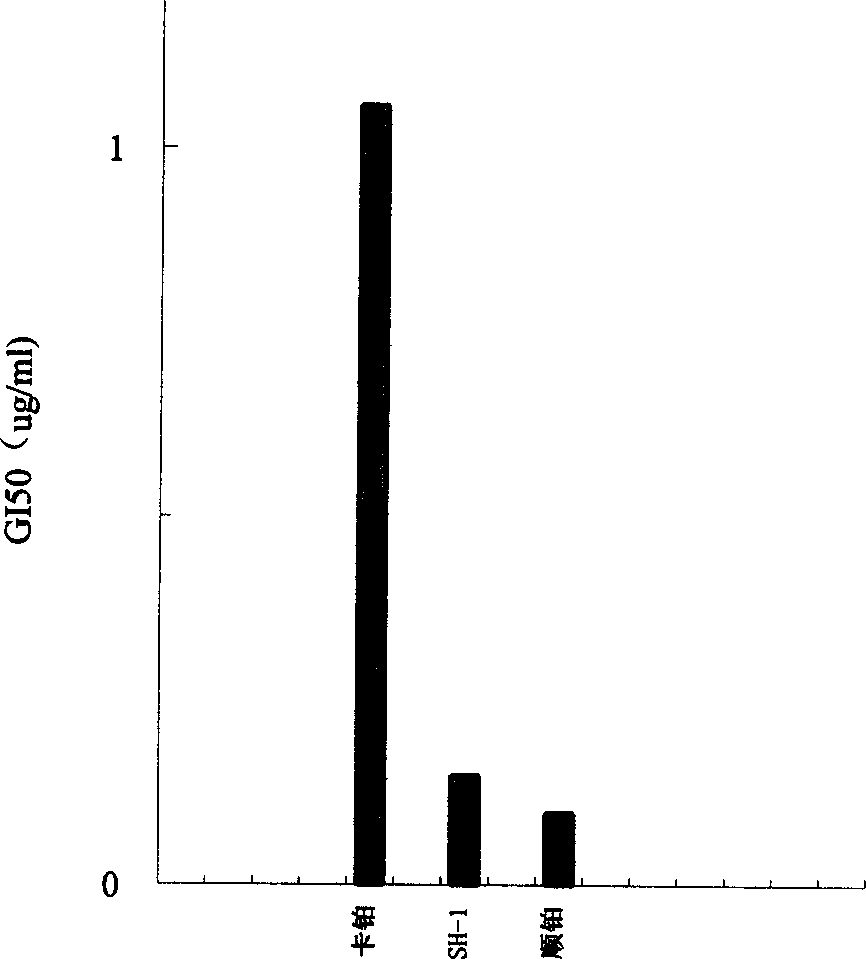
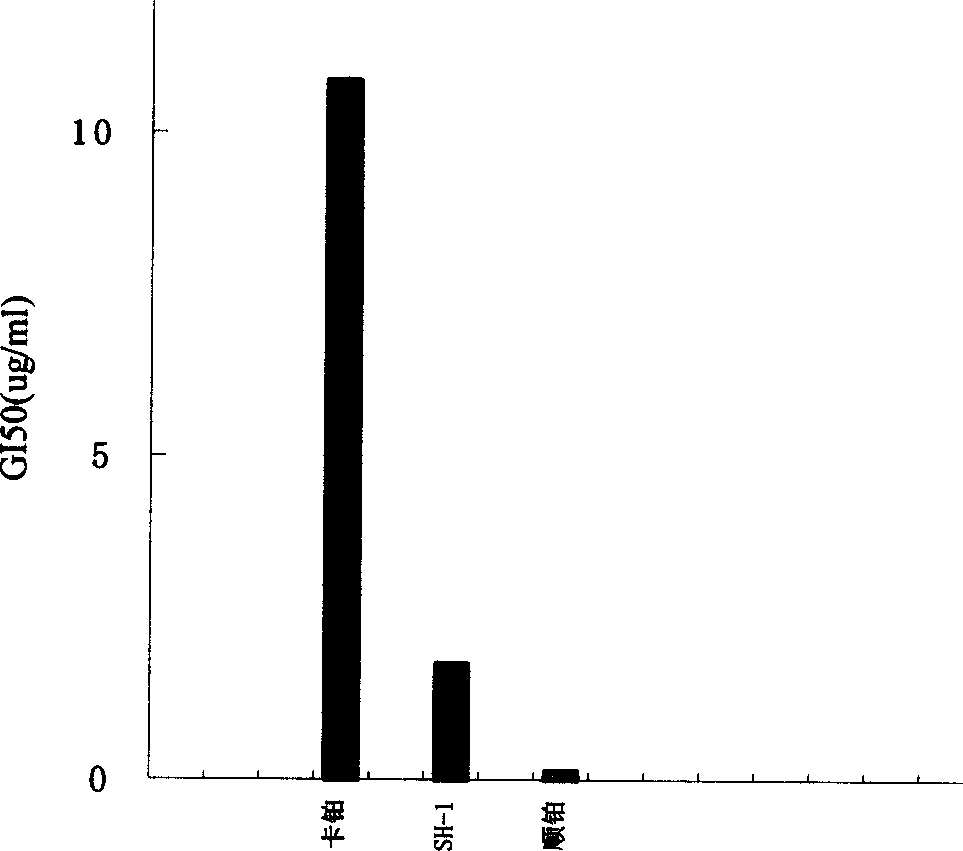
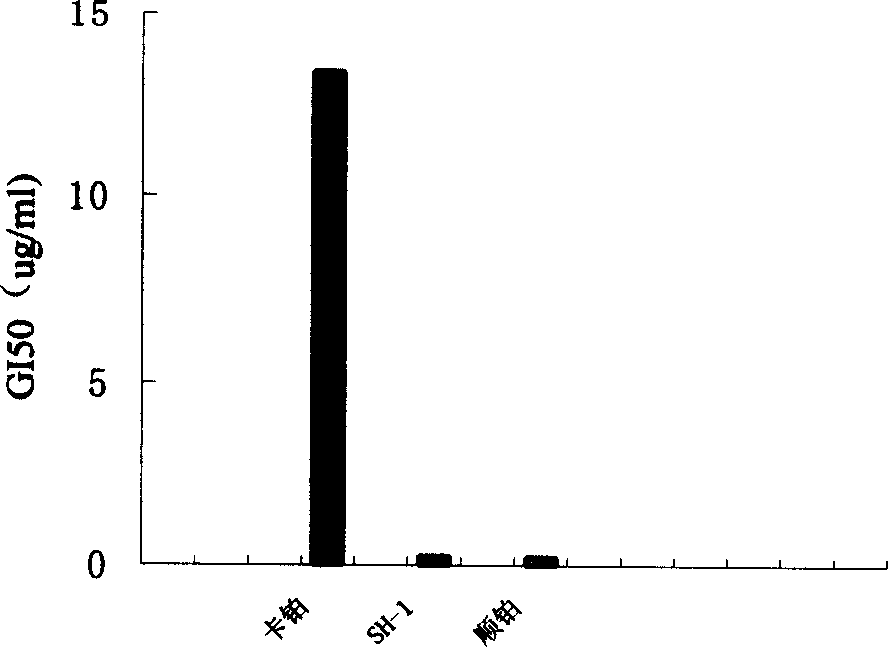
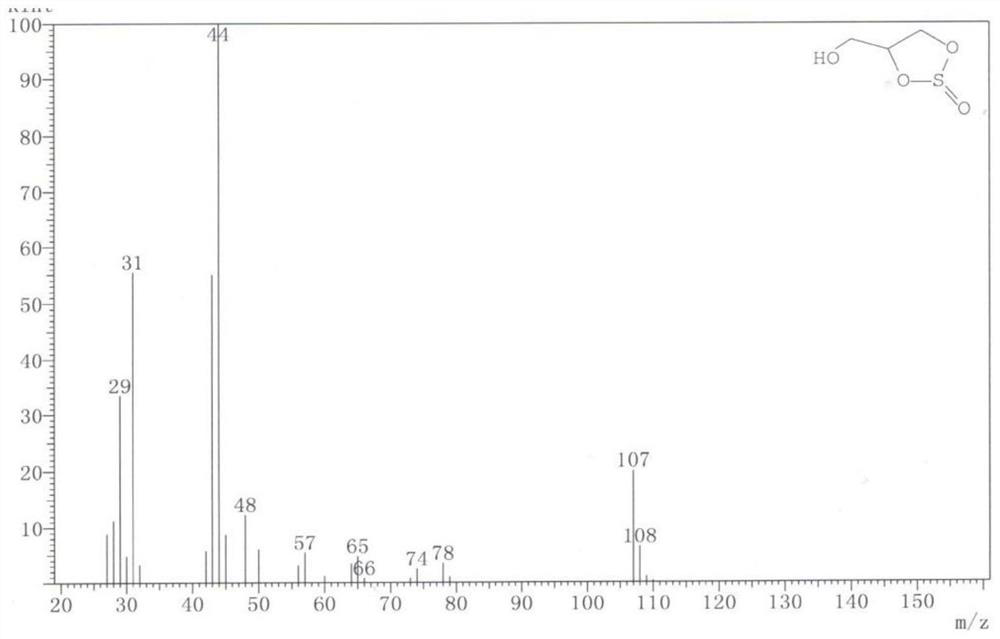
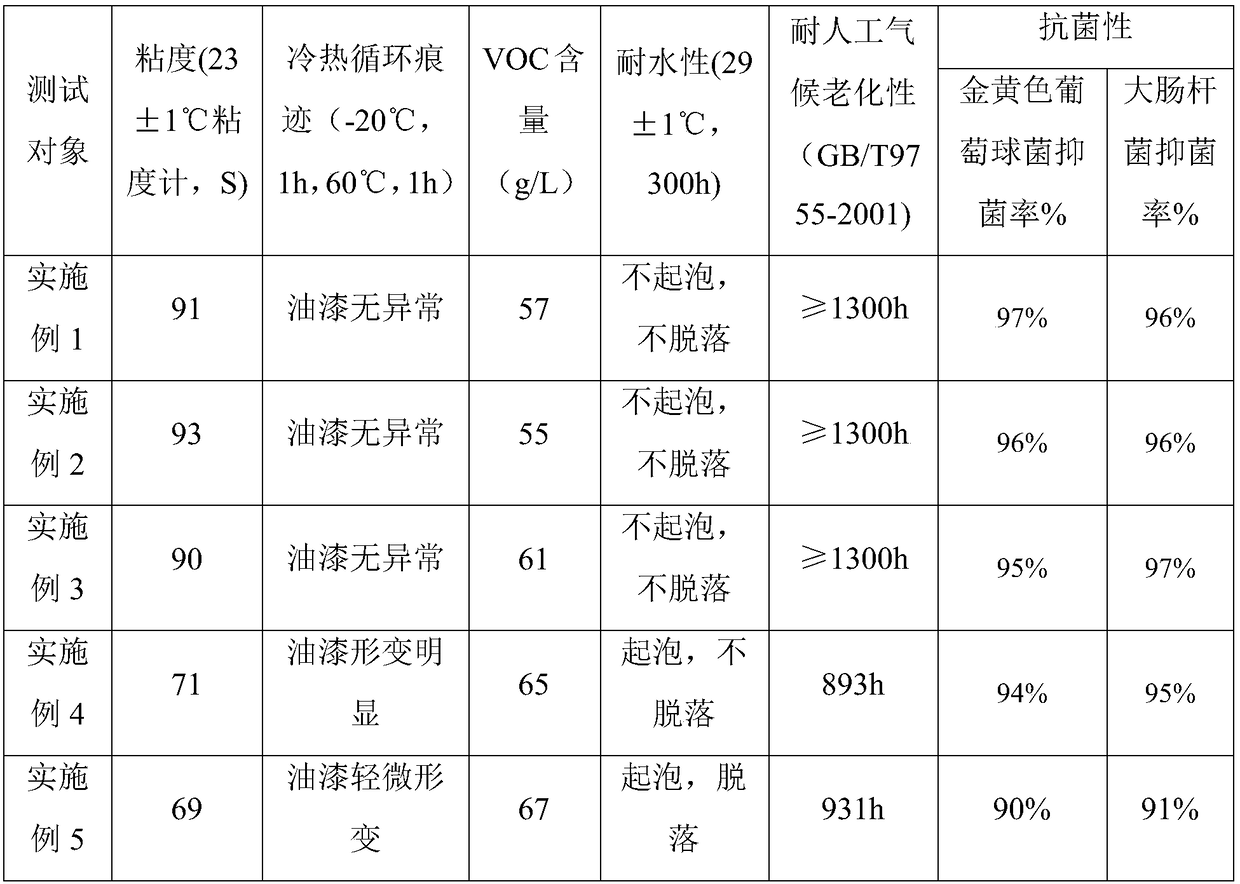
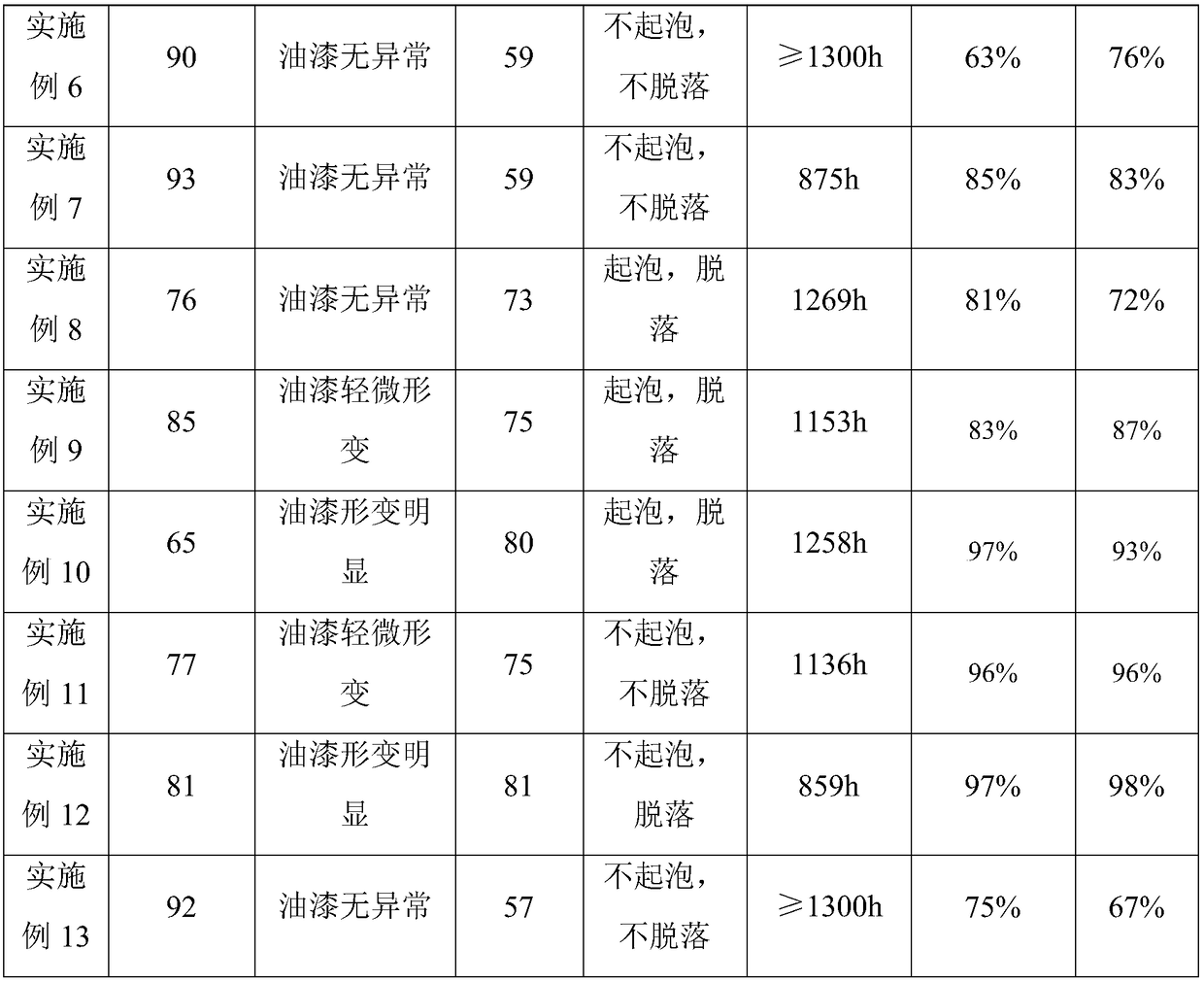
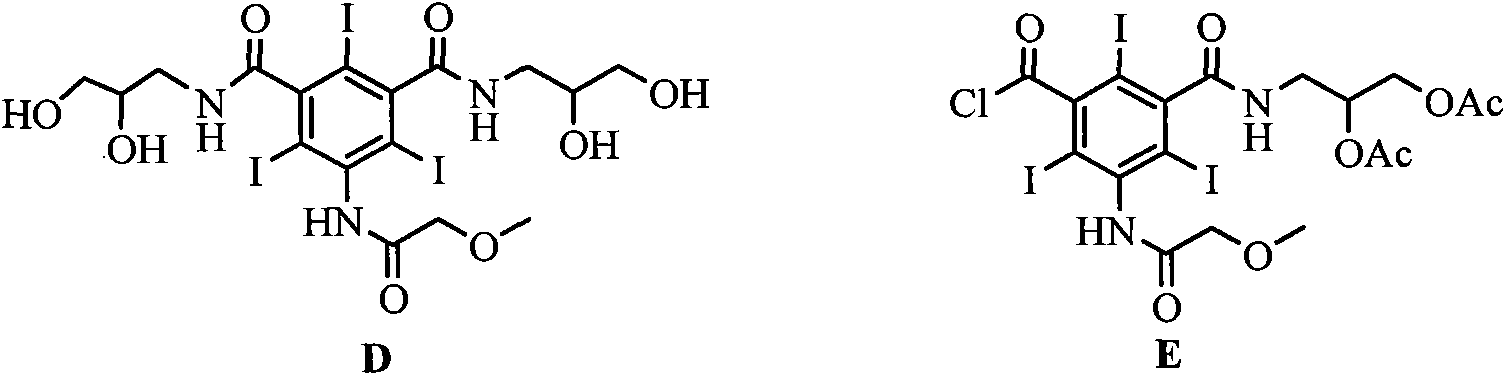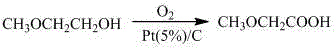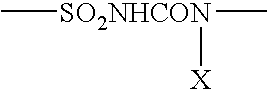Patents
Literature
70 results about "Methoxyacetic acid" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Methoxyacetic acid is a monocarboxylic acid that is acetic acid in which one of the methyl hydrogens is replaced by a methoxy group. It has a role as a human xenobiotic metabolite, an apoptosis inducer, a mutagen and an antineoplastic agent.
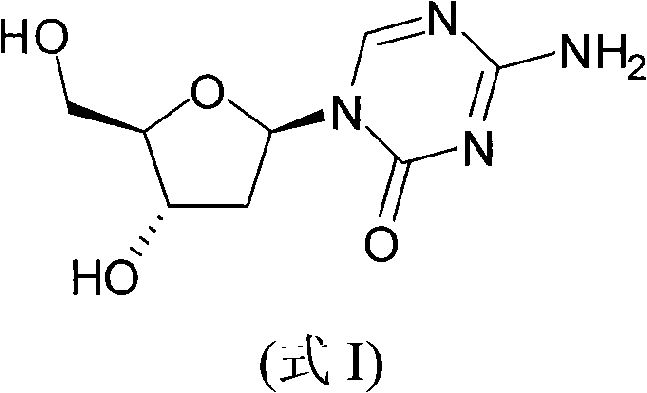
