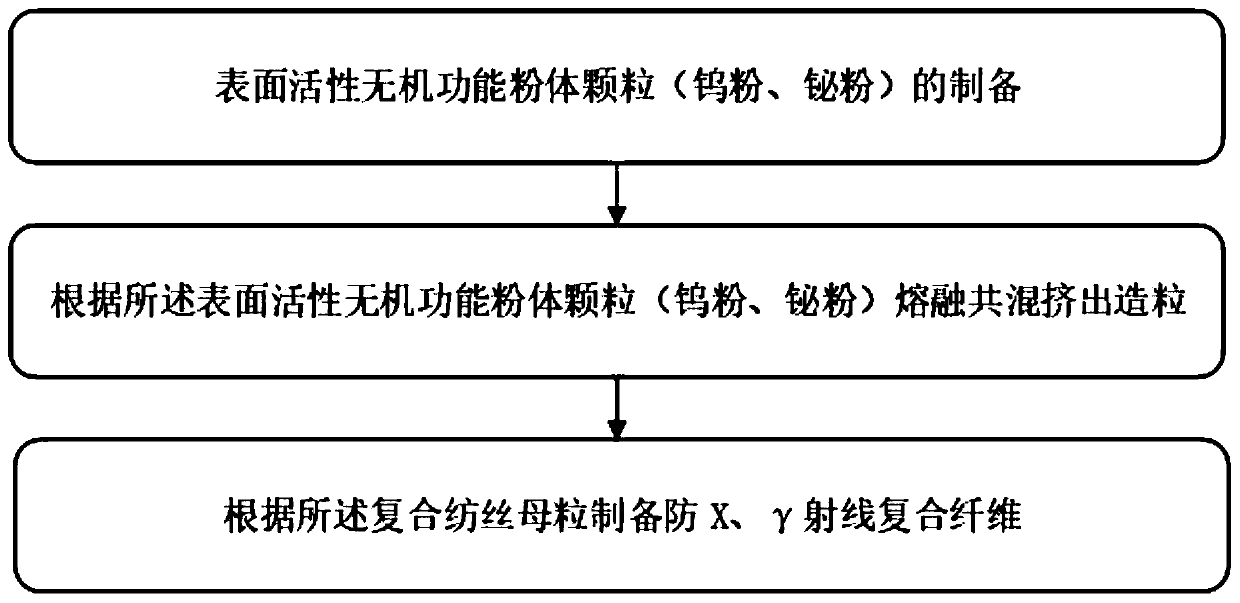
Preparation method of X-ray and gamma-ray shielding composite fiber
InactiveCN110219069ASimple processSimple and fast operationMonocomponent polyolefin artificial filamentMonocomponent polyesters artificial filamentFiberX-ray shield
The invention provides a preparation method of X-ray and gamma-ray shielding composite fiber. The preparation method includes the steps of S1, preparation of functional powder particles for surface active inorganic ray shielding; S2, blending granulation; S3, preparation of primary fiber. Polypropylene and polyethylene terephthalate are respectively adopted as the matrix of the composite fiber, tungsten powder and bismuth powder are taken as shielding agents for X-ray and gamma-ray, silane coupling agent VIII (aminophenyl trioxane) is taken as intermediates for surface modification of inorganic functional powder fillers in order to increase interfacial bonding between inorganic powder and organic matter, the lead-free composite protective fiber with high X-ray shielding agent content, gooddurability, excellent physical and mechanical properties and textile processing properties and good wearability is prepared, and broad application prospect is achieved.
Owner:NANTONG UNIVERSITY
Process for preparing trioxane
Owner:TICONA GMBH
Water-soluble trioxanes as potent and safe antimalarial agents
InactiveUS6136847AGood water solubilityImprove efficacyBiocideOrganic chemistryArylAntiparasite agent
