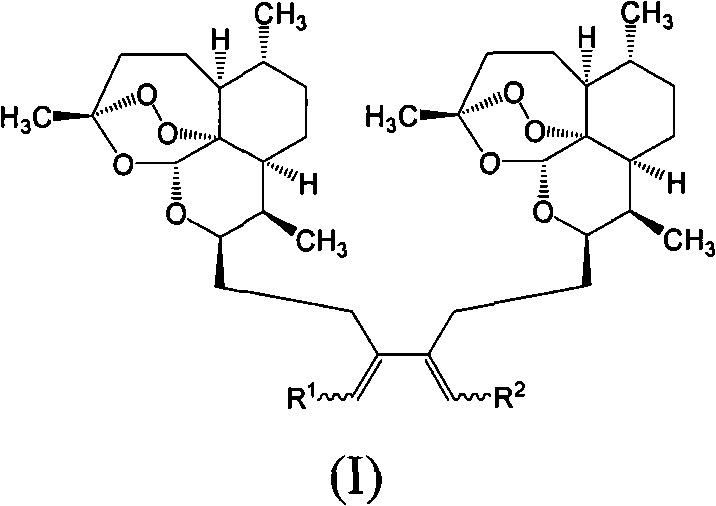
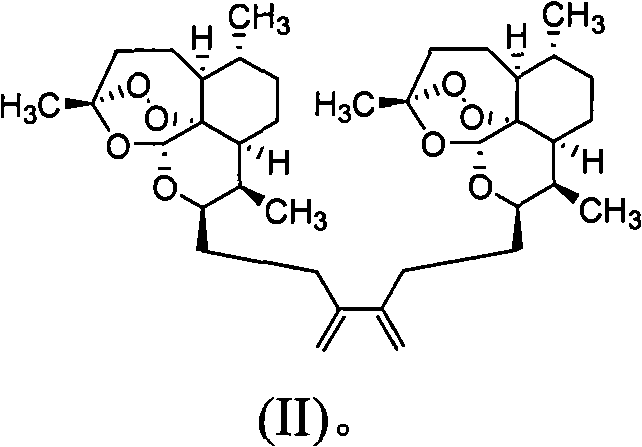
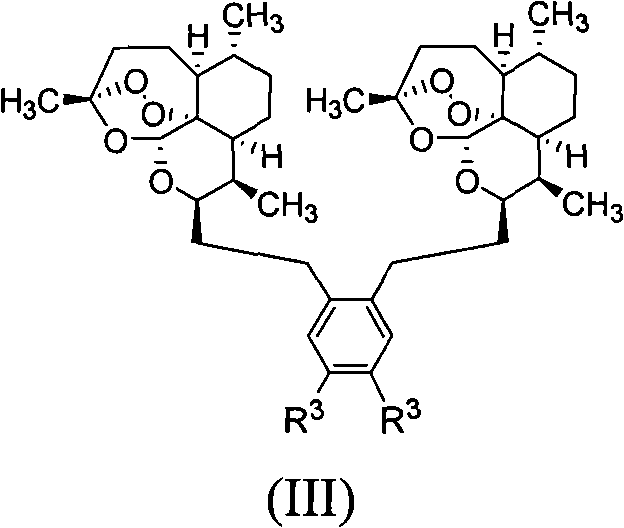
Trioxane dimers having high anticancer and long-lasting antimalarial activities
A compound and solvate technology, applied in the field of trioxane dimer with high anticancer activity and long-acting antimalarial activity, can solve the problem of not having long-acting antimalarial activity and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0198] The following examples are intended to illustrate, but not limit, the claimed invention.
[0199] All air- and moisture-sensitive reactions were performed under argon in oven-dried or flame-dried glass containers. Tetrahydrofuran (THF) and diethyl ether (diethyl ether) were distilled from sodium-benzophenone ketyl, and dichloromethane was distilled from calcium hydride under nitrogen. Dimethyl sulfoxide and hexamethylphosphoric acid triamide were tested under low pressure Molecular sieves are distilled from calcium hydride. Solvents and solutions for air- and moisture-sensitive reactions are transferred by syringe or cannula. All experiments were monitored by thin layer chromatography (tlc) on EM Science pre-coated silica gel 60F-254 glass support plates with a thickness of 0.25 mm. Flash chromatography was performed on EMD silica gel (40-63 μm). Yield was not optimized. The purity of the final product was verified by two different high performance liquid chromato...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com