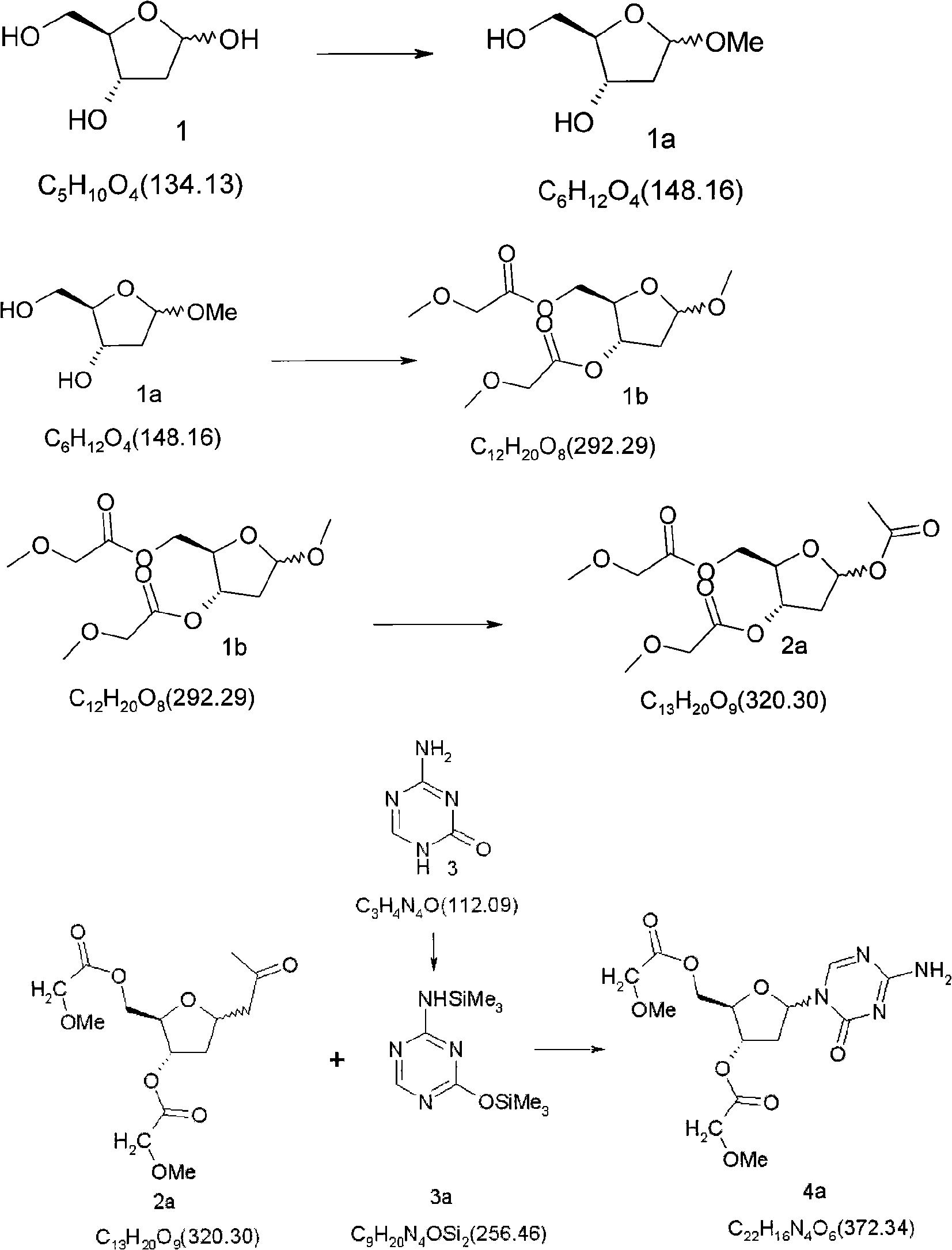
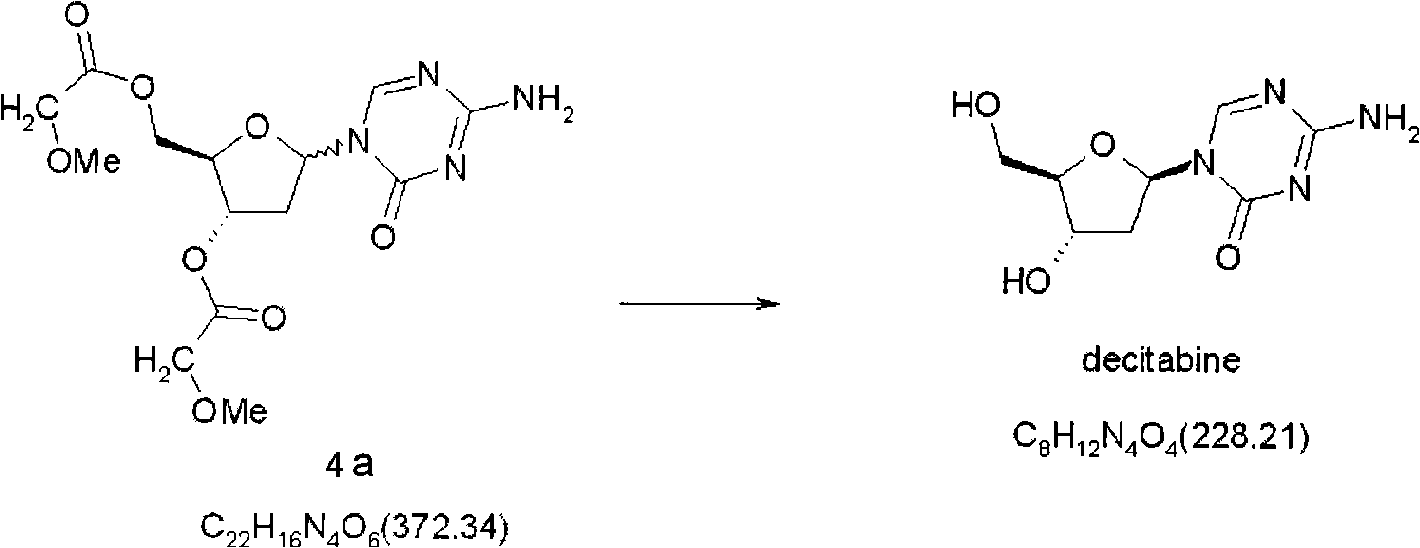
Synthetic process of decitabine
The invention relates to a method for preparing Decitabine. The particular proposal for solving the technical problem is as follows: 2-deoxidtion-D-ribose, 10 percent of HCL methanol solution, methoxyacetic acetic anhydride, HMDS, acetic anhydride, tri-silicyl tri-fluorine methane sulfonic acid ester, acetic acid amine, etc. are adopted as raw materials to synthesize the Decitabine; the target product of the Decitabine is obtained through the five steps of reactions, namely, methylation, acylation, trimethyl silication, ammoniation and deacylation with a total yield of above 18.4 percent and a product purity of above 99.7 percent.