Biologically-active, water soluble, 3-substituted trioxanes of the formula wherein R represents a COOH- substituted aryl group, a substituted or unsubstituted heteroaryl group or an alkyl group, and C12-(p-carboxy)benzyloxy trioxanes of formula wherein R represents a substituted or unsubstituted alkyl, alkenyl, aryl or heteroaryl group and methods for their use as antiparasitic agents, particularly for the treatment of malaria.
Owner:THE JOHN HOPKINS UNIV SCHOOL OF MEDICINE
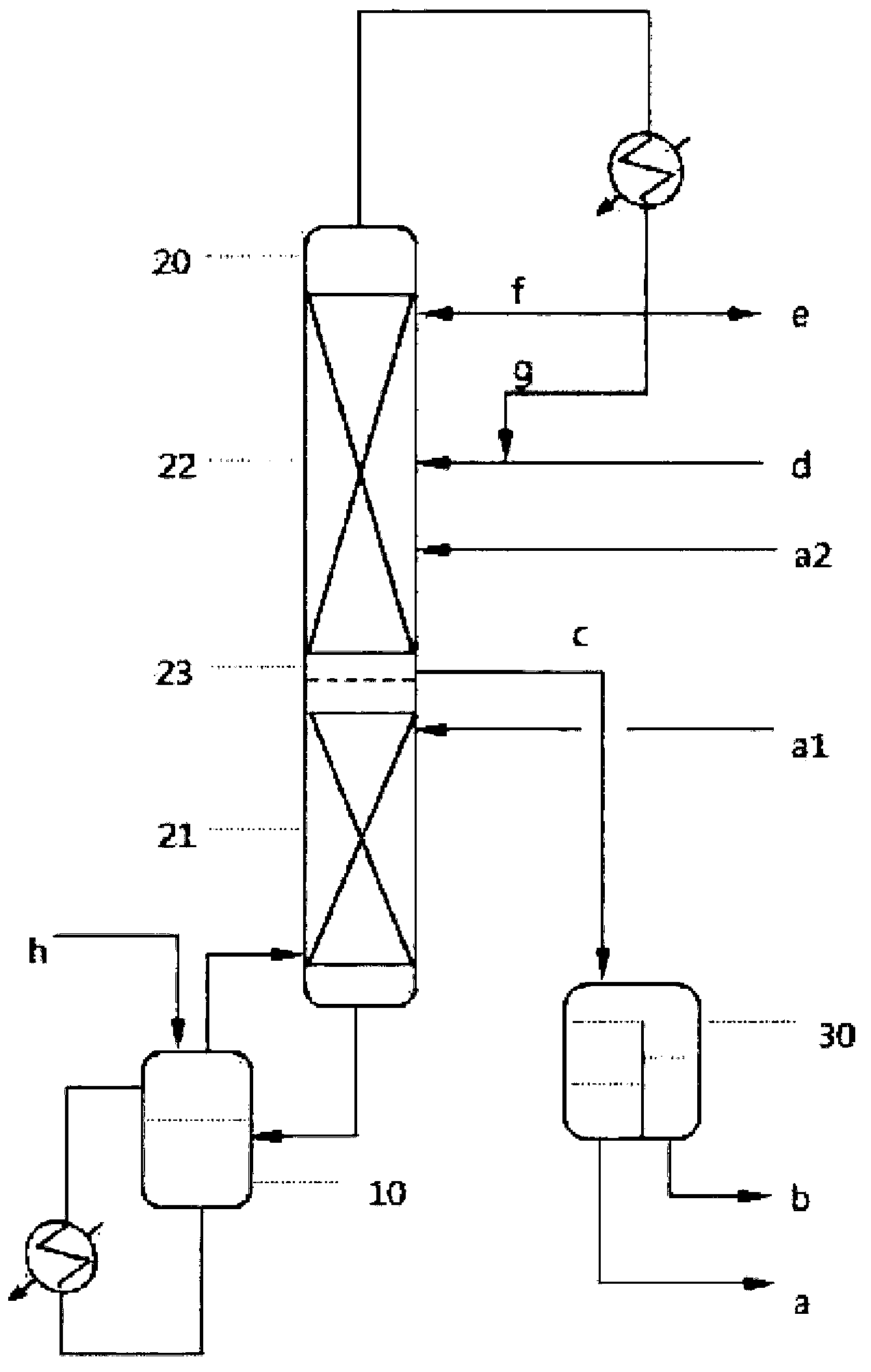
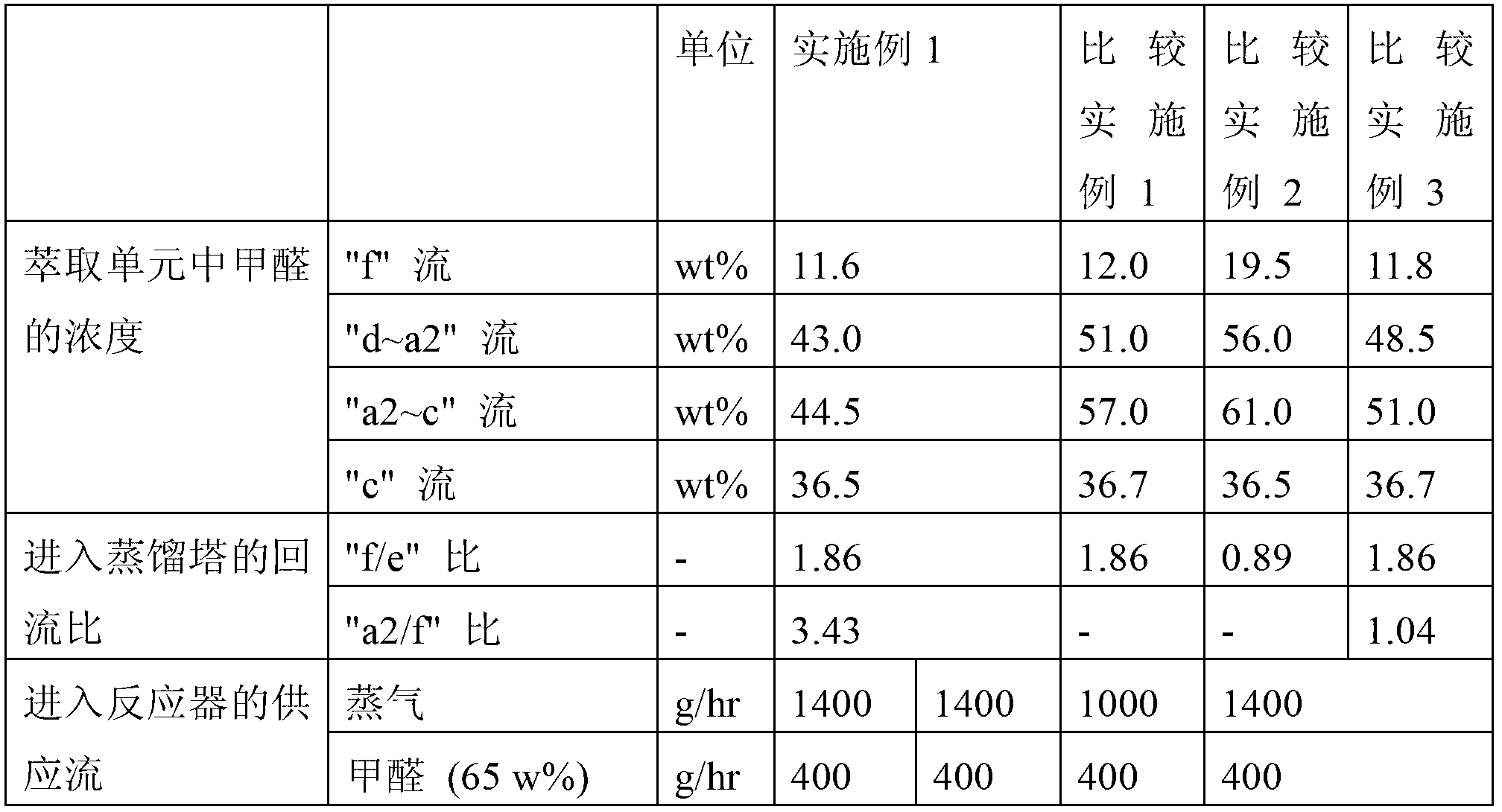
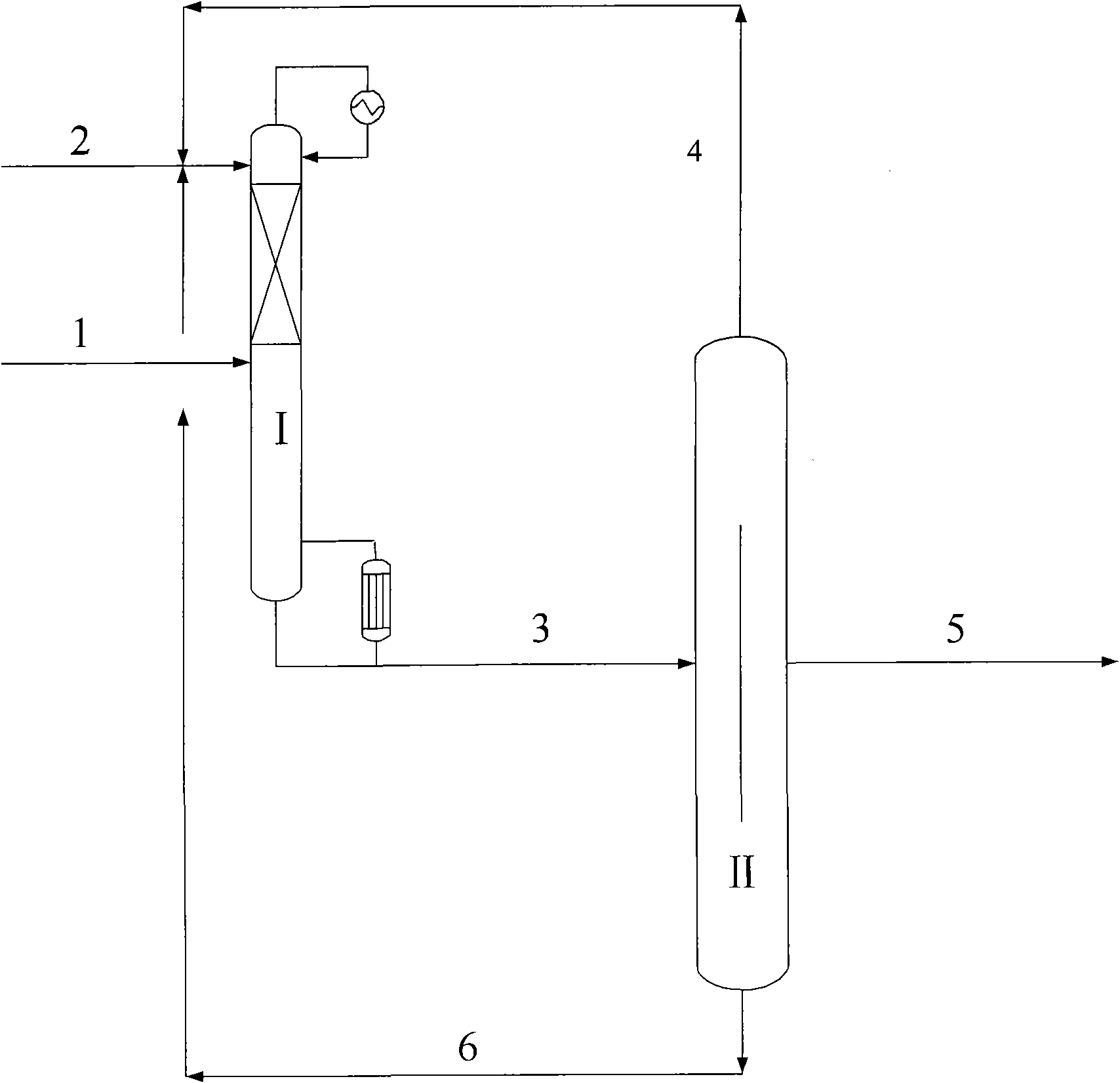
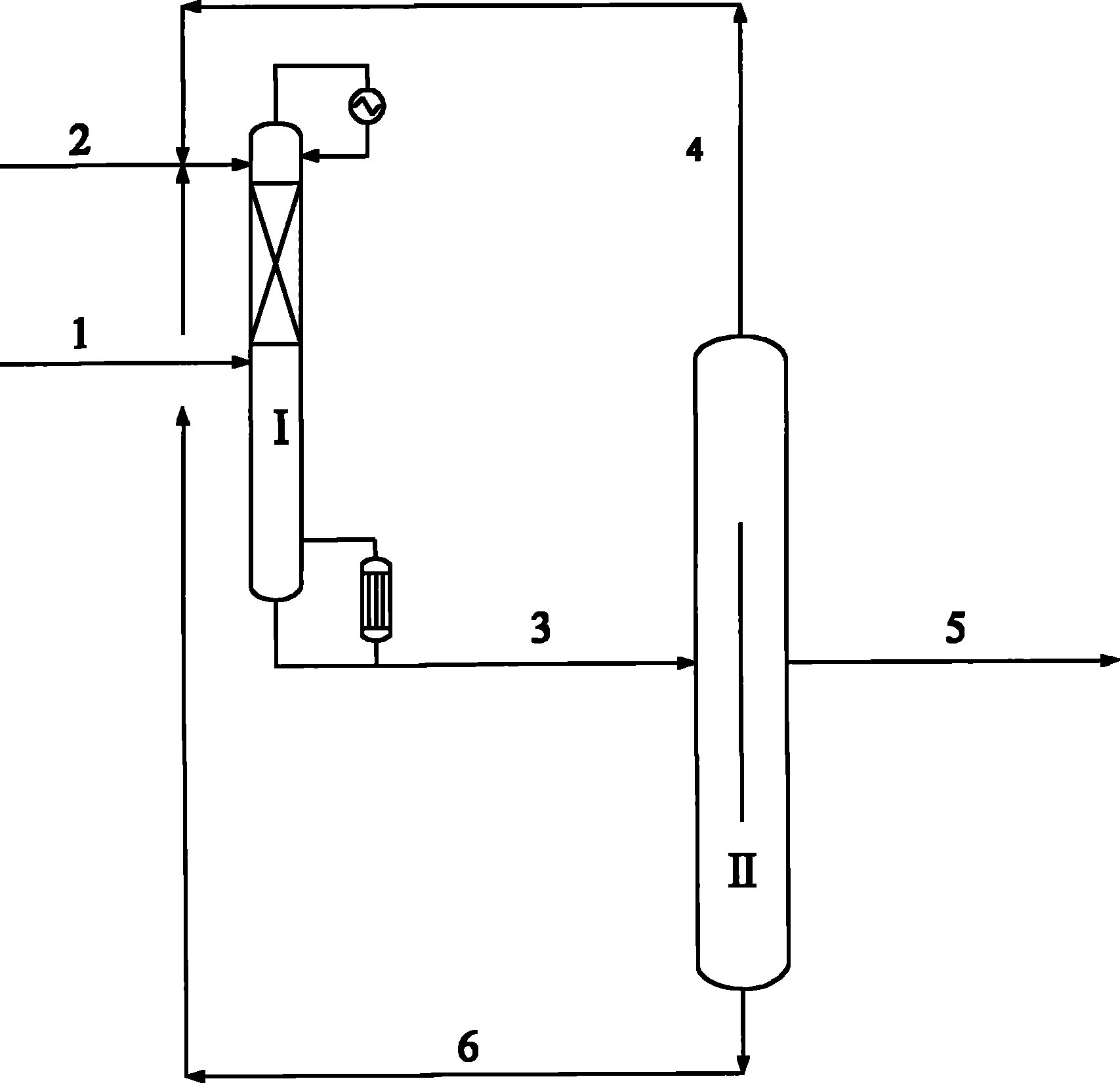
Process for the separation of an aqueous mixture of trioxane and formaldehyde and corresponding applications
InactiveUS7005529B2Stabilize solution obtainedOrganic compound preparationCarbonyl compound preparationDistillationVapor phase
Process for the separation of an aqueous mixture of trioxane and formaldehyde and corresponding applications. The aqueous trioxane and formaldehyde mixture has first trioxane:formaldehyde ratio. The process includes the steps of reacting the aqueous mixture of trioxane and formaldehyde with urea, and separation an exiting vapor phase having a second trioxane:formaldehyde ratio that it higher than the first trioxane:formaldehyde ratio. An aqueous mixture of trioxane and formaldehyde coming from a reactor in