Organic fuel cell methods and apparatus
a fuel cell and organometallic technology, applied in the direction of cell components, non-aqueous electrolyte cells, electrochemical generators, etc., can solve the problems of high cost, significant constraints on the construction materials of the fuel cell, and sulfuric acid electrolyte in the current-art direct methanol fuel cell, etc., to improve the wetting properties, reduce interfacial tension, and poor fuel wetting properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
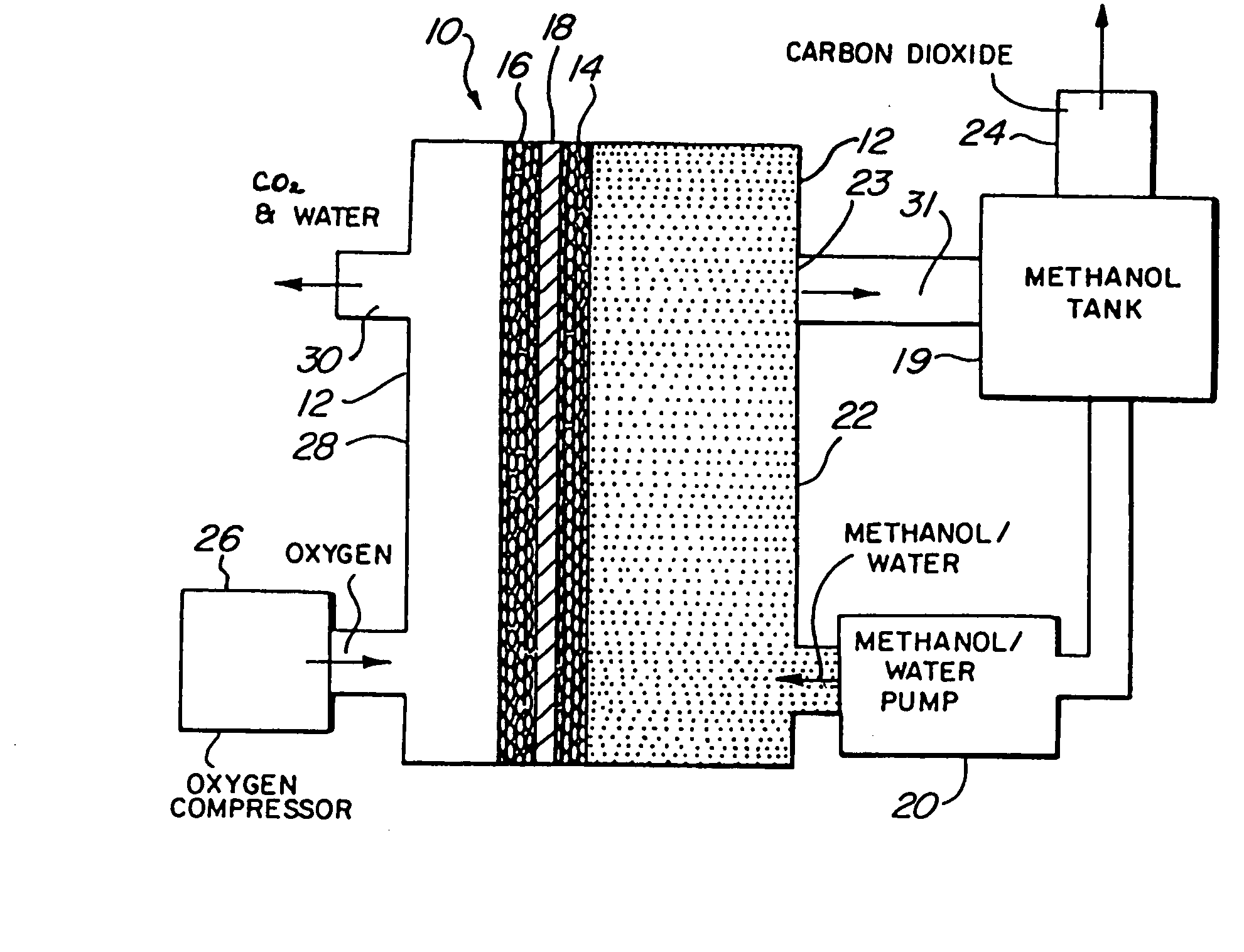
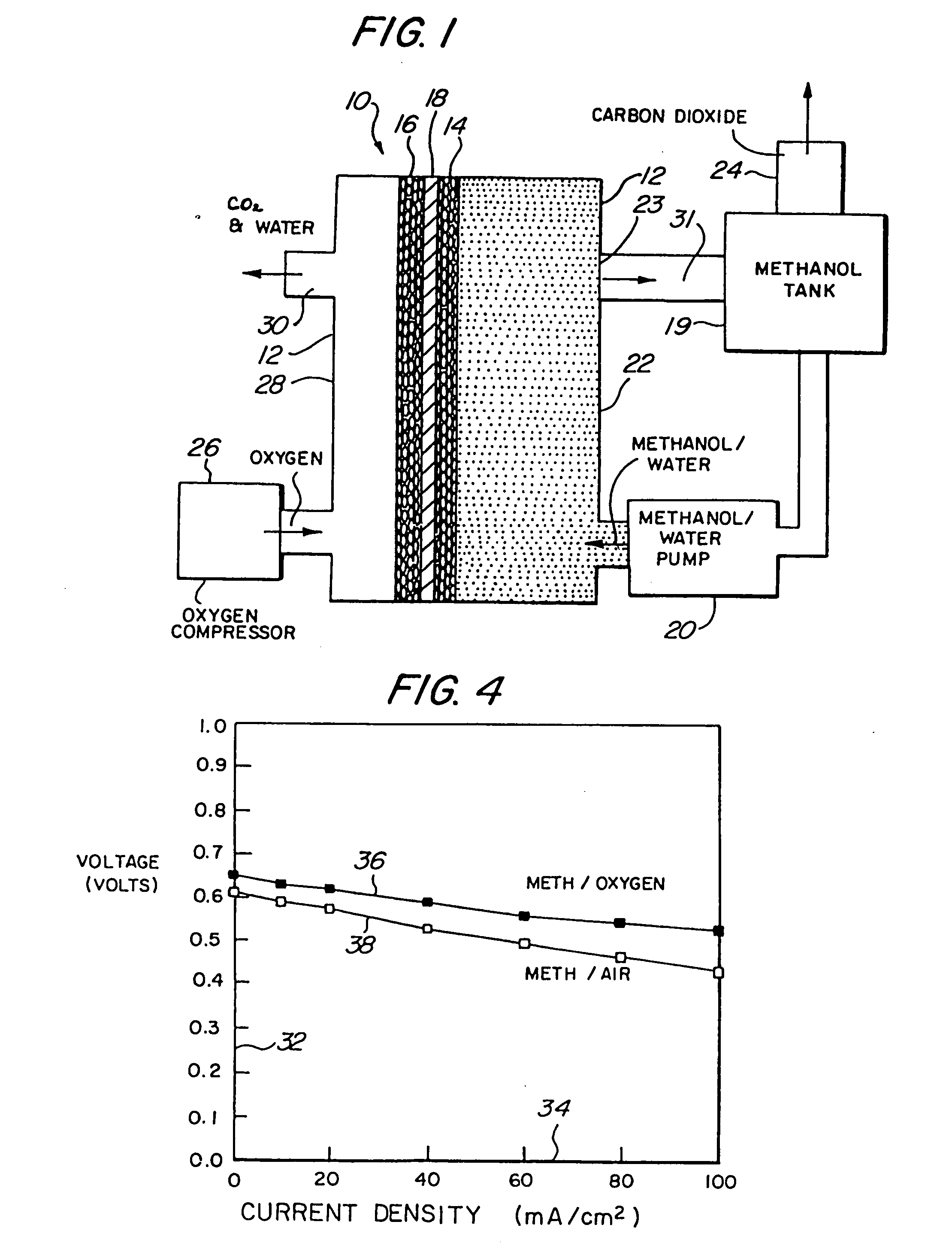