Owner:GUIZHOU UNIV
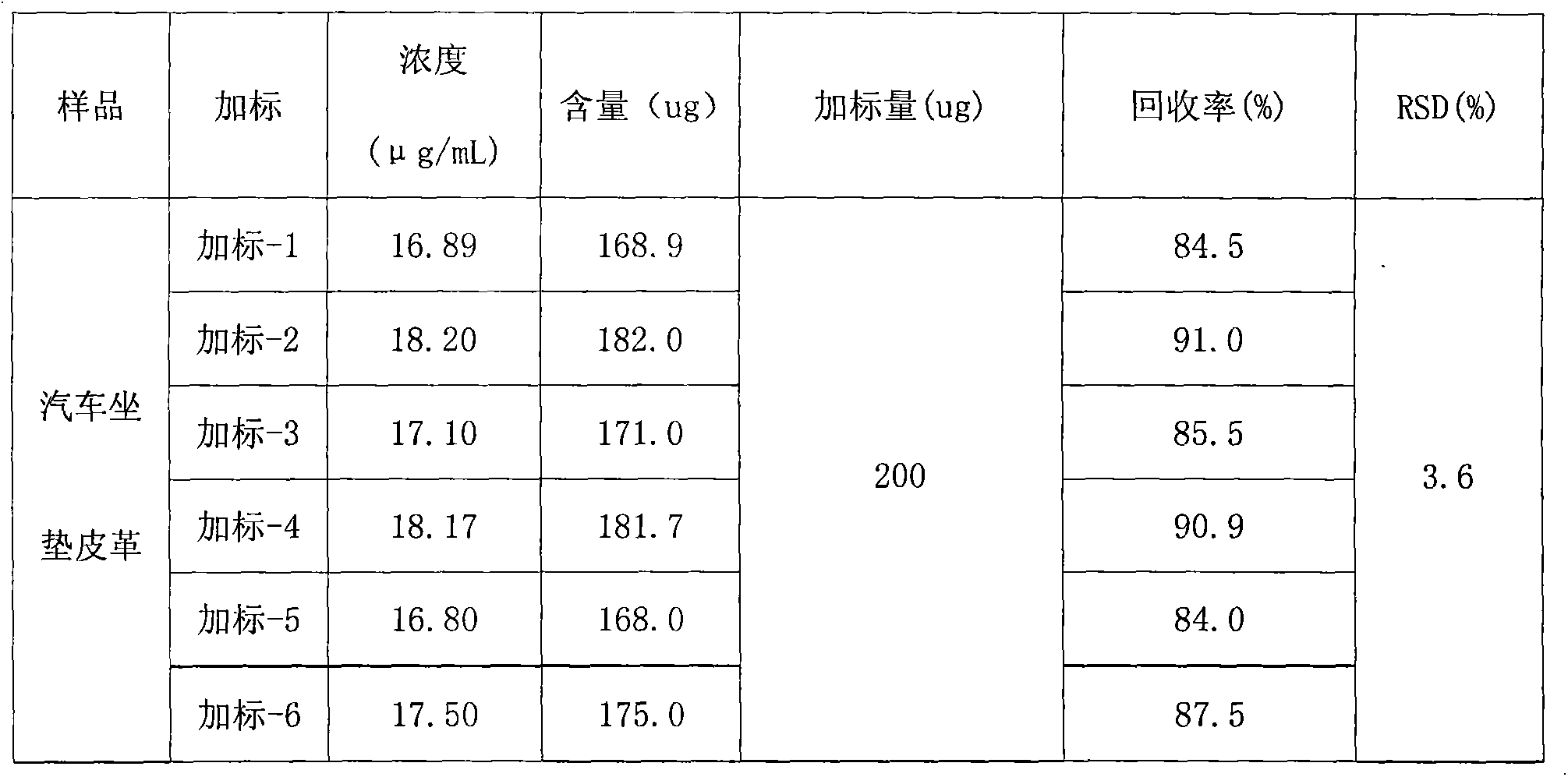
Determination method of harmful organic substance residue in toy sample
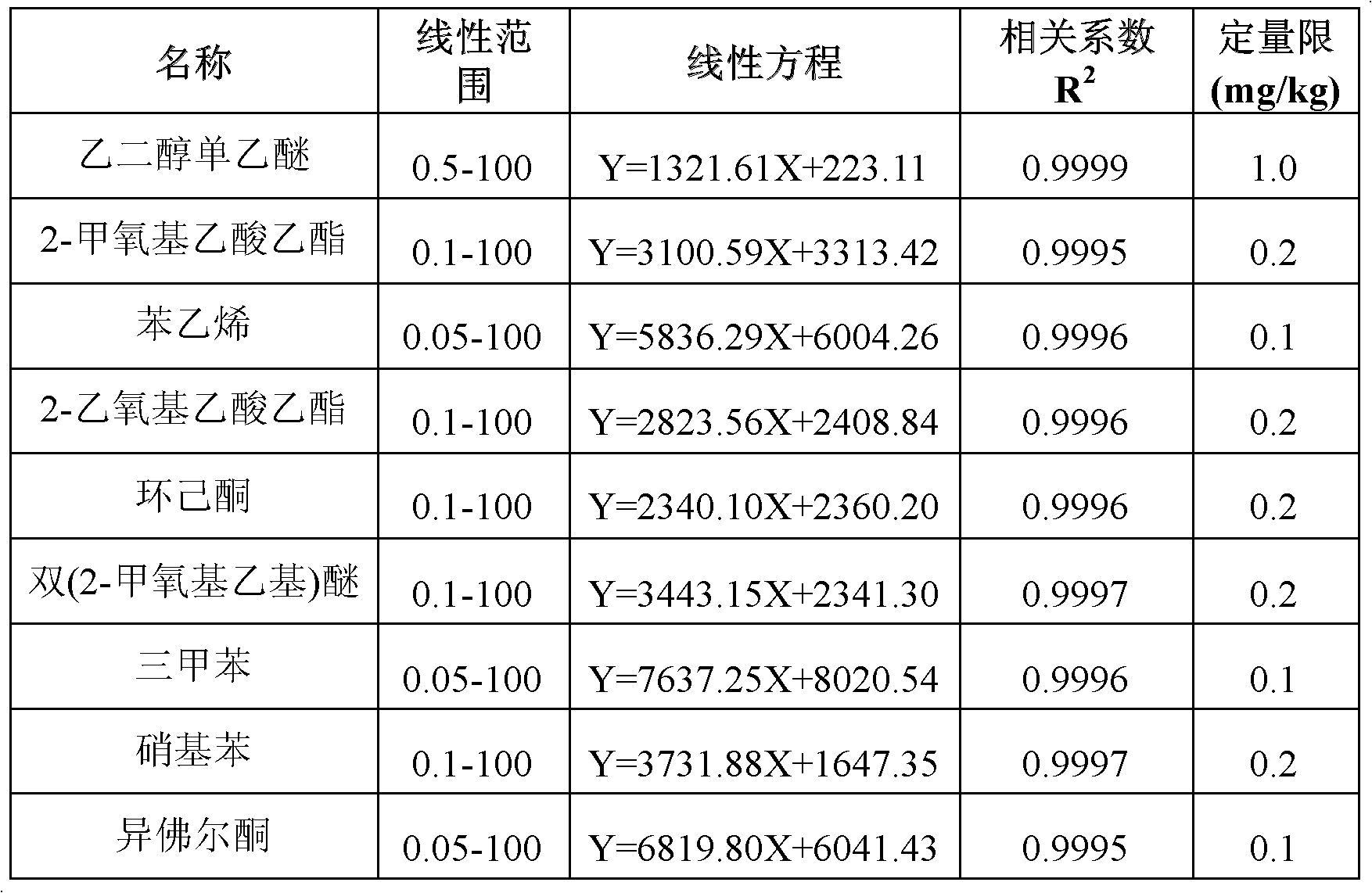
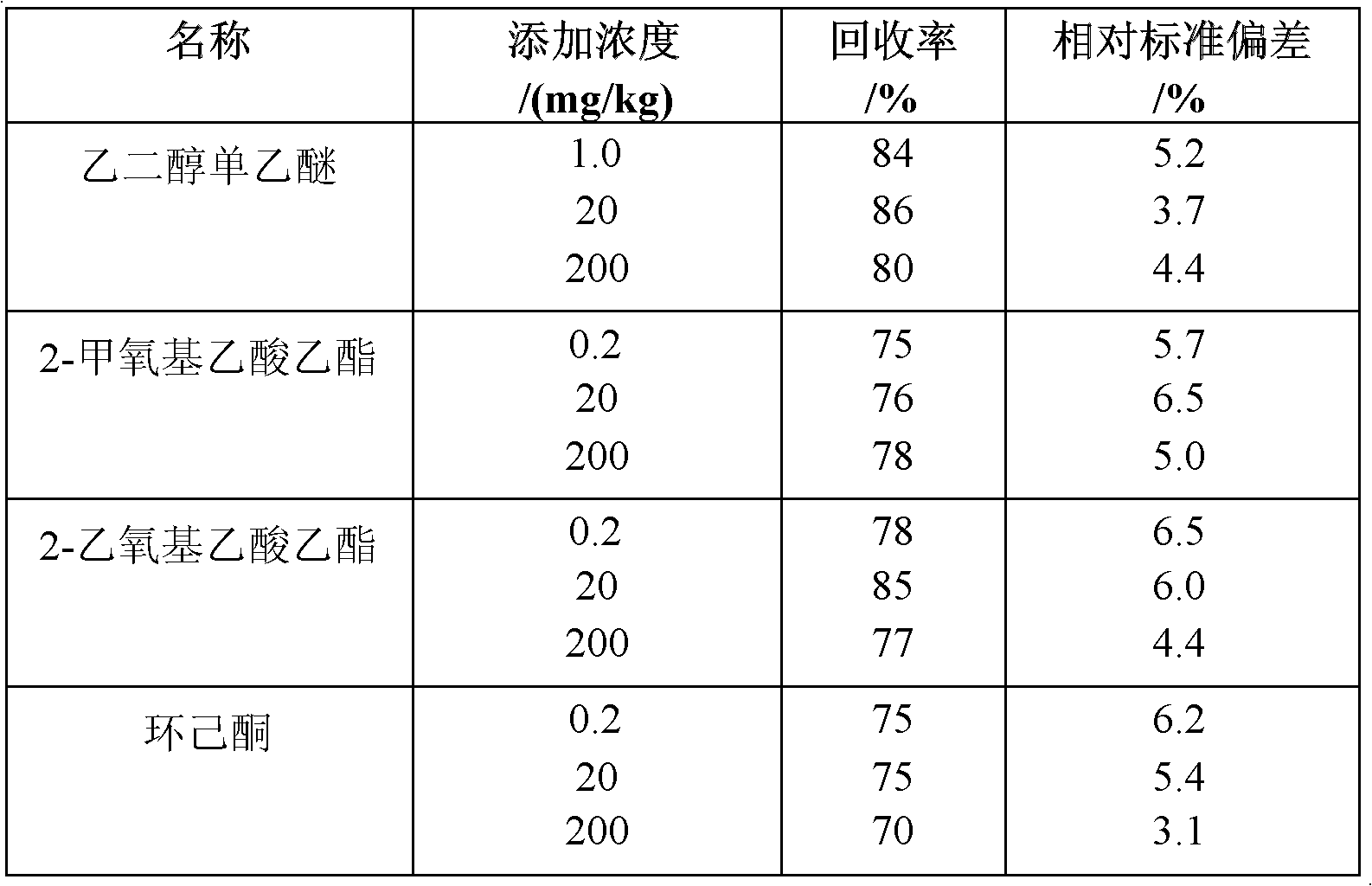
InactiveCN102608225AShorten the timeImprove efficiencyComponent separationCyclohexanoneMethoxyacetic acid
The invention adopts gas chromatography-mass spectrometry (GC-MS) to originally simultaneously determine residual contents of 9 kinds of organic substances in toy, including ethylene glycol monoethyl ether (EGEE), styrene, trimethylbenzene, isophorone, 2-methoxyethyl acetate, 2-ethoxyethyl acetate, cyclohexanone, bis(2-methoxyethyl) ether, and nitrobenzene. Common detection device is adopted in the method. The method provided by the invention has the advantages of easy popularization, simultaneous detection to save time, improved efficiency, low cost, high detection rate and sensitivity, and high application value in toy production and quality detection.