which trioxane is being synthesized or from a distillation column in which a prior aqueous mixture of trioxane and formaldehyde can be separated from the excess formaldehyde. Raw material for the production of urea-formaldehyde glues or resins can be formed.
Owner:PATENTES & NOVEDADES
Production process for s-trioxane and extraction reaction tower
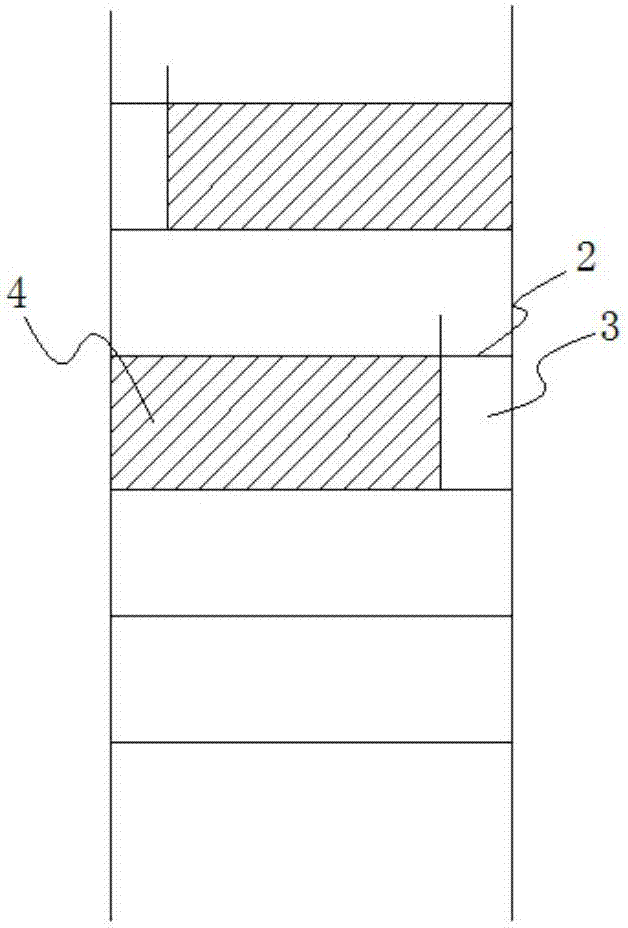
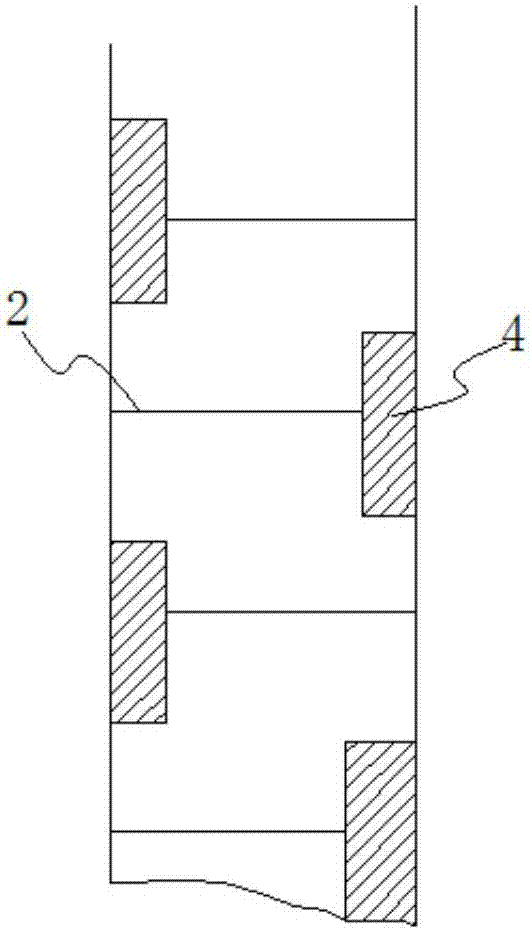
PendingCN107474036AImprove conversion rateReduce energy consumptionOrganic chemistryLiquid solutions solvent extractionPolyoxymethylenePtru catalyst
The invention discloses a production process for s-trioxane and an extraction reaction tower. Specifically, the extraction reaction tower includes an extraction agent inlet disposed at the bottom, a reactant recovery port arranged at the top, and multiple stages of extraction reaction units arranged sequentially from the bottom up. Adjacent extraction reaction units are separated by perforated tower plates, also downcomers are disposed between adjacent extraction reaction units, and the extraction reaction units are internally provided with a solid acid catalyst. The production process for s-trioxane and the extraction reaction tower provided by the invention can reach a formaldehyde conversion rate of more than 45%, and also have the characteristics of small steam consumption in the reaction process, and low equipment investment and the like.
