Method for preparation of methyl glycolate and by-product methyl methoxyacetate with catalyst
A by-product technology of methyl methoxyacetate and methyl glycolate, which is applied in the direction of carbon monoxide or formate reaction preparation, chemical instruments and methods, and the preparation of organic compounds, can solve the problem of low conversion rate of raw materials and product selectivity Low cost, environmental pollution and other problems, to achieve the effect of high conversion rate of raw materials, high product selectivity, and short synthetic route
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
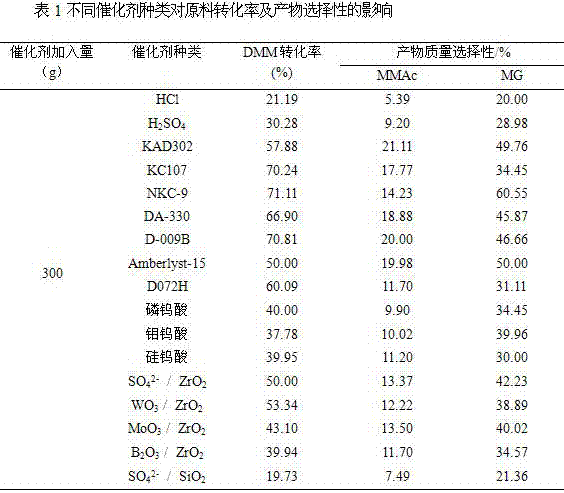
[0060] Weigh 20 kg of methylal (DMM), 3.9 kg of paraformaldehyde (the molar ratio of DMM to aldehyde group is 2:1), 300 g of catalyst, and 4 kg of water and put them into the reactor. The catalyst types are liquid acid (H 2 SO 4 , HCl), cation exchange resins (NKC-9, Amberlyst-15, DA330, KAD302, KC107, D072H), heteropoly acids (phosphotungstic acid, molybdenum tungstic acid, silicotungstic acid), solid super acid (SO 4 2 - / ZrO 2 , WO 3 / ZrO 2 , MoO 3 / ZrO 2 , B 2 o 3 / ZrO 2 ), impregnated solid acid (SO 4 2 - / SiO 2 ), and then introduce 1.0 MPa carbon monoxide gas, if there is no leakage, after emptying the gas in the kettle, repeat the above operation 2 times (replacing the air in the reactor). Introduce a certain amount of gas (6.0 MPa) again to test for leaks, and let it stand for 15 minutes. If the pressure indicator does not drop, it means that there is no air leakage in the device. Then empty the gas in the kettle and fill the kettle with 6.0 MPa CO g...
Embodiment 2
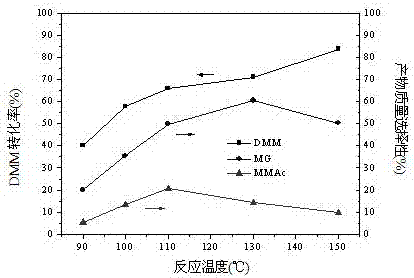
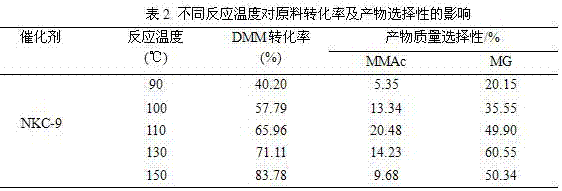
[0065] Weigh 20 kg of methylal (DMM), 3.9 kg of paraformaldehyde (the molar ratio of DMM to aldehyde group is 2:1), 4 kg of water, and 300 g of NKC-9 resin catalyst into the reaction kettle, and then pass it into the reaction vessel at 1.0 MPa Carbon monoxide gas, if there is no leakage, after emptying the gas in the kettle, repeat the above operation twice (replace the air in the reactor). Introduce a certain amount of gas (6.0 MPa) again to test for leaks, and let it stand for 15 minutes. If the pressure indicator does not drop, it means that there is no air leakage in the device. Then empty the gas in the kettle and fill the kettle with 6.0 MPa CO gas again. , heated up, the stirring speed of the reactor was 500 rpm, the reaction pressure was 6.0 MPa, the reaction temperatures were 90°C, 100°C, 110°C, 130°C, 150°C, and the reaction time was 6 h. The reaction results are shown in Table 2.
[0066]
[0067] Reaction conditions: 20 kg methylal, 3.9 kg paraformaldehyde, 4 kg...
Embodiment 3
[0070] Weigh 20 kg of methylal (DMM), 3.9 kg of paraformaldehyde (the molar ratio of DMM to aldehyde group is 2:1), 4 kg of water, and 300 g of NKC-9 resin catalyst into the reaction kettle, and then pass it into the reaction vessel at 1.0 MPa Carbon monoxide gas, if there is no leakage, after emptying the gas in the kettle, repeat the above operation twice (replace the air in the reactor). Introduce a certain amount of gas (6.0 MPa) again to test for leaks, and let it stand for 15 minutes. If the pressure indicator does not drop, it means that there is no air leakage in the device. Then empty the gas in the kettle and fill the kettle with 6.0 MPa CO gas again. , heated up, the stirring speed of the reactor was 500 rpm, the reaction pressures were 2.0 MPa, 3.0 MPa, 4.0 MPa, 5.0 MPa, 6.0 MPa, 8.0 MPa, 10.0 MPa, 15.0 MPa, the reaction temperature was 130°C, and the reaction time was 6 h , and the reaction results are shown in Table 3.
[0071]
[0072] Reaction conditions: 2...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com