Owner:CHINESE ACAD OF INSPECTION & QUARANTINE
Conductive silk fibroin material and preparation method thereof
ActiveCN106046390AImprove conductivityPromote regenerationTissue regenerationProsthesisAcetic acidBiological materials
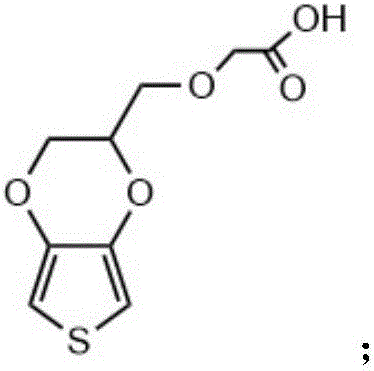
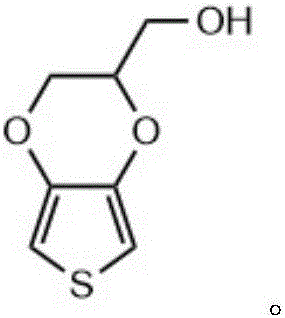
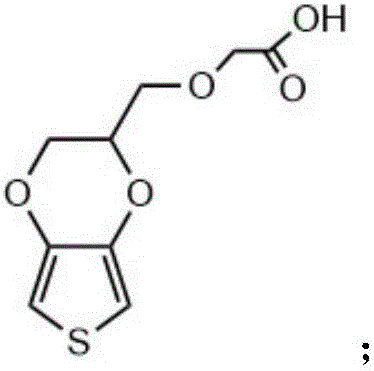
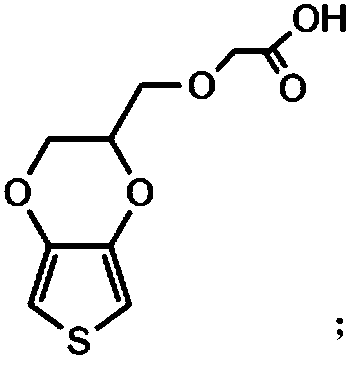
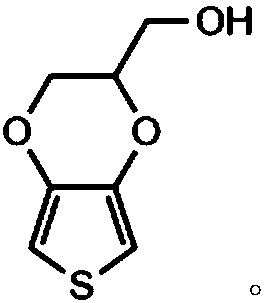
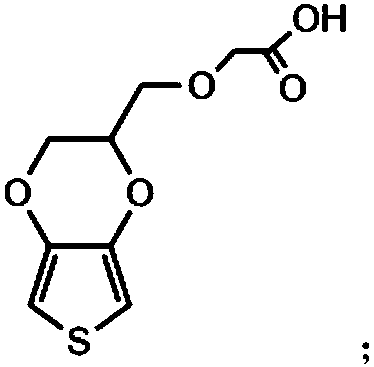
The invention relates to a conductive silk fibroin material and a preparation method thereof. The preparation method mainly comprises the following steps: (1) insoluble treatment of silk fibroin material; (2) 2-((2,3-dihydrothieno[3,4-b][1,4]dioxene-2-yl)methoxy)acetic acid activation product graft modification of silk fibroin material subjected to insoluble treatment; and (3) in-situ oxidation polymerization of thieno[3,4-b]-1,4-dioxin-2-methanol on silk fibroin material surface subjected to graft modification. The method can be used for preparing the silk fibroin conductive composite material with the polythieno[3,4-b]-1,4-dioxin-2-methanol grafted on the surface; the surface sheet resistance is 0.9*10<5>-5*10<7>ohm; the preparation technique is simple and mild; and the obtained novel biological material has high application value, and especially can be used as a nervous tissue engineering material and a neural restoration material.
Owner:DONGHUA UNIV
Method for separating rare earth from aluminum by complexation
ActiveCN108950206AImprove separation efficiencyLow costProcess efficiency improvementAcetic acidMethoxyacetic acid
The invention discloses a method for separating rare earth from aluminum by complexation. One or more of acetic acid, methoxyacetic acid, amino acetic acid, triamino acetic acid, methyl acetic acid and hydroxyacetic acid are added as complexing agents into a solution containing the rare earth and aluminum, and the adding amount of the complexing agents is controlled; aluminum impurities are settled and separated out by adjusting the pH value of the complexing solution to 5.6-6.6; filtering and washing are conducted to obtain a pure rare earth solution and aluminum slag; and a sodium hydroxideprecipitator is added to the rare earth solution, the pH value of a precipitation end point is controlled to be 9.0-12.0, and a rare earth hydroxide precipitate is obtained. Addition of the complex can preferentially conduct complexation of the rare earth in the solution containing the rare earth and aluminum, precipitation of the rare earth hydroxide and aluminum hydroxide is not influenced, andtherefore difference of the precipitation pH value between the aluminum hydroxide and rare earth hydroxide is enlarged, and efficient separation of rare earth from aluminum is realized.
Owner:GANZHOU ZHANHAI IND & TRADING
Method for preparation of methyl glycolate and by-product methyl methoxyacetate with catalyst
ActiveCN107501091AImprove conversion rateRaise the ratioOrganic compound preparationPreparation by carbon monoxide or formate reactionMethoxyacetic acidMethyl methoxyacetate
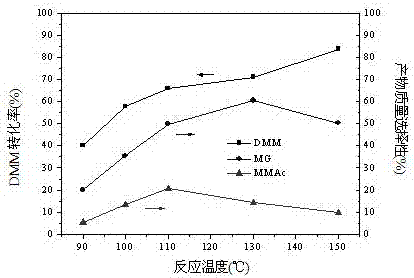
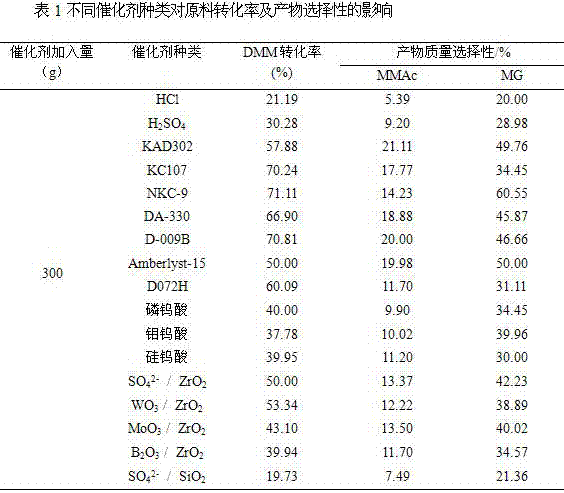
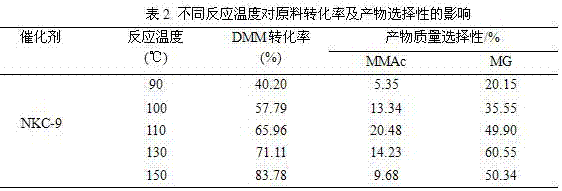
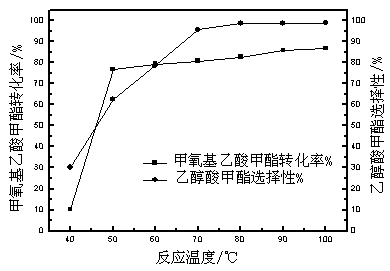
Relating to a preparation method of chemical raw materials, the invention provides a method for preparation of methyl glycolate and by-product methyl methoxyacetate with a catalyst. The method adopts methylal as the solvent, s-trioxane, tetrameric or paraformaldehyde and methylal are taken as the source of formaldehyde, H2SO4, HCl and other liquid acids or cation exchange resin, heteropoly acid, impregnation solid acid, solid super acid and other solid acids are employed as the catalyst, one-step synthesis with high conversion rate and high selectivity is carried out to obtain methyl glycolate and the by-product methyl methoxyacetate. In a fixed bed reactor, 300g of an NKC-9 sulfonic acid resin catalyst is used, the raw materials include: 20kg of methylal, 4kg of water and 2.9kg of s-trioxane, the mass space velocity of the raw materials is 30h<-1>, reaction is carried out under a reaction temperature of 130DEG C and a reaction pressure of 6.0MPa, the catalyst is stable without inactivation for 2000h, the methylal conversion rate is 89.83%, the product methyl glycolate has mass selectivity of 28.88%, the methyl methoxyacetate has mass selectivity of 67.68%. The method provided by the invention has the advantages of short synthetic route, high raw material conversion rate or high product selectivity, and the synthesis process has no pollution to the environment.