Owner:湖北三里枫香科技有限公司
Method for preparing 1,3,5-trioxane
The present invention relates to a method for preparing 1,3,5-trioxane using a distillation tower including a reactor, a distillation section, and an extraction section. Particularly, the present invention relates to a method for preparing 1,3,5-trioxane, which involves utilizing the water phase separated from the stream discharged through the extraction section of the distillation tower in the process for extracting 1,3,5-trioxane.
Owner:KOLON PLASTICS INC
Orally active, antimalarial, anticancer, artemisinin-derived trioxane dimers
InactiveUS7417156B2Easy to transformPotent anticancer activityBiocideAnimal repellantsCancer cellPhosphoric acid
In only two steps and in 65% overall yield, natural trioxane artemisinin (I) was converted on gram scale into C-10-carba trioxane dimer (3). This new, very stable dimer was then transformed easily in one additional step into four different dimers (4-7). Alcohol and diol dimers (4 and 5) and ketone dimer (7) are 10 times more antimalarially potent in vitro than artemisinin (I), and alcohol and diol dimers (4 and 5) are strongly inhibitory but not cytotoxic toward several human cancer cell lines. Water-soluble carboxylic acid derivatives (8a-10c and 12) were easily prepared from dimers (4-6); they are thermally stable even at 60° C. for 24 hours, are more orally efficacious as antimalarials than either artelinic acid or sodium artesunate, and have potent and selective anticancer activities. Further derivitization of the alcohol dimers (4 and 17), diol dimer (5) and ketone (7) has produced a number of analogs also antimalarially active in vitro at sub-nanomolar concentrations (most notably: pyridine N-oxides (13, 15, 18, 23, 24 and 25), phosphoric acid triesters (26 and 27), sulfonamide (40) and cyclic carbonate (41)). In addition, dimers (13 and 19) are more efficacious (when administered both orally and i.v.) and less toxic (when administered intraperitoneally to mice as a single dose) than clinically-used sodium artesunate, thereby giving them a better antimalarial therapeutic index than sodium artesunate.
Owner:THE JOHN HOPKINS UNIV SCHOOL OF MEDICINE
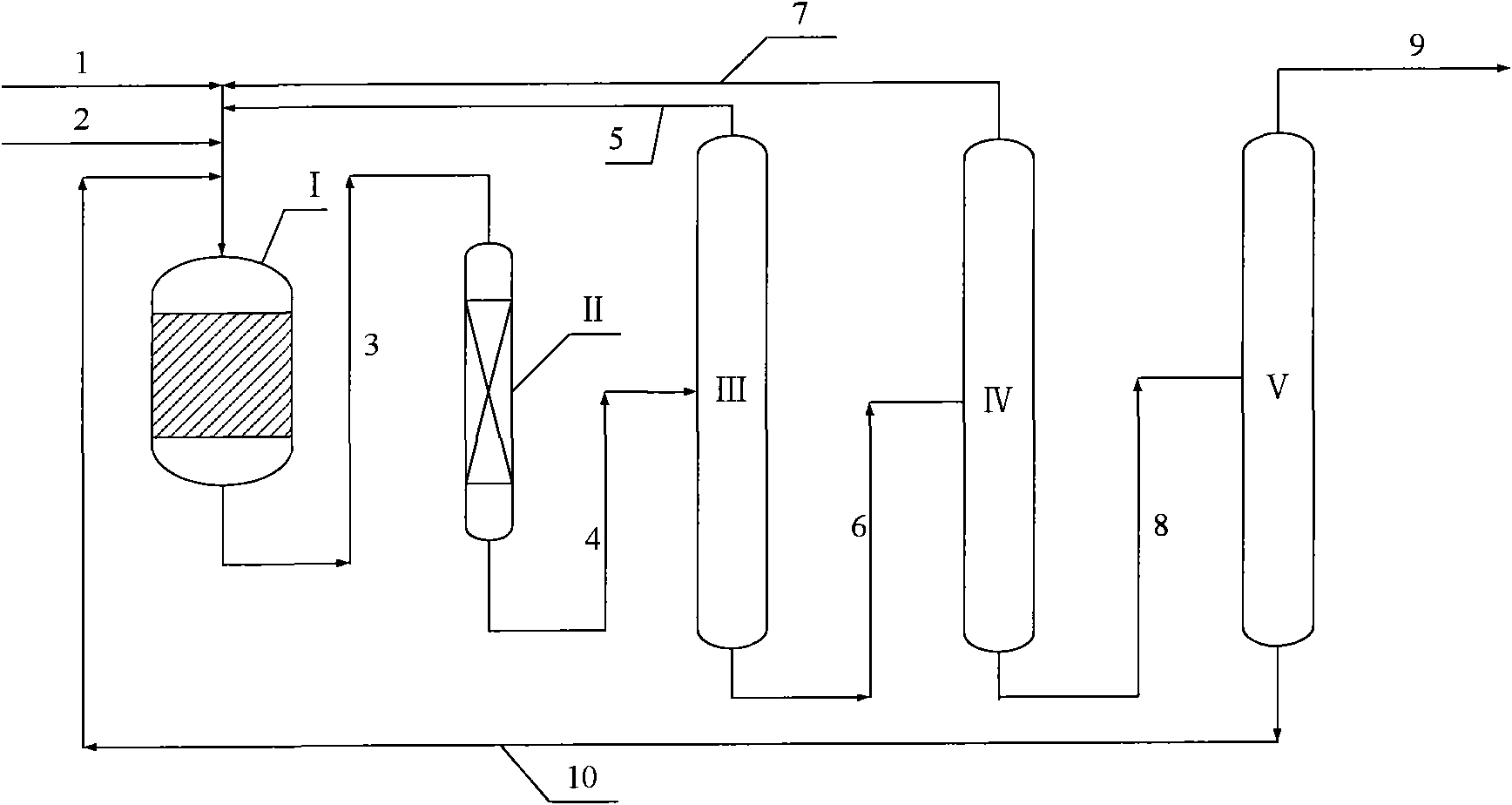
Device and method for preparing 1,3,5-trioxane continuously from concentrated formaldehyde as raw material
The invention discloses a device and a method for preparing 1,3,5-trioxane continuously from concentrated formaldehyde as a raw material. The device comprises a reaction and concentration system, a crystallization system and a refining system. The reaction and concentration system is mainly used for preparing 1,3,5-trioxane from concentrated formaldehyde as the raw material through a reaction under the action of a catalyst and concentrating 1,3,5-trioxane into a 1,3,5-trioxane solution with the concentration of 63% through a concentrating tower; the crystallization system is mainly used for concentrating the 1,3,5-trioxane solution with the concentration of 63% into a 1,3,5-trioxane solution with the concentration of 93% through crystallization; the refining system is mainly used for refining the 1,3,5-trioxane solution with the concentration of 93% into a 1,3,5-trioxane solution with the concentration of 99.9%, and the 1,3,5-trioxane solution serving as an intermediate product is conveyed to other devices for use. Compared with a 1,3,5-trioxane preparing route adopting extraction, the device and the method have the advantages that the flow and the operation are simple, energy consumption is low, environmental pollution caused by benzene is reduced effectively, and the device and the method are quite suitable for large-scale industrial production.
Owner:苏州双湖化工技术有限公司
Production method of polyoxymethylene dimethylether
ActiveCN102372614AOvercome the shortcoming of short lifeHigh selectivityOrganic chemistryOrganic compound preparationPolyoxymethyleneReflux
The invention relates to a production method of polyoxymethylene dimethylether and mainly solves the problems of low selectivity of polyoxymethylene dimethylether, complex technology and high energy consumption during the present production process of polyoxymethylene dimethylether. The production method provided by the invention comprises the following steps of: carrying out a reaction between methylal and trioxane in a catalytic distillation column while separating ingredients, condensing steam on the top of the column, followed by reflux, allowing a first part of the materials on the bottom of the column to return to the catalytic distillation column after vaporizing the first part through a reboiler while a second part is used as a production stream I and the weight ratio of the first part materials to the second part materials is 1-10: 1; allowing the production stream I to enter into a divided wall distillation column for separation, and collecting polyoxymethylene dimethylether DMM3-8 from the produced central part in the divided wall distillation column. The technical scheme provided by the invention greatly solves the problems and can be used in the industrial production of polyoxymethylene dimethylether.