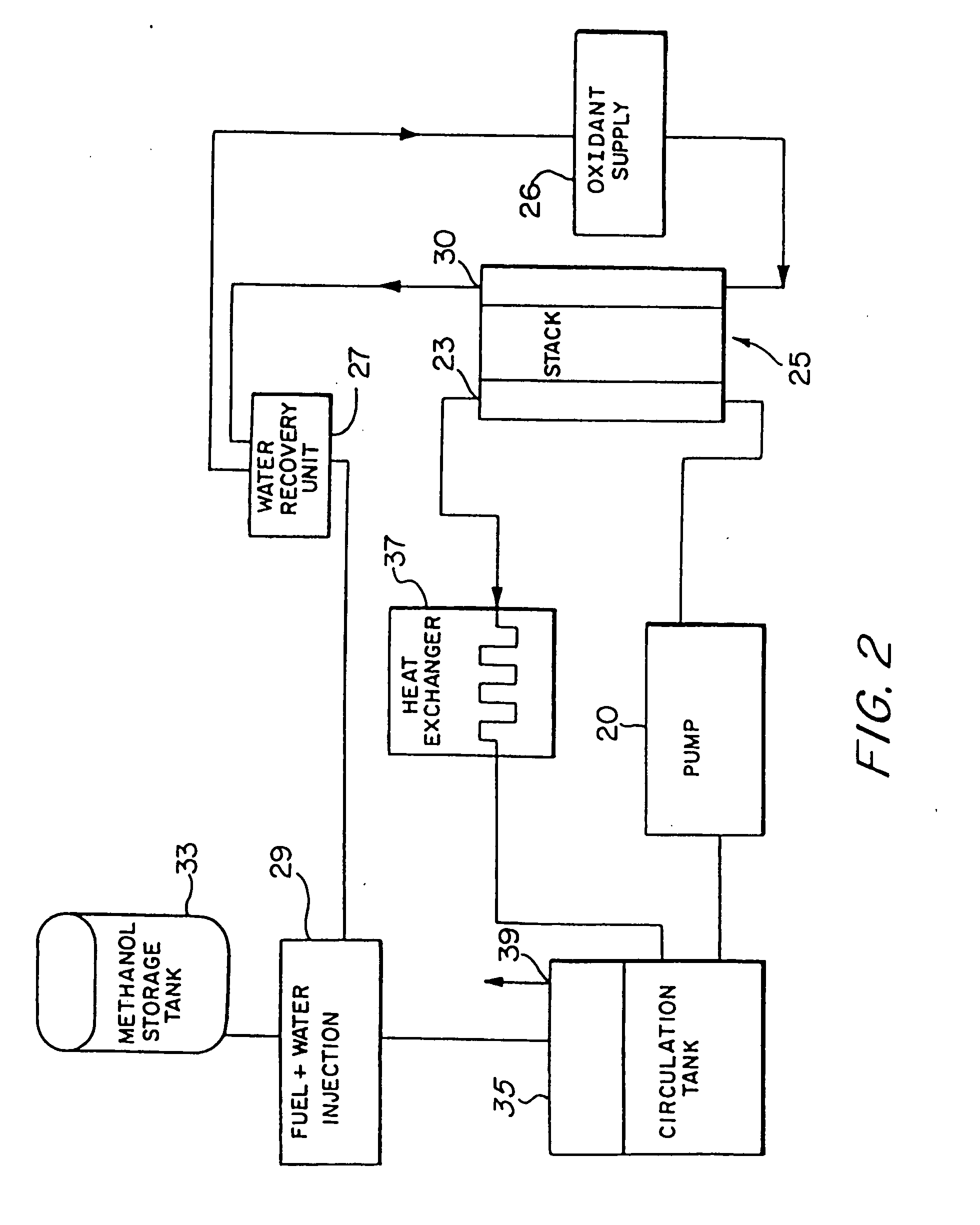
[0054] Referring to the figures, the preferred embodiments of the invention will now be described. Initially, an improved liquid feed organic fuel Cell using a solid polymeric electrolyte membrane and a ionomeric anode additive is described, primarily with reference to FIGS. 1-6. Then, a method for fabricating the anode having the ionomeric additive is described with reference to FIGS. 7-8. A method for achieving improved wetting by fabricating an electrode within a bath containing perfluorooctanesulfonic acid is described with reference to FIGS. 9-11. A fuel cell employing perfluorooctanesulfonic acid as a fuel additive is described with reference to FIG. 12. Fuel cells employing dimethoxymethane, trimethoxymethane and trioxane as fuels are described with referen&to FIGS. 13-21.
Fuel Cell Employing Solid Proton Conducting Elecrolyte Membrane.
[0055]FIG. 1 illustrates a liquid feed organic fuel cell 10 having a housing 12, an anode 14, a cathode 16 and a solid polymer proton-conduc...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Area | aaaaa | aaaaa |
| Hydrophilicity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com