Owner:SHENYANG INSTITUTE OF CHEMICAL TECHNOLOGY
Body wash with sunscreen
InactiveUS20080317688A1Effective solar protectionEfficient use ofCosmetic preparationsHair removalMethoxyacetic acidCleansers skin
Owner:ARIZONA SUNWASH
Novel synthesis method for iopromide
ActiveCN103965074AFew stepsProduct quality is easy to controlOrganic compound preparationCarboxylic acid amides preparationMethoxyacetic acidSynthesis methods
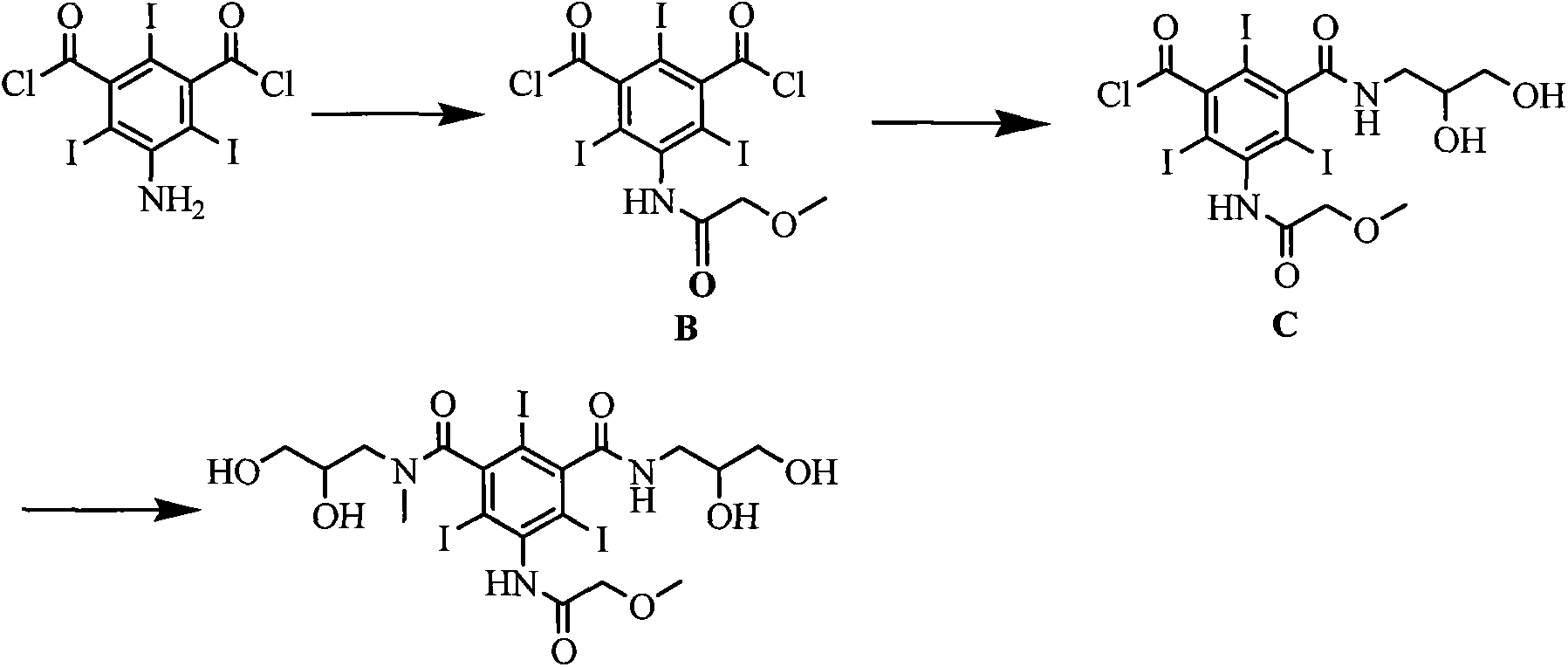
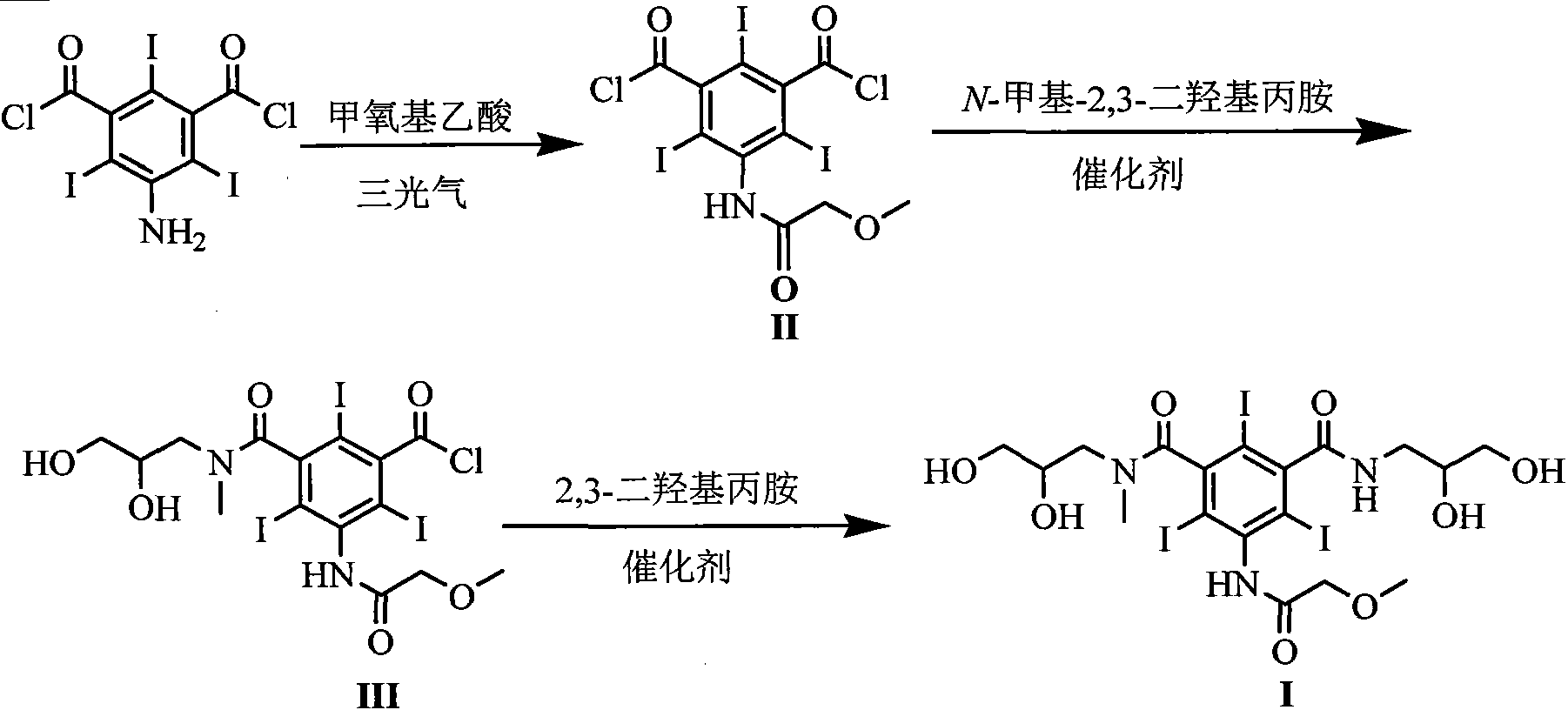
The invention discloses a novel synthesis method for iopromide. The novel synthesis method comprises the following steps of 1, enabling methoxyacetic acid to react with triphosgene to obtain a reaction product, and then, enabling the reaction production to be directly subjected to one-pot reaction with 5-amino-2, 4, 6-triiodo-isophthalicacyl chloride to prepare a compound 5-[(2-methoxyl) acetamido]-2, 4, 6-triiodo-isophthalicacyl chloride as shown in the formula (II); 2, condensing the compound as shown in the formula (II) and N-methyl-2, 3-dihydroxyl propylamine under the action of a solid catalyst ZrO2-Cr2O3 to obtain a compound 5-[(2-methoxyl) acetamido]-3-(2, 3-dihydroxyl-N-methylpropylaminoformoxyl)-2, 4, 6-triiodo-benzoyl chloride as shown in the formula (III); 3, condensing the compound as shown in the formula (III) and 2, 3-dihydroxylpropylamine under the action of a catalyst to prepare a compound iopromide as shown in the formula (I). The synthesis method for iopromide, disclosed by the invention, is few in byproduct, easy to control the product quality, high in product purity, cheap and easily-obtained in used reagent, few in step, simple in operation, relatively high in total yield and suitable for industrial production, and provides a novel approach for preparing iopromide.
Owner:HUAIHAI INST OF TECH
Solvent type ultraviolet photocureable coating capable of shielding Wi-Fi signals and production method of photocureable coating
ActiveCN103013301AFunction increasePrevent hand sweatPolyurea/polyurethane coatingsEpoxy resin coatingsPolyesterEpoxy
The invention relates to a production method of solvent type ultraviolet photocureable coating capable of shielding Wi-Fi (wireless fidelity) signals. The method is characterized in that the method comprises the steps as follows: (1) putting diphenyl ketone, 3-methyl-3-methoxyacetic acid butyl ester and polyethylene wax slurry into a reaction cylinder for mixing and stirring to form a premixed material, (2) adding 2-hydroxy-3-phenoxy propyl acrylic ester and modified fatty acid epoxy dimethyl acrylate, and further adding four-functional group polyester methyl acrylate and modified fatty group polyurethane methyl acrylate to form a mixture by stirring, (3) adding an acrylic acid flatting agent, a siloxane flatting agent, propylene oxide pentaerythritol tetrapropylene acid ester and vacuum aluminizing pigment, and (4), filtering the mixture acquired in Step (3) and obtaining the solvent type ultraviolet photocureable coating capable of shielding the Wi-Fi signals. The solvent type ultraviolet photocureable coating can shield the Wi-Fi signals effectively, and meet the requirements of the RoHS (Restriction of Hazardous Substances).
Owner:卡秀万辉(无锡)高新材料有限公司
Methoxyactic acid preparation method
InactiveCN104892390ANo pollution in the processHigh catalytic efficiencyOrganic compound preparationCarboxylic compound preparationState of artMethoxyacetic acid
The present invention relates to a methoxyactic acid preparation method, wherein Pt / C is adopted as a catalyst, oxygen is adopted as an oxidant, water is adopted as a solvent, and 2-methoxyethanol oxidation is performed to prepare the methoxyactic acid in a one-step manner. Compared with the method in the prior art, the method of the present invention has the following advantages that (1) the used solvent is the water with characteristics of low price, easy obtaining and green environmental protection, such that environment pollution can not generated; (2) the used catalyst Pt / C has the high catalysis efficiency, and can be reused only through the simple filtration after completing the reaction; (3) the reaction conditions are mild, and the operation is operate; and (4) the used oxidant is the O2 with characteristics of safety, environmental protection, low price and easy obtaining, such that waste gas and waste liquid can not be generated.
Owner:HUBEI UNIV +1
Antistatic textile fabric and preparing method thereof
ActiveCN110528141ASuitable for large-scale productionWide variety of sourcesElectroconductive/antistatic filament manufactureConjugated cellulose/protein artificial filamentsMethoxyacetic acidFiber
The invention discloses an antistatic textile fabric. The antistatic textile fabric is characterized by being prepared from, by weight, 40-60 parts of antistatic functional fiber, 20-30 parts of otherfiber and 7-15 parts of alginic acid fiber, The antistatic functional fiber is prepared by mixing a methyl 2-methoxy-2-(prop-2-enoylamino)acetate / 1-allyl-3-methylimidazolium chloride / vinyl-functionalized Zr-MOFs / 1-[3-(triethoxysilyl)propyl]-1H-pyrrole-2,5-dione / acrylonitrile / monovinyl-terminated polydimethylsiloxane copolymer with carboxymethyl cellulose sodium. The invention further discloses apreparing method of the antistatic textile fabric. The antistatic textile fabric has the advantages of remarkable antistatic effect, excellent sweat absorbing property, high conductivity, good thermal insulation effect, high wearing comfort level and high water absorption and decontamination capacity.