Owner:CHINA PETROLEUM & CHEM CORP +1
Trioxane dimers having high anticancer and long-lasting antimalarial activities
Owner:THE JOHN HOPKINS UNIV SCHOOL OF MEDICINE +1
Polyacetal copolymer
To provide a resin material in which various properties of a polyacetal resin such as excellent appearance, slidability and thermal stability are maintained and a rigidity is improved as well. That is, a polyacetal copolymer which is prepared by a copolymerization of 100 parts by weight of trioxane (A), 0.0005 to 2 parts by weight of the component (B), which is a compound (B-1) having at least three glycidyl groups in the molecule or a compound (B-2) having at least two epoxy groups in the molecule, and 0 to 20 parts by weight of a cyclic ether compound (C) copolymerizable with trioxane, and which has a total terminal group amount of 15 to 150 mmol / kg, when (B) is (B-2).
Owner:POLYPLASTICS CO LTD +1
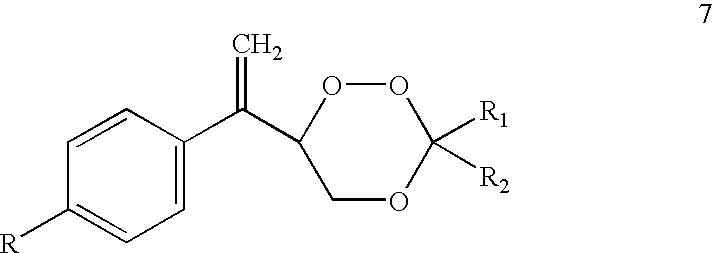
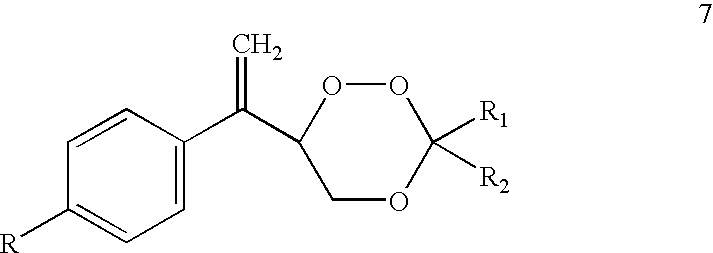
Substituted 1,2,4-trioxanes as antimalarial agents and a process of producing the substituted 1,2,4-trioxanes
InactiveUS6316493B1Promising anti-malarial activityPromising activityBiocideOrganic active ingredientsArylHydrogen
The present invention relates to novel substituted 1,2,4-trioxanes that are useful as anti-malarial agents, and have a general formula 1,wherein R1 and R2 are selected from the group consisting of a hydrogen, a C1-11 alkyl group; R3 and R4 are selected from the group consisting of a hydrogen, a C1-11 alkyl group, a C3-10 aryl group, a C1-2CO2H carboxyalkyl group; and X represents hydrogen or a lower alkoxy group having 1 to 6 carbons.
Owner:COUNCIL OF SCI & IND RES
Wear-resisting coating
The invention discloses a wear-resisting coating. The wear-resisting coating comprises the following raw materials in parts by weight: 20-30 parts of vinyl triisopropoxysilane, 3-5 parts of silicon dioxide aerogel, 15-20 parts of trioxane, 5-9 parts of water, 3-7 parts of acetic anhydride, 10-15 parts of acetylcholine, 0.1-0.3 part of polyoxyethylene anhydro-sorbit monoglyceride, 10-15 parts of crystallizing