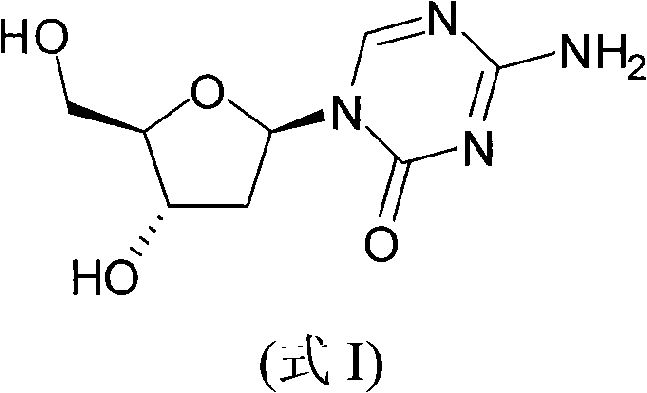
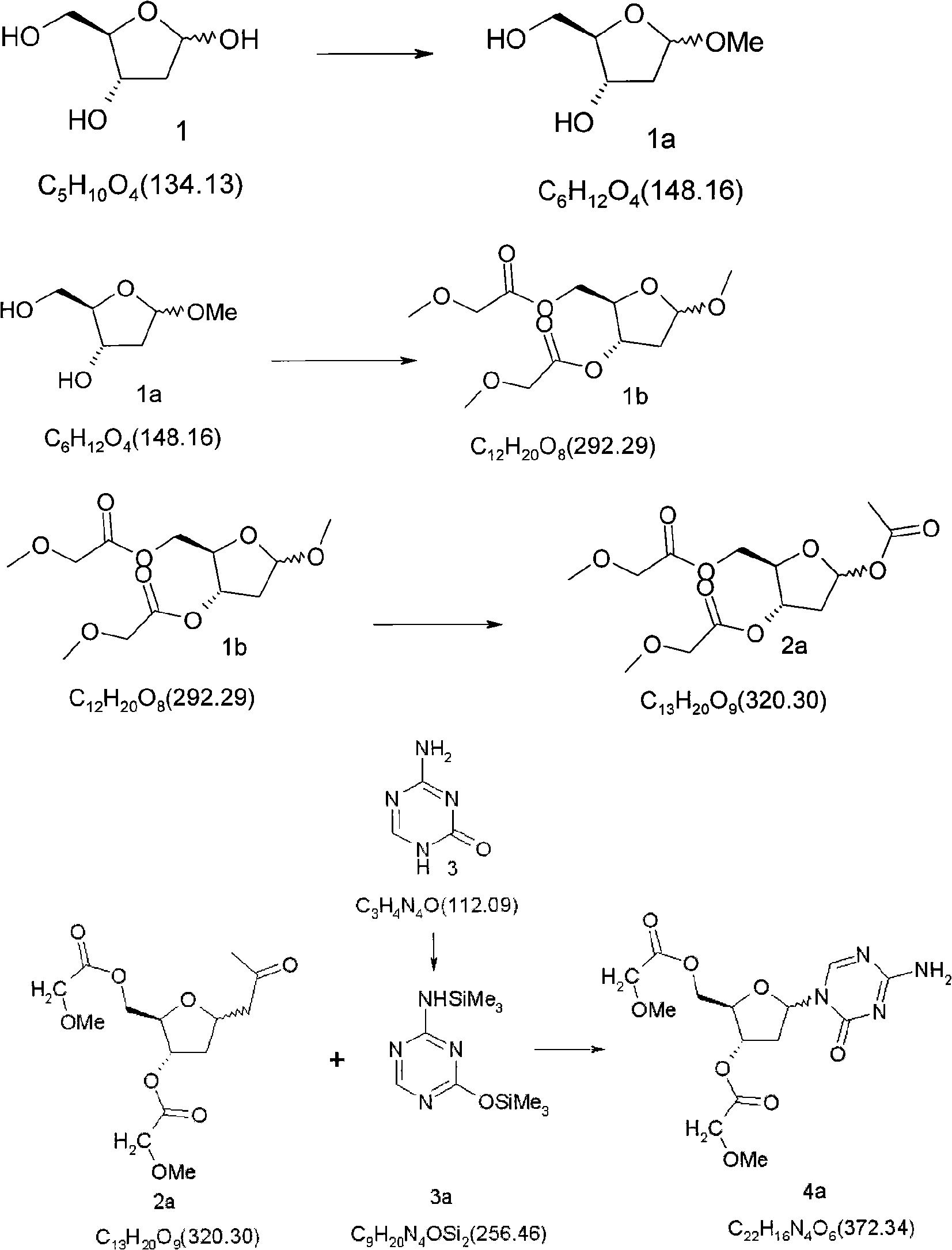
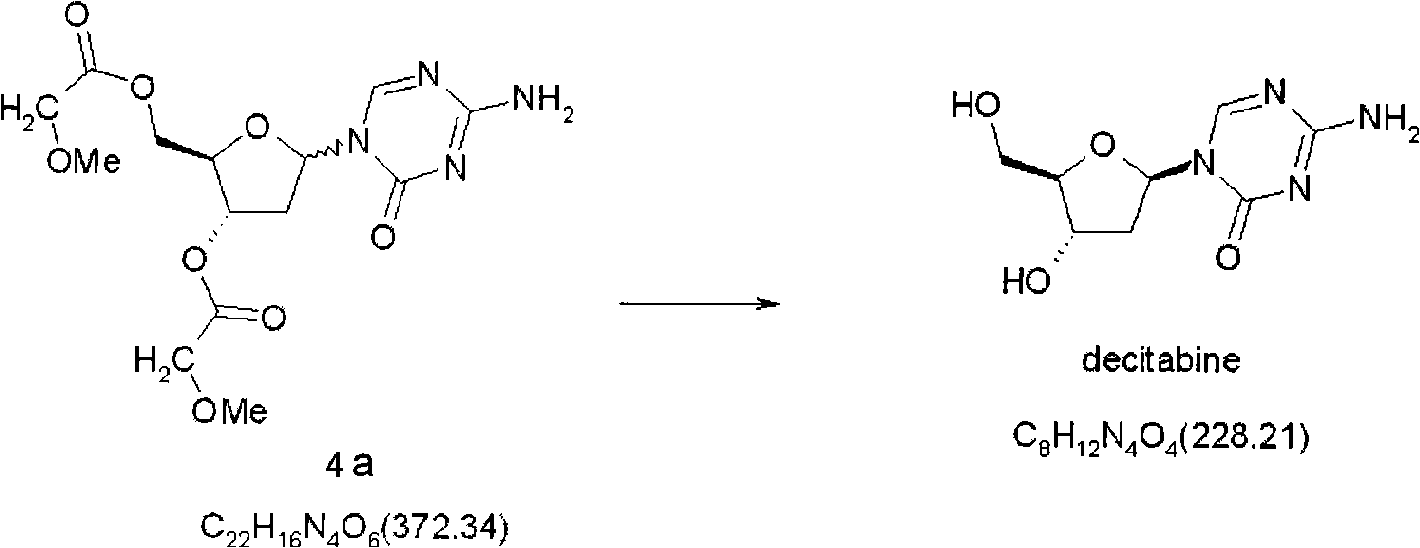
Synthetic process of decitabine
A technology of decitabine and its synthetic method, which is applied in the field of chemical drug synthesis, and can solve the problems that ammonia gas is not easy to control, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0058] 1.3 5-Acetyl-2-methoxyacetylmethyl-3-methoxyacetyl-2-deoxy-D-ribose (2a)
[0059] Dissolve 292.29 g of 5-methoxy-2-methoxyacetylmethyl-3-methoxyacetyl-2-deoxy-D-ribose in 850 mL of glacial acetic acid and 425.4 mL of acetic anhydride. Under nitrogen protection, a mixed solution of 36.08 g of concentrated sulfuric acid and 50 mL of glacial acetic acid was slowly added dropwise at 5°C to 12°C, and the temperature was controlled at 12°C to 15°C. The dropwise addition was completed in about 45 minutes, and the reaction was continued for 15 minutes to 30 minutes (such as 15 minutes for blackening, 30 minutes for non-blackening). The resulting mixture was poured into 2 L of ice water with stirring, and extracted with dichloromethane (2000 mL×2). The organic phase was saturated with Na 2 CO 3 The pH of the aqueous solution was adjusted to 7-8, several layers were separated, and the aqueous layer was extracted with 1000 mL of dichloromethane. The organic layers were c...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com