Owner:无锡兴之盛针织有限公司
Etching liquid composition
InactiveCN100543188CImprove wettabilitySolution to short lifeDetergent mixture composition preparationSemiconductor/solid-state device manufacturingAcetic acidPhosphoric acid
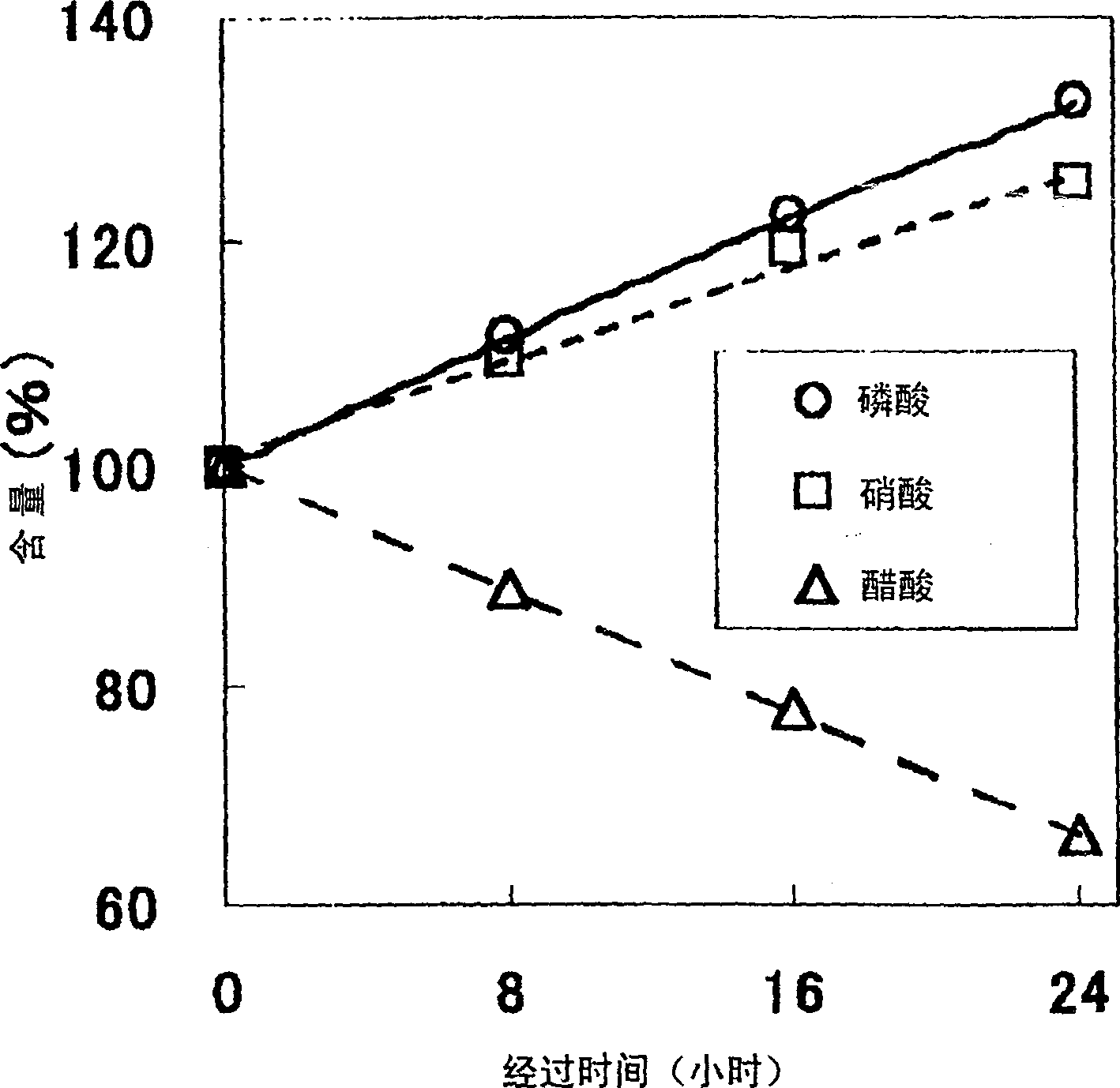
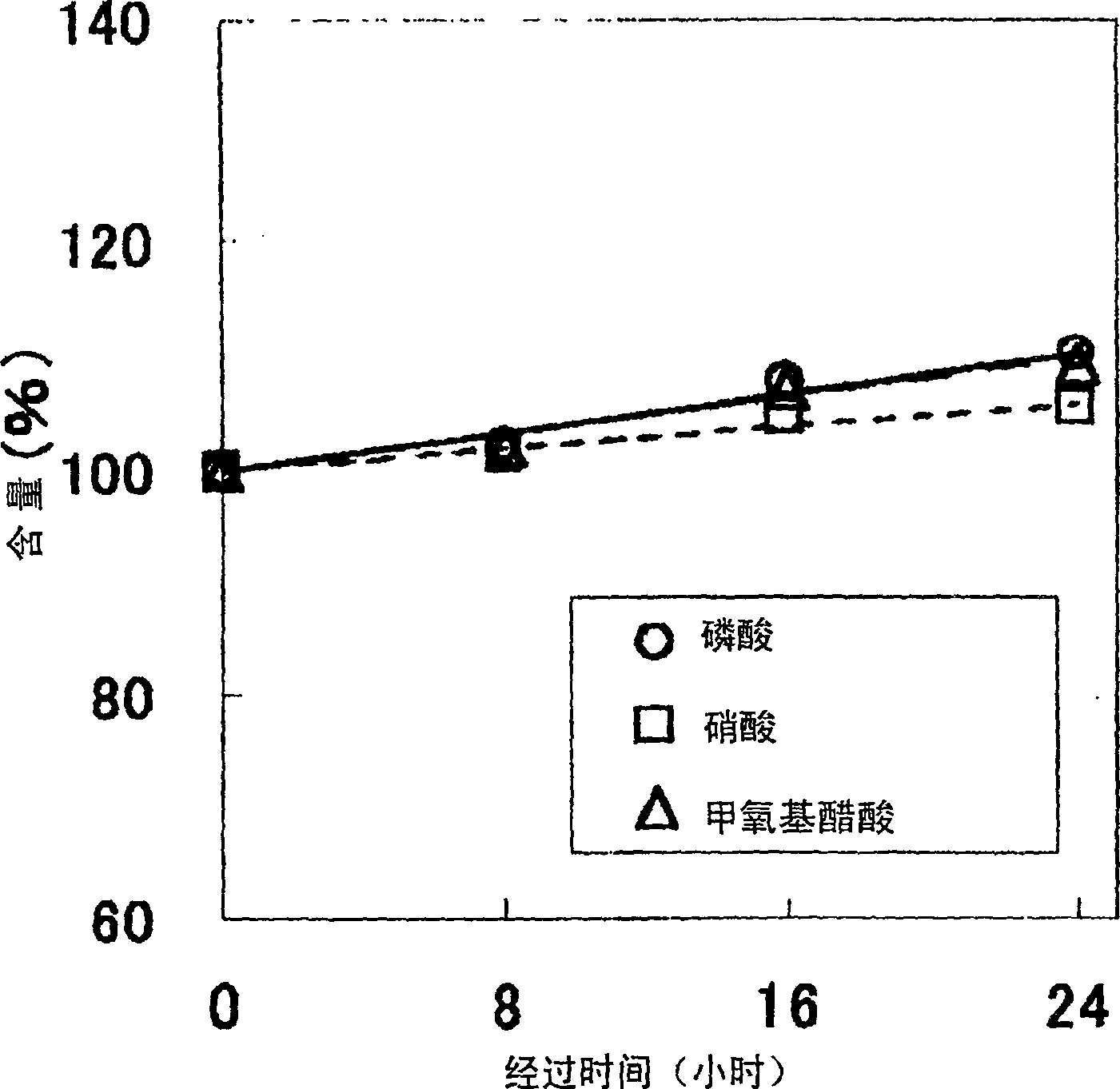
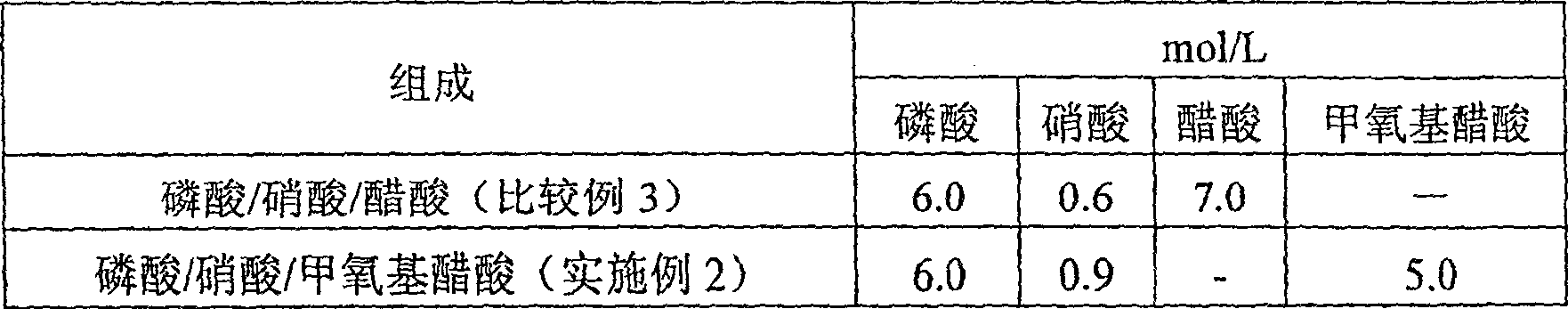
The present invention provides an etching liquid composition for various metal thin films and metal oxide thin films that does not contain acetic acid, has no pungent odor, has little performance change, and can be used in the manufacturing process of electronic devices such as semiconductor devices and flat panel display devices. The etching liquid composition contains phosphoric acid, nitric acid, methoxyacetic acid and water.
Owner:KANTO CHEM CO INC
Water-based herbicidal suspension
Not so many reports on the practical use of herbicidal sulfonylurea compounds have been made since they easily decompose in water or a process for production of their suspensions is complicated. Therefore, it is desired to prepare a water-based herbicidal suspension in which a herbicidal sulfonylurea compound will not decompose in water and excellent suspensibility of which is maintained, without complicated process. A water-based herbicidal suspension comprising (1) a herbicidal sulfonylurea compound (excluding 1-[3-[(4,6-dimethoxypyrimidin-2-ylcarbamoyl)sulfamoyl]-2-pyridyl]-2-fluoropropyl methoxyacetate and N-[(4,6-dimethoxypyrimidin-2-yl)aminocarbonyl]-2-(2-fluoro-1-hydroxypropyl)-3-pyridinesulfonamide) or its salt, (2) an inorganic salt, (3) at least one sulfonate selected from the group consisting of an aryl sulfonate, an alkylaryl sulfonate and their formaldehyde condensates and (4) water.
Owner:ISHIHARA SANGYO KAISHA LTD
Anti-tumor platinum (II) complex
InactiveCN1569862AGroup 8/9/10/18 element organic compoundsAntineoplastic agentsMethoxyacetic acidAbsolute configuration
The invention discloses a group of platinum (II) coordinated complex having effective anticancer activity, which is represented by the structural formula (1) disclosed in the specification, wherein R groups are the same, which are hydrogen atoms or C1-5 alkyl, the cyclohexane diamine is 1,2-trans-cyclohexane diamine, R3 and R4 in formula (3) can be identical or different, which are hydrogen atom or C1-5 alkyl, or can connect with a carbon atom to form a cycloalkyl.
Owner:NANJING UNIV
Gas chromatography method for detecting methoxy acetate in electronic products
InactiveCN103267809AQuick checkSimple and relatively safe detectionComponent separationMethoxyacetic acidOrganic solvent
The invention relates to a gas chromatography method for detecting methoxy acetate in electronic products. The method comprises weighing pre-treated samples, extracting with organic solvents through ultrasonic, acidifying extracted solution, adding methyl tert-butyl ether, uniformly mixing, performing a methyl ester derivative reaction, after the derivative reaction, filtering the methyl tert-butyl ether layer, analyzing the filtrate with a gas chromatograph, and quantifying with an external-standard calibration curve method, wherein methanol is used as an extraction solvent and a derivative reagent simultaneously, the derivative is easy to prepare, the reagent is easily available, a reaction selectivity is good, the derivative reagent does not disturb separation and detection of target compounds, and a recovery rate is high. The method is good in analysis effect, and accurate and rapid in determination, and can meet needs of detection work.
Owner:谱尼测试集团深圳有限公司
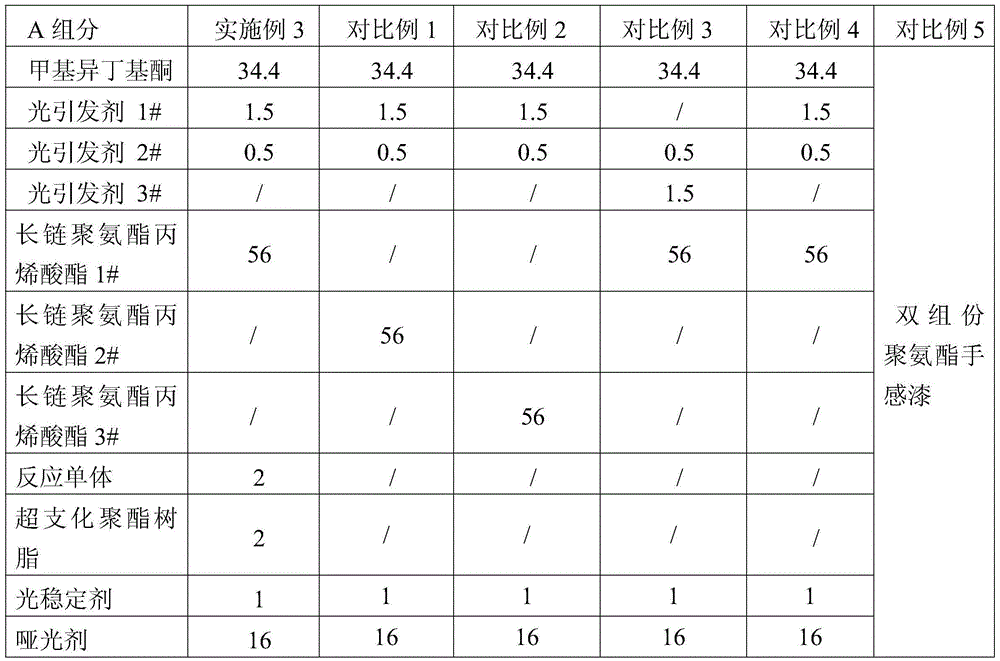
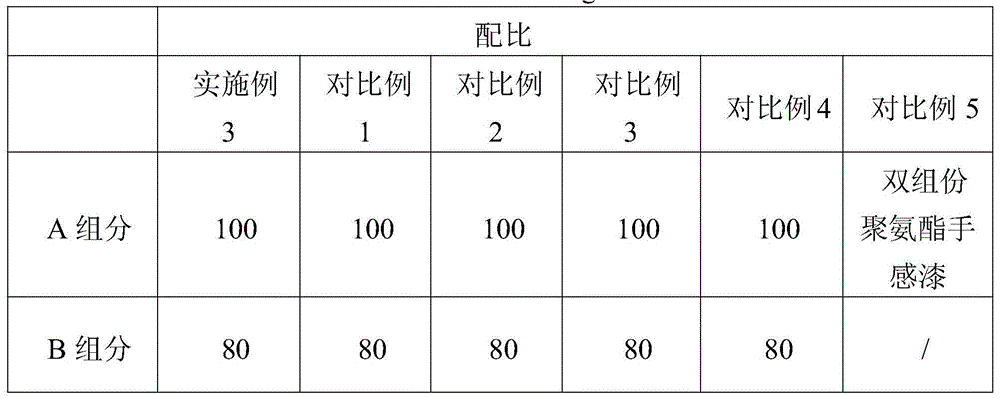
UV solidified elastic hand feeling paint and production method
The invention relates to UV solidified elastic hand feeling paint and a production method. The method is characterized by including the following steps of firstly, sequentially throwing part of methyl isobutyl ketone, photoinitiator and light stabilizer in a small reaction still under a shielding condition, and conducting stirring till the solution is clear; secondly, sequentially throwing long-chain urethane acrylate, reactive monomer and branched polyester resin in a large reaction still, and conducting stirring; thirdly, adding matte agent into the large reactions till while stirring, and conducting stirring; fourthly, adding the mixture in the small reaction still into the large reaction still, adding the rest of methyl isobutyl ketone, and conducting stirring to obtain a component A; fifthly, throwing isobutanol, butyl cellosolve, ethyl acetate, 3-methyl-3-ethyl methoxyacetate, butanone and methyl isobutyl ketone in the large reaction still, and conducting stirring to obtain a component B; sixthly, packaging the component A and the component B independently, and mixing the component A and the component B for use in the use process. The hand feeling and performance of PU elastic paint can be achieved, and meanwhile no organic tin or other harmful substances are contained.