silicon oxide, and 7-13 parts of graphite powder, wherein the porosity of the silicon dioxide aerogel is 75-79%, and the weight ratio of silicon dioxide aerogel to vinyl triisopropoxysilane is 1:(5-7). The wear-resisting coating can improve the degree of crosslinking of a coating and enhance the resistance to scratching, and has low cost.
Owner:ANHUI JINDUN PAINT
Production method for polyacetal copolymer
A production method for a polyacetal copolymer that makes deactivation of a catalyst simple and efficient and that achieves a high polymerization yield and high quality using equipment that does not require a cleaning step and a process that involves a simple operation technique. The production method for a polyacetal copolymer uses trioxane as a main monomer and a cyclic ether and / or a cyclic formal having at least one carbon-carbon bond as a comonomer. In the production method, a predetermined heteropoly acid is used as a polymerization catalyst to perform copolymerization, a predetermined salt is added to the reaction product, melt kneading processing is performed, and the polymerization catalyst is deactivated.
Owner:POLYPLASTICS CO LTD
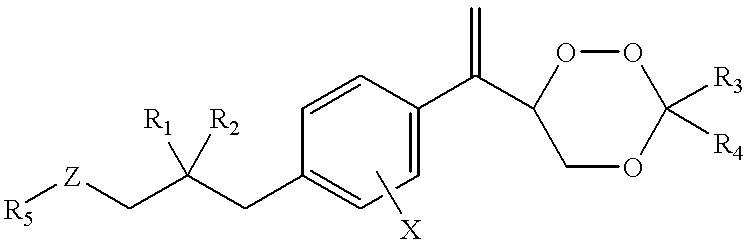
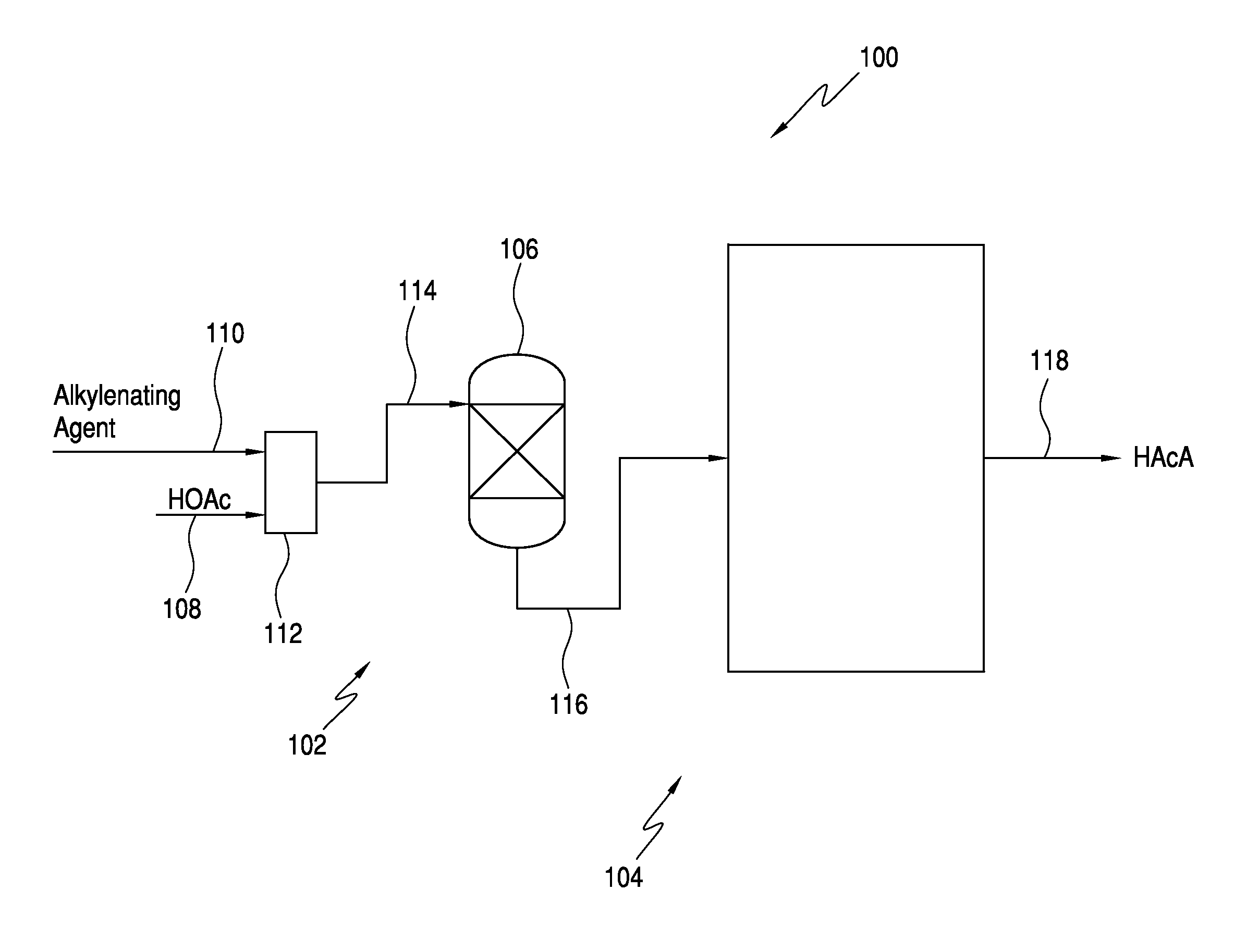
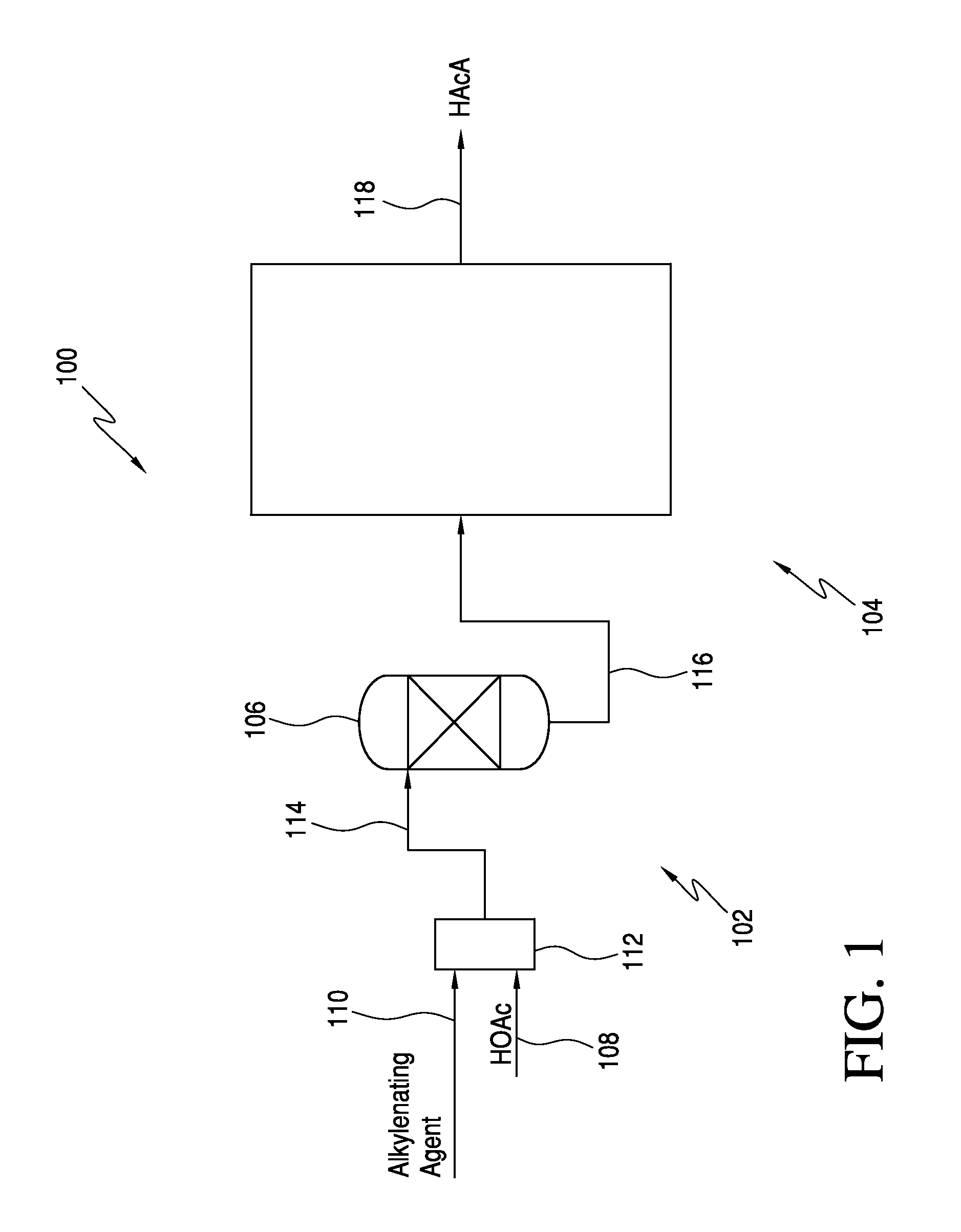
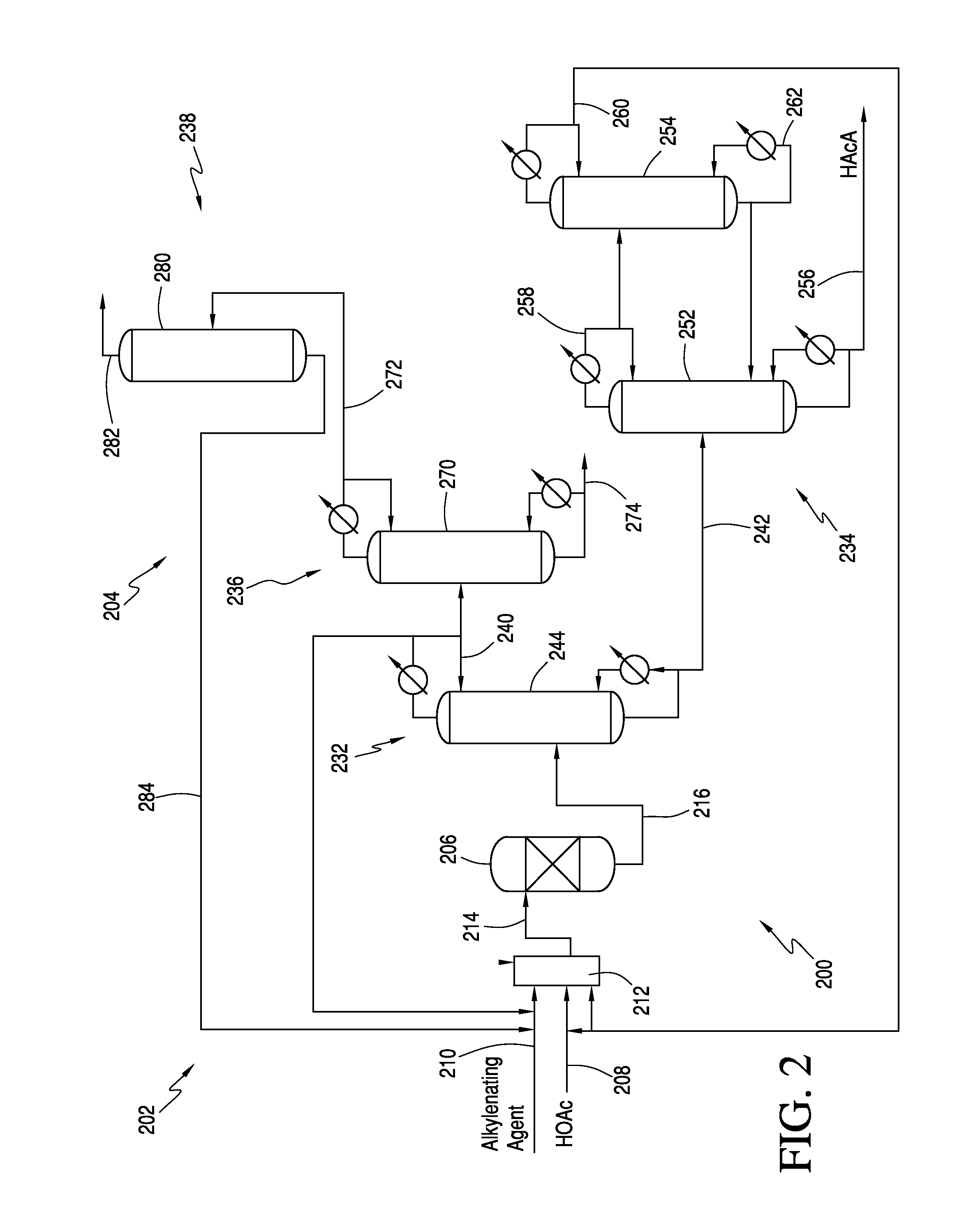
Processes for the production of acrylic acids and acrylates from a trioxane feed
In one embodiment, the invention relates to a process for producing an acrylate product. The process comprises the step of reacting a reaction mixture comprising an alkanoic acid and an alkylenating agent composition under conditions effective to form a crude acrylate product. The alkylenating agent composition comprises at least 1 wt % of a ring-structured compound comprising at least two oxygen atoms and at least one carbon atom, e.g., trioxane and / or tetraoxane. The process further comprises the step of separating at least a portion of the crude acrylate product to form at least one alkylenating agent stream and at least one purified acrylate product stream comprising acrylate product.
