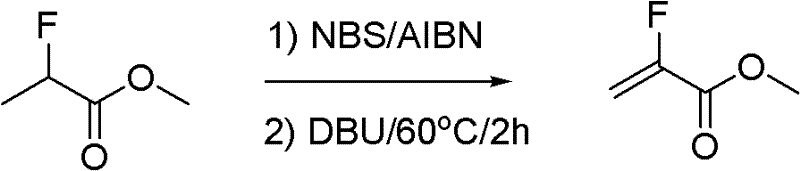
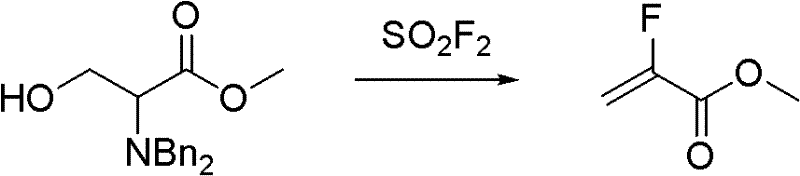Synthesis method of methyl-alpha-fluoroacrylate and analogues thereof
A technology for the synthesis of methyl fluoroacrylate, which is applied in the preparation of carboxylic acid esters, chemical instruments and methods, and the preparation of organic compounds, can solve problems such as low yield, insufficient product purity, and unsuitability for industrial production, and achieve The effect of low preparation cost and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Example 1: Synthesis of α-methyl fluoroacrylate - no separation of intermediates
[0031] Methyl fluoroacetate (20 g, 0.217 mol) and dimethyl oxalate (25.6 g, 0.217 mol) were added to 100 ml of diphenyl ether, sodium methoxide solid (11.7 g, 0.217 mol) and hydroquinone (0.2 g), heated to 30-40°C and stirred for 5 hours, added paraformaldehyde (6.5g, 0.217mol), kept the temperature at 30-40°C and stirred for 5 hours, directly steamed the product under reduced pressure with a water pump, washed with 5 ml of saturated brine for 2 Once, dried over sodium sulfate to obtain 6.77 grams of product with a yield of 30% and a gas phase purity of 95%. Its reaction formula is:
[0032]
Embodiment 2
[0033] Example 2: Synthesis of α-methyl fluoroacrylate intermediate enol sodium salt, tert-butyl methyl ether as solvent
[0034] Add methyl fluoroacetate (92g, 1mol) and dimethyl oxalate (118g, 1mol) into 500ml of tert-butyl methyl ether, add solid sodium methoxide (54g, 1mol), heat to 30-40°C and stir for 5 hours, Cool to 20° C., filter, wash with 100 ml of tert-butyl methyl ether, and vacuum-dry at 50° C. for 5 hours to obtain 198 g of white or light yellow solid powder with a yield of 99%.
Embodiment 3
[0035] Example 3: Synthesis of α-methyl fluoroacrylate intermediate enol sodium salt, using ethylene glycol dimethyl ether as a solvent
[0036] Add methyl fluoroacetate (92g, 1mol) and dimethyl oxalate (118g, 1mol) to 500ml of ethylene glycol dimethyl ether, add solid sodium methoxide (54g, 1mol), heat to 30-40°C and stir for 5 hours, cooled to ℃, filtered, washed with 100 ml of ethylene glycol dimethyl ether, and vacuum-dried at 50°C for 5 hours to obtain 190 g of white or light yellow solid powder with a yield of 95%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| boiling point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com