Owner:卡秀万辉(无锡)高新材料有限公司
Method for preparing methyl glycolate and haloalkane as byproduct
ActiveCN107602388AHigh selectivityNo pollution in the processOrganic compound preparationCarboxylic acid esters preparationMethoxyacetic acidMethyl methoxyacetate
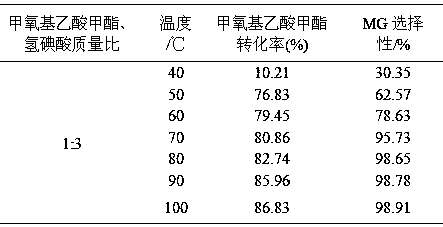
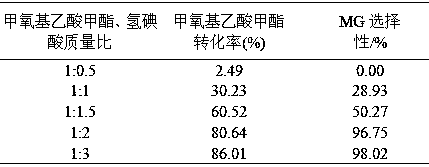
The invention relates to a method for preparing methyl glycolate and haloalkane as a byproduct, which relates to the method for preparing methyl glycolate. The method takes methoxyacetic acid methyl ester and halogen (or its hydride) as raw materials, and methyl glycolate and haloalkane as the byproduct are synthesized with high selectivity through an ether bond cracking reaction. During a synthesis route, halogen hydride is strong acid, and is taken as a reaction raw material and a reaction catalyst, compared with a traditional methyl glycolate synthesis technology, The reaction path of a brand new reaction route is short, reaction condition is mild, an extra catalyst is not required, separating problem of the catalyst is not existed, the byproduct is little, the raw material conversion rate and the product selectivity are high, no pollution is generated on environment during synthesis, and the method has the advantages of energy saving and environmental protection. When the mass ratio of methoxyacetic acid methyl ester to hydroiodic acid is 1:3, the reaction temperature is 80 DEG C, the reaction time is 5 h, the raw material methoxyacetic acid methyl ester conversion rate is 86.01%, and the product methyl glycolate selectivity can reach 98.02%.
Owner:SHENYANG INSTITUTE OF CHEMICAL TECHNOLOGY
Acrylic resin paint for reflecting light and its production process
The present invention relates to one kind acrylic resin paint for reflecting light and its production process. The paint includes liquid A, which consists of acrylic resin, mixed solvent of xylene, acetone and isophorone, organic silicone solution, superfine silica powder, titanium dioxide powder, methyl-(N-beta-amino ethyl gamma-amino propyl)-dimethoxy siloxane solution, gamma-methyl acrylyloxy propyl tromethoxy siloxane solution and fluorocarbon modified siloxane; and liquid B, which consists of hexamethylene diisocyanate, polydiisocyanate, xylene, and methoxy propyl acetate. When the paint is used, two components are mixed together. The paint has low cost and convenient construction, and its coating has excellent sunproof heat insulating performance and sunlight reflecting rate of 0.90. The paint may be used in the outer surface of oil tank and roof to form sunproof coating.
Owner:BC P INC CHINA NAT PETROLEUM CORP +1
Solvent-based two-component elastic polyester/polyurethane sandstorm resistant train coating and preparation method thereof
ActiveCN104130691AGood storage stabilityHigh mechanical strengthPolyurea/polyurethane coatingsMethoxyacetic acidMass ratio
A solvent-based two-component elastic polyester / polyurethane sandstorm resistant train coating comprises a component A main agent and a component B curing agent, and the component A comprises 6-15% of an elastic polyester resin, 48-62% of an acrylic modified polyester resin, 2-4% of a leveling agent, 1-3% of a light stabilizer, 0.2-0.4% of a catalyst, 15-20% of propylene glycol methyl ether acetate, 2-5% of butyl acetate and 3-5% of 3-methyl-3-methoxybutyl acetate; the component B comprises 75-100% of hexamethylene diisocyanate and 0-25% of propylene glycol methyl ether acetate; and a mass ratio of the component A to the component B is 5:1. The coating has the advantages of good elasticity, strong impact resistance, high gloss retention, weather resistance, corrosion resistance and good sandstorm impact resistance, and is especially suitable for high-cold sandstorm resistant trains.
Owner:卡秀万辉(无锡)高新材料有限公司
Method for preparing 5-methoxy-4,6-dihydroxy pyrimidine disodium
ActiveCN103864700AReduce generationSave operating timeOrganic chemistryMethoxyacetic acidSodium methoxide
The invention discloses a method for preparing 5-methoxy-4,6-dihydroxy pyrimidine disodium. The method comprises the following steps: 1) performing Clancy reaction, namely, feeding solid sodium ethoxide into a reaction kettle, further feeding methoxyacetic acid methyl acetate and diethyl oxalate for reaction, after the reaction, adding trichloro ethylene, stirring and diluting, adding water, 30% hydrochloric acid, adjusting the pH value, separating trichloro ethylene, extracting a water layer by using trichloro ethylene, recycling a trichloro ethylene solvent, decarbonizing, and then performing reduced pressure distillation to obtain methoxy-malonic acid methyl ethyl ester; and 2) performing cyclization reaction, namely, firstly, feeding sodium methylate into a reaction pot, adding a silicon dioxide-aluminum oxide catalyst, stirring and heating, adding formamide and methoxy-malonic acid methyl ethyl ester, after that, keeping the temperature to react, after keeping the temperature, recycling methanol till no liquid is discharged, subsequently adding water, cooling, discharging, and rotating a damp product to dry under reduced pressure so as to obtain the 5-methoxy-4,6-dihydroxy pyrimidine disodium. The method disclosed by the invention is high in yield, can greatly reduce energy consumption, and is applicable to industrial production.
Owner:CHANGSHU JINSHEN MEDICAL PROD CO LTD
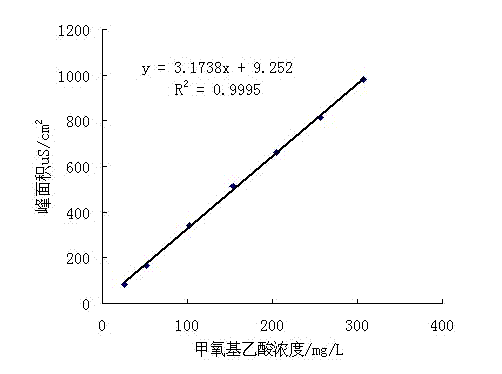
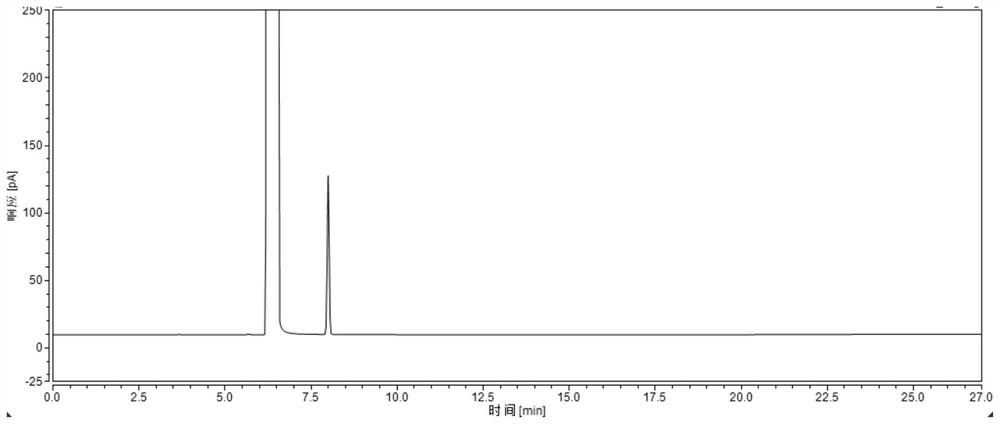
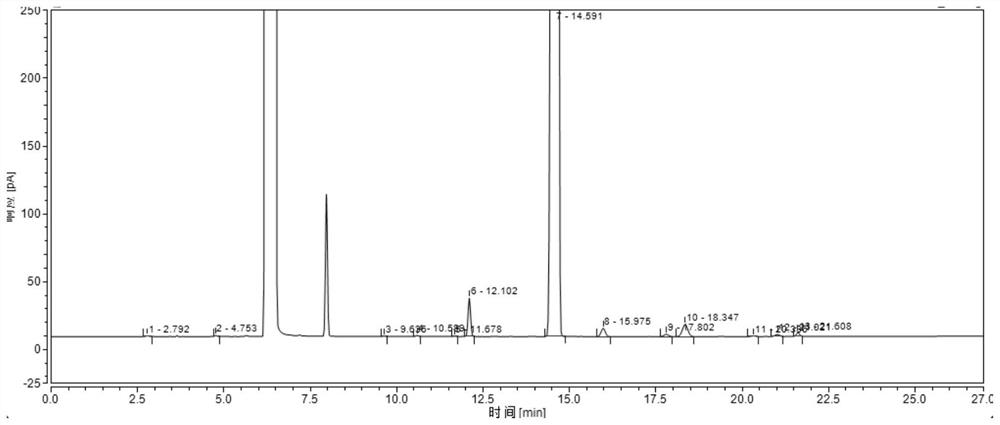
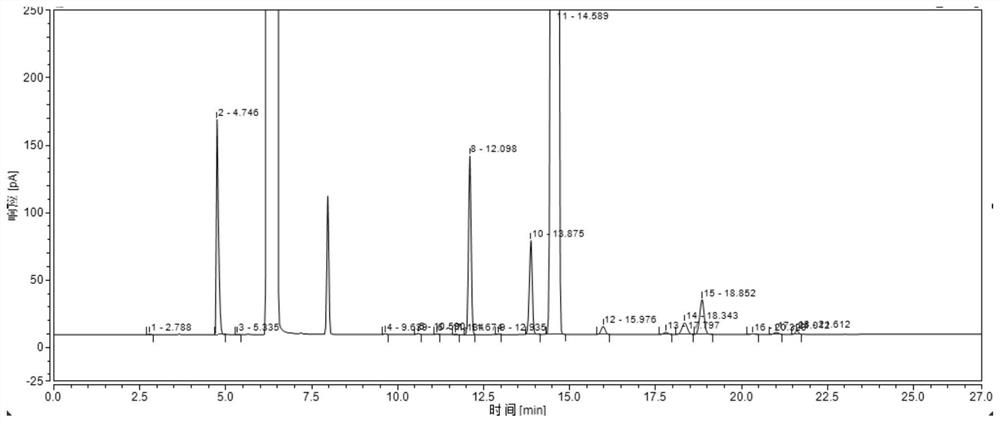
Method for analyzing and detecting methoxyacetic acid
InactiveCN102539557AHigh sensitivityGood reproducibilityComponent separationMethoxyacetic acidIon chromatography
The invention discloses a method for analyzing and detecting methoxyacetic acid. Ion chromatography analysis is performed on a methoxyacetic acid sample, and an external standard method is used for calculating content of the methoxyacetic acid in the sample. Eluent is 2.5-4.0 mmol / L sodium carbonate, flow velocity of the eluent is 0.70-0.90 mL / min, and regenerated liquid is 50-100 mmol / L sulfuricacid. Ion chromatography adopts a negative ion chromatographic column, a detector is a chemical inhibition electric conductivity detector, and an ion chromatographic column temperature box is at 40 DEG C. The method has the advantages of being strong in specificity, high in accuracy, good in repeatability, high in sensibility, simple to operate, large in linear range, rapid, low in pollution, accurate and reliable in methoxyacetic acid analysis and detection and capable of being used for guiding research and production of the methoxyacetic acid.
Owner:CHONGQING UNISPLENDOUR CHEM
Body wash with sunscreen
InactiveUS7824662B2Effective protectionEfficient use ofCosmetic preparationsHair removalMethoxyacetic acidCleansers skin
There is provided a body wash composition that includes sun screen materials. The body wash composition is formulated so that it may be applied during normal hygiene activities, such as washing. However, the composition applies an effective of sun screen material to the body such that the sun screen continues to provide effective solar protection even after rinsing or washing of the human body. Further, the material is a non greasy, easy to apply material that may be used during showering activities in a manner similar to a bar soap or cleanser. The composition includes a variety of materials that assist in the processing and storage of the body wash. Effective amounts of solar protective material include octyl methoxycinamate, octyl salycilate, and titanium dioxide. Testing shows that the product provides a solar protective level of at least approximately 14 even after multiple rinsings.