Owner:CELANESE INT CORP
Initiator
InactiveUS20080125566A1High standardOther chemical processesCationic polymerizationSolution polymerization
An initiator for cationic polymerization comprises a salt of a protic acid as well as a protic acid. The molar ratio of protic acid to salt is in the range from 1:0.01 to 1:2000. The initiator is used for example for cationic homo- or copolymerization of trioxane, and permits stable and flexible operation of the polymerization.
Owner:CELANESE SALES GERMANY
Manufacturing method of polyacetal copolymer
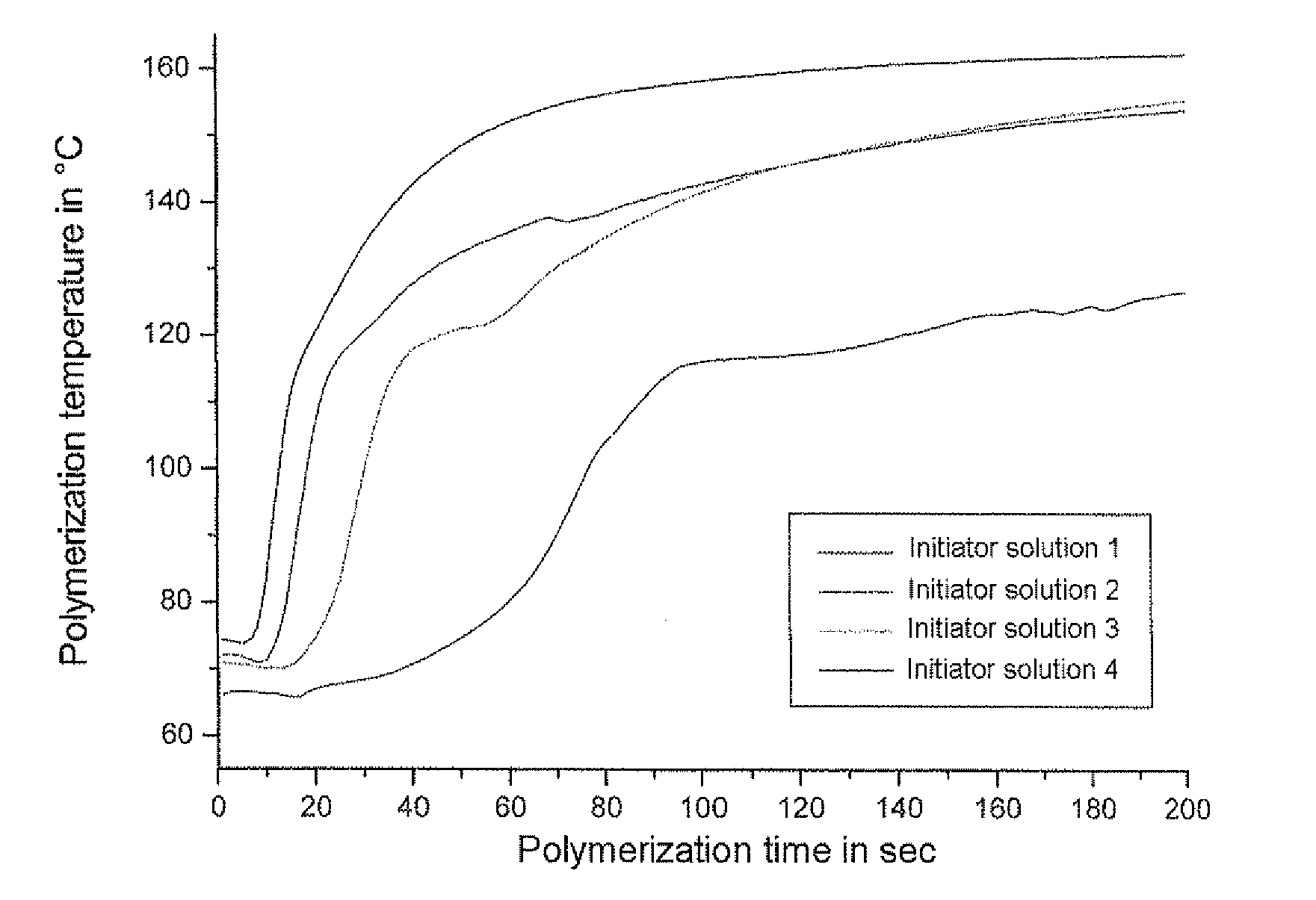
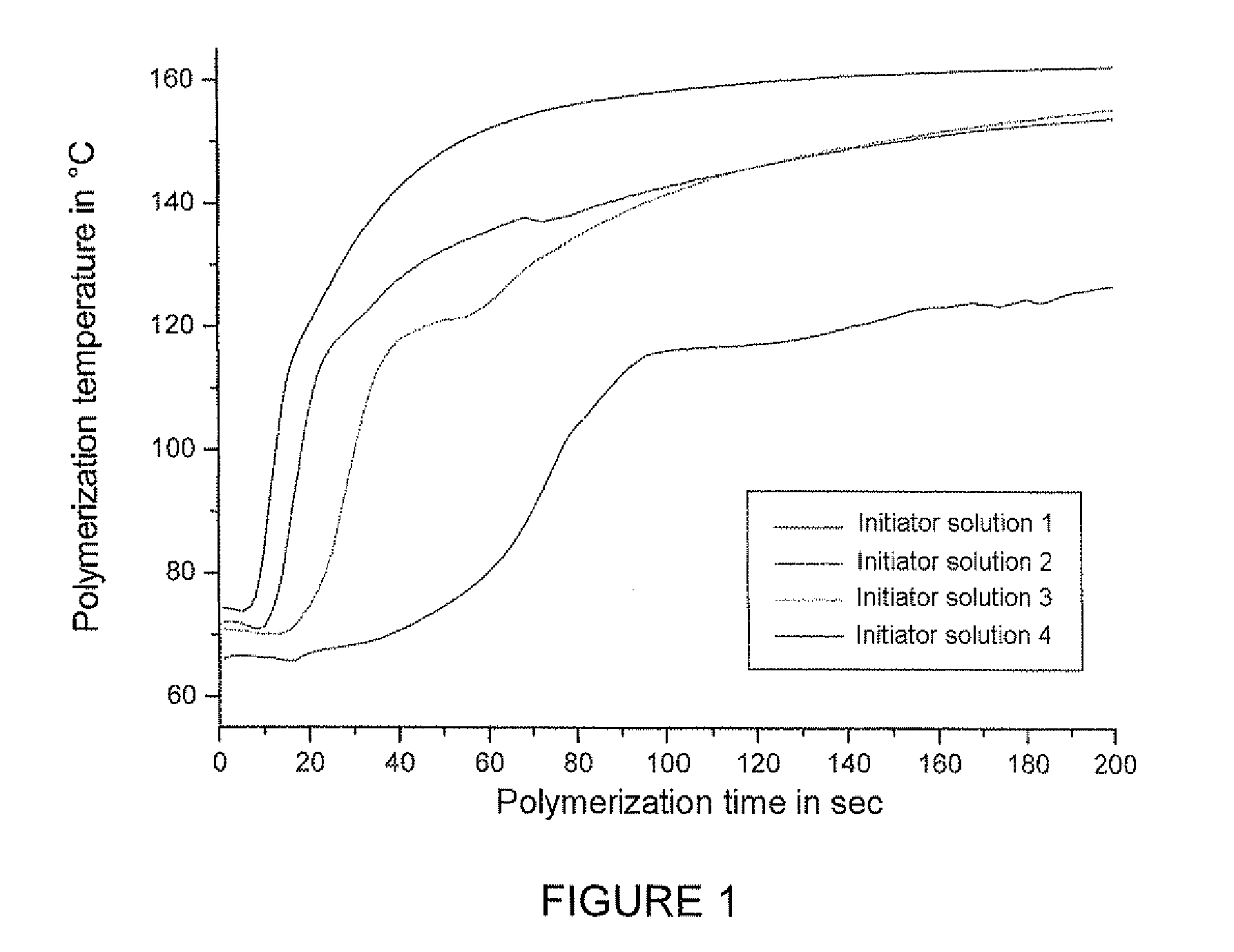
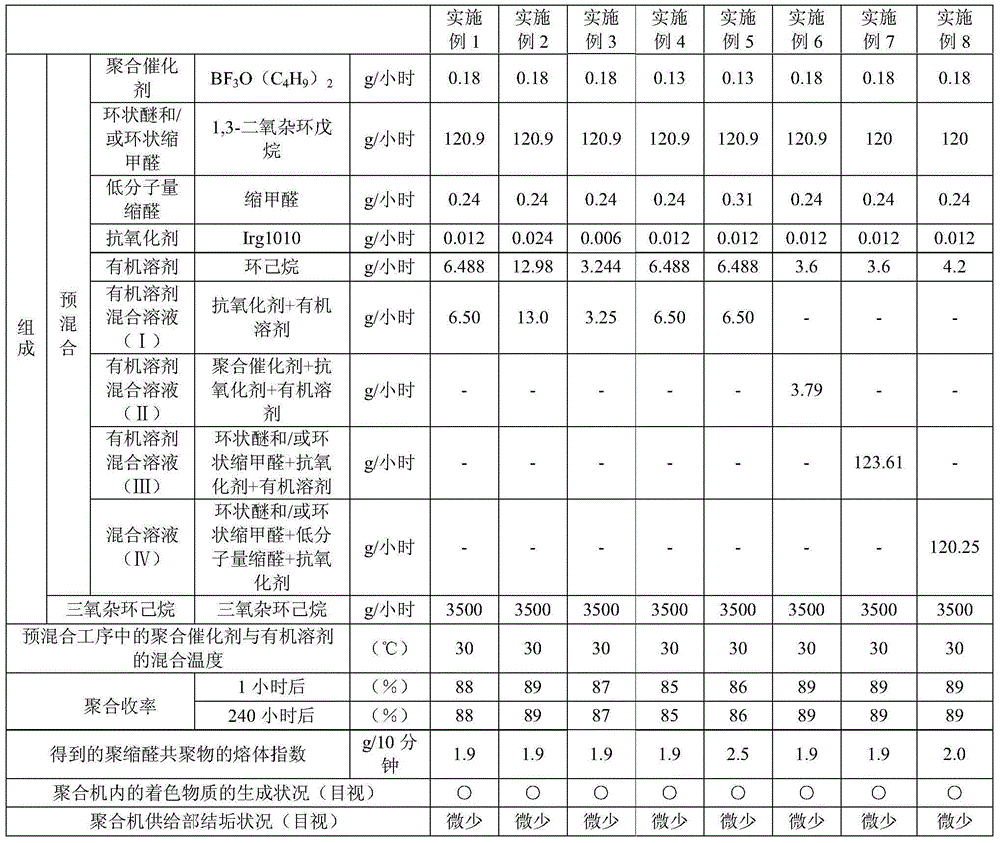
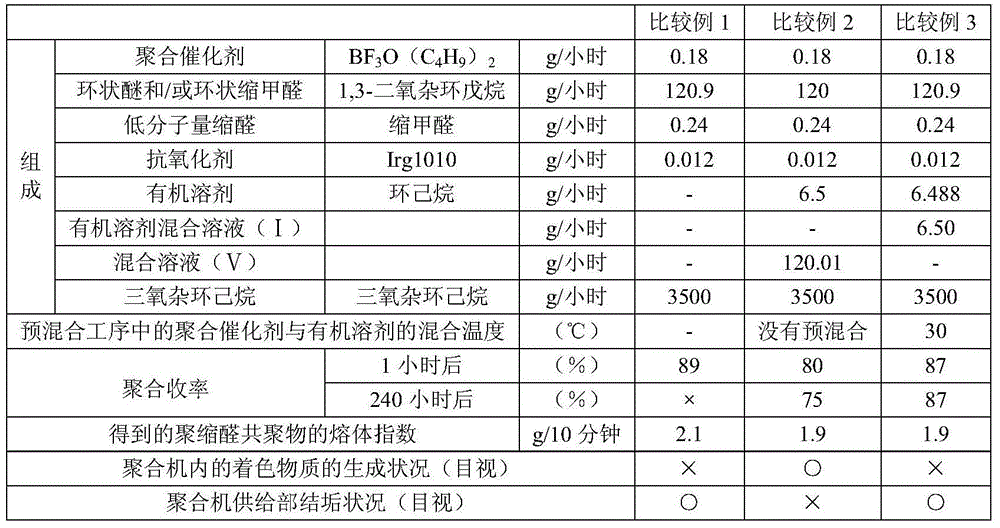
The invention relates to a manufacturing method of polyacetal copolymer. The manufacturing method is capable of reducing scales, and can stably and continuously produce high quality polyacetal copolymer in a long term in a high yield. The manufacturing method comprises the following steps: mixing cyclic ether and / or cyclic condensed formaldehyde, a polymerization catalyst, acetal, which is represented by the formula (1), with a low molecular weight, an organic solvent, and an antioxidant to obtain a pre-mixed solution; and then feeding the pre-mixed solution and trioxane into a polymerization machine to carry out polymerization. Formula (1): R-(CH2-O)n-R.... in the formula (1), the R represents a free hydrogen atom, a straight chain alkyl group or a branched alkyl group, a straight chain alkoxyl group or a branched alkoxyl group, or a hydroxyl group, and n represents an integer in a range of 1 to 20.
Owner:ASAHI KASEI KK
1,2,4-Trioxanes and 1,2,4-trioxepanes
Novel substituted 1,2,4-trioxanes and 1,2,4-trioxepanes useful as anti-malarial and / or anticancer agents, and an improved method for their preparation, preferably involving a thiol-olefin co-oxygenation (TOCO) reaction between an allylic alcohol and a ketone.
Owner:ONEILL PAUL M +3
Process for producing polyacetal copolymer
ActiveUS20170073451A1Improve thermal stabilityLittle formaldehyde formationPolymer sciencePtru catalyst
A process for producing a polyacetal copolymer, the process making catalyst deactivation easy and efficient. Trioxane as a major monomer is copolymerized with one or more comonomers that are a cyclic ether and / or cyclic formal having at least one carbon-carbon bond, using a nonvolatile protonic acid as a polymerization catalyst at 100° C. or lower until the conversion reaches 50% and thereafter at a polymerization environment temperature of 115° C. to 140° C. This process includes: a crushing step in which a dry-process crusher is used to obtain a crude polyacetal copolymer crushed to such a degree that when the crude copolymer is screened with a sieve having an opening size of 11.2 mm, 90 parts by weight or more thereof passes therethrough; and a deactivation step in which a basic compound (e) is added to the crude copolymer and the mixture is melt-kneaded to thereby deactivate the polymerization catalyst.
Owner:POLYPLASTICS CO LTD
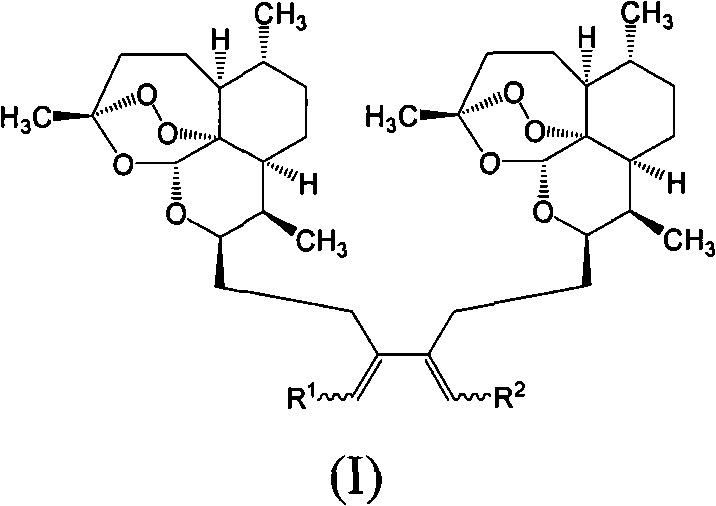
Trioxane dimer compound having antiproliferative and antitumor activities
InactiveUSRE38117E1Pharmaceutical non-active ingredientsPhosphorus organic compoundsAntitumor activityTrioxane
Described herein are novel trioxane dimers of structurewhich possess antiproliferative and antitumor activities.
Owner:THE JOHN HOPKINS UNIV SCHOOL OF MEDICINE
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com