Owner:ARIZONA SUNWASH
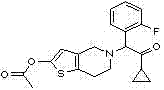
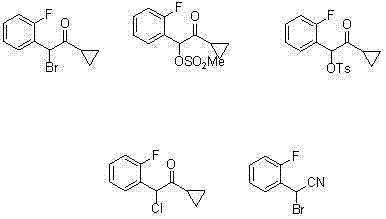
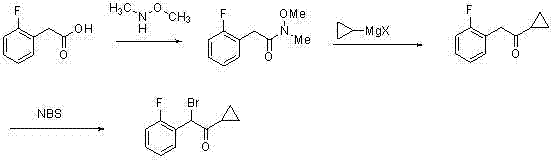
Synthetic method of compound 2-cyclopropyl-1-(2-fluorophenyl)-2-carbonyl ethyl p-methylbenzensulfonate
InactiveCN102241612AHigh yieldLow costSulfonic acid esters preparationMethoxyacetic acidChemical synthesis
The invention discloses a synthetic method of a medicinal prasugrel intermediate, i.e., compound 2-cyclopropyl-1-(2-fluorophenyl)-2-carbonyl ethyl p-methylbenzensulfonate, belonging to the technical field of chemical synthesis. The method comprises the following steps of: making o-fluorobenzaldehyde serving as an initial raw material with chloroform and sodium hydroxide to obtain o-fluoromandelicacid; esterifying, protecting with methoxyl chloromethane and hydrolyzing to obtain 2-(2-fluorophenyl)-2-( methoxyl) acetic acid; performing amidation, Grignard reaction and deprotection to obtain 1-cyclopropyl-1-(2-fluorophenyl)-2-hydroxyacetophenone serving as a key intermediate; and making the 1-cyclopropyl-1-(2-fluorophenyl)-2-hydroxyacetophenone react with paratoluensulfonyl chloride to obtain a target product. The method has the advantages of readily-available raw materials, low cost, high yield, short reaction period, high efficiency, low pollutant emission and environmental friendliness.
Owner:NORTHWEST NORMAL UNIVERSITY
Guanidyl functionalized ionic liquid as well as preparation method and application thereof
PendingCN113861081ARapid responseHigh yieldGas treatmentOrganic compound preparationMethoxyacetic acidPtru catalyst
The invention discloses guanidyl functionalized ionic liquid as well as a preparation method and application thereof. Cations of the ionic liquid are 1,1,3,3-tetramethylguanidine cations, and anions are methoxyacetic acid anions or ethoxyacetic acid anions. The preparation method comprises the following steps: adding 1,1,3,3-tetramethylguanidine into absolute ethyl alcohol, slowly dropwise adding methoxyacetic acid or ethoxyacetic acid in an ice bath environment, carrying out stirring reaction, carrying out rotary evaporation to remove absolute ethyl alcohol and water to obtain an ionic liquid primary product containing trace water, and carrying out vacuum drying to obtain the guanidyl functionalized ionic liquid. When the guanidyl-containing ionic liquid is used for trapping sulfur dioxide, the guanidyl-containing ionic liquid has the advantages of high absorption capacity, reusability and the like, can promote effective conversion of SO2 under low-temperature and solvent-free conditions, and can be used as an effective absorbent for flue gas desulfurization and a catalyst for cycloaddition reaction of SO2 and an epoxy compound.
Owner:CHINA PHARM UNIV
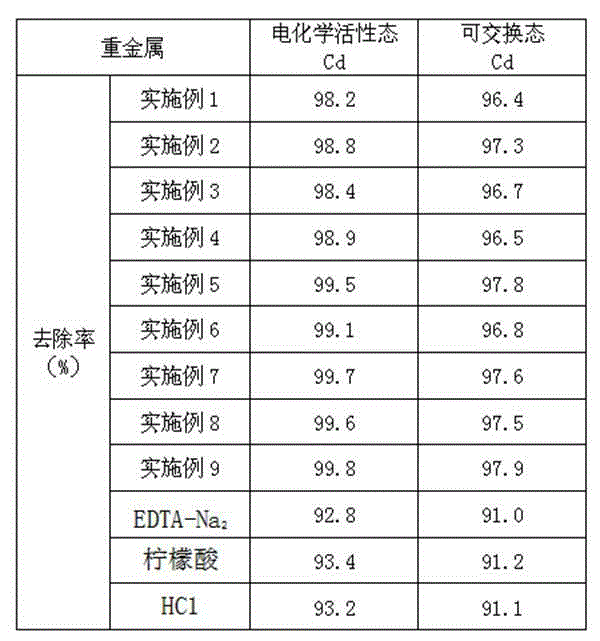
Detergent for cadmium-polluted building waste
InactiveCN104673551ASolve the problem of poor cleaning effectSurface-active non-soap compounds and soap mixture detergentsMethoxyacetic acidMethyl methoxyacetate
The invention relates to a detergent, and in particular relates to a detergent for a cadmium-polluted building waste. The detergent is prepared from the following raw materials: dimethoxyacetic acid methyl ester, 1-(5-bicyclo[2.2.1]heptyl-2-alkenyl)-1-phenyl-3-(1-piperidyl)propyl-1-alcohol, ethyl 3-methyl-3-phenyl glycidate, meso-2,3-Dibromosuccinic acid, bis(2,4-dicumylphenyl)pentaerythritol diphosphite, and 2-diethyaminoethyl3-amino-4-propoxy-benzoate hydrochloride. The detergent for the cadmium-polluted building waste can directly wash the building waste, and has the removing effect of more than 96% to cadmium elements in the cadmium-polluted building waste; and the cleaning efficiency is high, the removing effect is good, and the detergent is suitable for being widely popularized and applied in the cadmium-polluted building waste environmental protection field.
Owner:烟台顺隆化工科技有限公司
A kind of conductive silk fibroin material and preparation method thereof
ActiveCN106046390BImprove conductivityPromote regenerationTissue regenerationProsthesisMethoxyacetic acidPolymer science
The invention relates to a conductive silk fibroin material and a preparation method thereof. The preparation method mainly comprises the following steps: (1) insoluble treatment of silk fibroin material; (2) 2-((2,3-dihydrothieno[3,4-b][1,4]dioxene-2-yl)methoxy)acetic acid activation product graft modification of silk fibroin material subjected to insoluble treatment; and (3) in-situ oxidation polymerization of thieno[3,4-b]-1,4-dioxin-2-methanol on silk fibroin material surface subjected to graft modification. The method can be used for preparing the silk fibroin conductive composite material with the polythieno[3,4-b]-1,4-dioxin-2-methanol grafted on the surface; the surface sheet resistance is 0.9*10<5>-5*10<7>ohm; the preparation technique is simple and mild; and the obtained novel biological material has high application value, and especially can be used as a nervous tissue engineering material and a neural restoration material.
Owner:DONGHUA UNIV
Preparation method for 2-methoxy-N-(2-nitro-5-phenylthio) phenylacetamide
The invention discloses a preparation method for 2-methoxy-N-(2-nitro-5-phenylthio) phenylacetamide. The preparation method comprises the following steps: in a non-polar organic solvent, under catalysis of an acid binding agent, 2-nitro-5-phenylthio-aniline and methoxyacetic acid are added, and have a chlorination reaction with thionyl chloride under reflux; after the reaction ends, excess thionylchloride is decomposed by water, standing is performed for layering; the solvent is concentrated, a solvent with poor dissolvability is added for cooling crystallization, and suction filtration and drying are performed to obtain the 2-methoxy-N-(2-nitro-5-phenylthio) phenylacetamide. The preparation method adopts a one-pot method, and uses the thionyl chloride to replace phosphorus trichloride, so that the product purity reaches 99.8% or more, and the yield reaches 93.5% or more. The method has the advantages of being simple to operate, environmental-friendly, easy to industrially produce andthe like.
Owner:CHANGZHOU YABANG QH PHARMACHEM +2
High temperature coated acrylic acid resin paint and its manufacturing method
ActiveCN1912030ALow costWide range of construction applicationsAnti-corrosive paintsMethoxyacetic acidAcrylic resin
The invention relates to a high temperature-coated crylic resin coating and the manufacturing method thereof, using acrylic resin, anhydrous dimethylbenzene, butyl acetate and isophorone mixed solvent, n-butyl alcohol etherified amino resin, zinc iso-octoate solution, superfine talcum powder, titanium dioxide, calcined kaoline, organic modified bentone solution, gamma-ethyl propylene acyloxy propyl trimethyl silane solution, vinyl resin copolymer solution to compose solution A, and using hexamethylene diisocyanate polyisocyanate, dimethylbenzene and methoxy propyl acetate mixed solution to compose solution B, and as using, mixing the two solutions. And it has low cost, convenient to construct, able to normally construct when the surface temperature of workpiece is up to 70 deg.C and forms a coating with excellent appearance, and can be used to make external anticorrosive coatings for petrochemical storage tanks and roofs in high temperature environment.
Owner:BC P INC CHINA NAT PETROLEUM CORP +1
Detection method of methyl bromoacetate
ActiveCN114264752AQuality improvementEasy to separateComponent separationBulk chemical productionMethoxyacetic acidAcetic acid
The invention belongs to the technical field of medicine detection, and particularly relates to a detection method of methyl bromoacetate. The content of methanol, methoxyacetic acid, bromoacetic acid and methyl methoxyacetate in methyl bromoacetate is measured by adopting a gas chromatographic method. According to the method, methanol, methyl methoxyacetate, methoxyacetic acid, methyl bromoacetate and bromoacetic acid can be effectively separated, and the contents of methanol, methoxyacetic acid, bromoacetic acid and methyl methoxyacetate in methyl bromoacetate can be rapidly, efficiently and accurately determined.
Owner:BEIJING JINCHENG TAIER PHARMA CO LTD
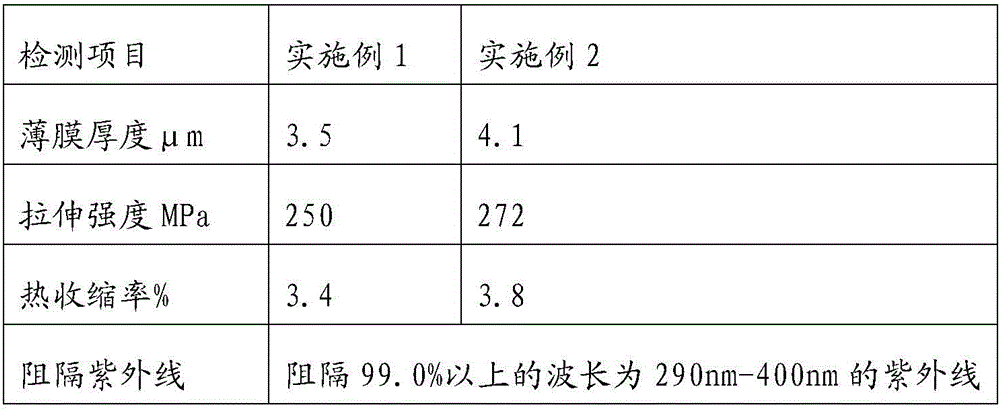
Anti-ultraviolet, heat-insulating and radiation-proof PET membrane and preparation method thereof
The invention relates to an anti-ultraviolet, heat-insulating and radiation-proof PET membrane and a preparation method thereof. The PET membrane is prepared from the following raw materials in parts by weight: 50-100 parts of PET polyester chips, 2-10 parts of methoxypropyl acetate, 2-10 parts of ethyl acrylate, 1-20 parts of bis(2,2,6,6-tetramethyl-4-piperidyl)sebacate, 0.1-20 parts of bisbenzoxazole, 5-20 parts of nano-titania, 1-10 parts of RC-E60 silicon-free release agent, 0.1-4 parts of HANKINS NCP-2 and 3-20 parts of 2-(2-hydroxyl-3-tert butyl-5-methoxyphenyl)-5-chlorobenzotriazole. The PET membrane prepared by the preparation method can be used for blocking 99.0% or more of ultraviolet rays with the wavelength of 290nm to 400nm; the PET membrane is transparent, also has the functions of resisting ultraviolet rays, insulating heat and proofing radiation and has an excellent effect.
Owner:周开雄
Composite environmentally-friendly antibacterial paint and preparation method thereof
InactiveCN109021791AStrong adhesionGood weather resistanceAntifouling/underwater paintsPaints with biocidesPolyesterMethoxyacetic acid
The invention provides composite environmentally-friendly antibacterial paint and a preparation method thereof. The composite environmentally-friendly antibacterial paint comprises polyester modifiedorganosilicone resin, polypropylene grafted glycidyl acrylate, polyester modified acrylic resin, zirconium-containing composite silicate powder, cellulose acetate, nanometer zinc oxide powder, bamboofiber ultrafine powder, dibutyltin dilaurate, boiled tung oil, limonene, phthalic anhydride, a titanium dioxide suspension, methoxypropyl acetate, polyethylene wax, palm wax, nickel slag powder, pearlpowder, a leveling agent, a toughening agent, a defoaming agent, a coupling agent and a solvent. The composite environmentally-friendly antibacterial paint has the advantages of wide raw material source, low cost, low VOC content in paint construction and use, environmental friendliness, strong adhesion, weather resistance, pollution resistance, antibacterial property, low surface friction coefficient, scratch resistance, scrub resistance, long service life, and no problems such as foaming, cracking, whitening or even delamination after long-term use.
Owner:陈慧颖
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com