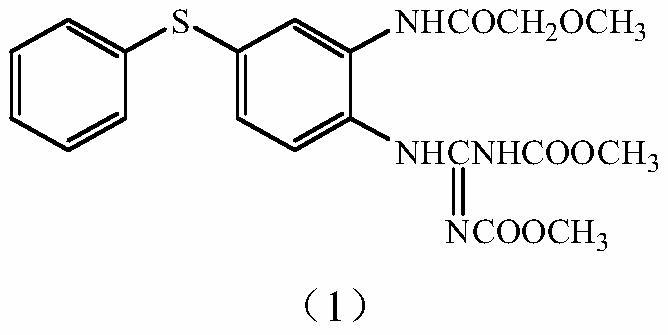
Preparation method for 2-methoxy-N-(2-nitro-5-phenylthio) phenylacetamide
A technology of phenylacetamide and phenylthioaniline, which is applied in the field of preparation of 2-methoxy-N-phenylacetamide, can solve the problems affecting the conversion rate of reduced product quality, reduction catalyst poisoning, wastewater treatment difficulties, etc. , to achieve the effects of green and environmentally friendly industrialized production, lower wastewater treatment costs, and easy industrialized production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
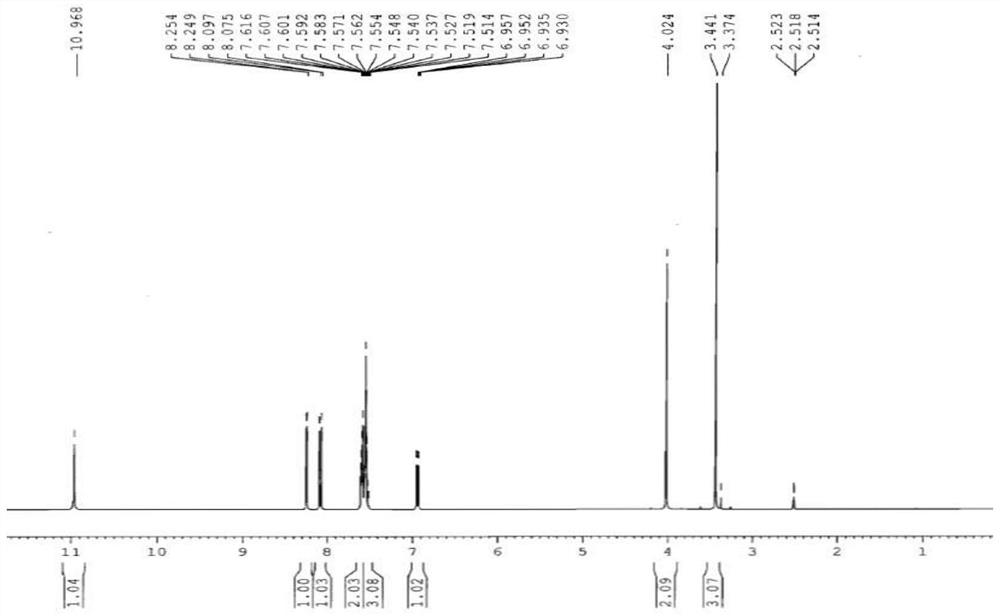
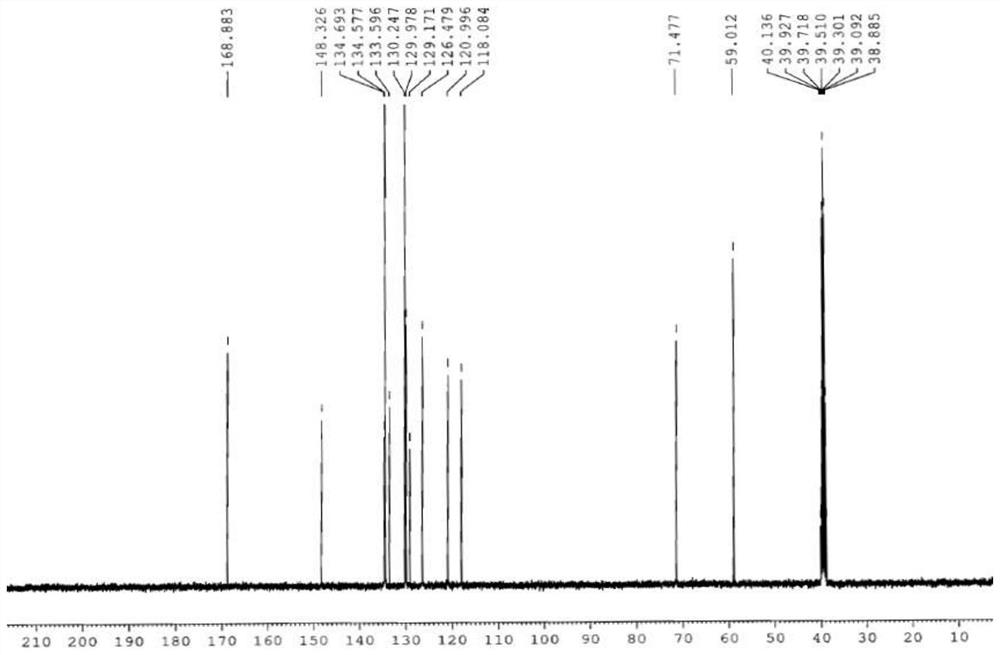
[0029] In a 500ml four-necked flask, add 190g xylene and 75.0g condensate (0.3mol), 27.02g (0.3mol) methoxyacetic acid and 7.32g (0.06mol) 4-dimethylaminopyridine, start stirring, and heat up to 65 °C, 41.04 g (0.345 mol) of thionyl chloride was added dropwise, and the addition was completed in about 2 hours. After the dropwise addition, the temperature was raised to reflux at 140° C., and the temperature was kept at reflux for 5 hours. The temperature was lowered to 35° C., and 38 g of water was added dropwise for 0.5 hours. After static separation, the lower aqueous layer was separated, the upper organic layer was concentrated under reduced pressure, and 152 g of xylene was collected. Add 240 g of methanol, lower the temperature to 15° C., and stir for 0.5 hours. Filter with suction, wash with 30 g of methanol, and dry the filter cake at 70° C. for 10 hours. 91.8 g of light yellow solid 2-methoxy-N-(2-nitro-5-phenylthio)phenylacetamide was obtained, with a yield of 94.6%....
Embodiment 2
[0036] Add 450g cyclohexane and 75.0g condensate (0.3mol), 32.43g (0.36mol) methoxyacetic acid and 18.21g (0.18mol) triethylamine into a 1000ml four-necked flask, start stirring, and heat up to 70°C. 44.61 g (0.375 mol) of thionyl chloride was added dropwise, and the addition was completed in about 4 hours. After the dropwise addition, the temperature was raised to reflux at 80° C., and the temperature was kept at reflux for 8 hours. The temperature was lowered to 50° C., and 50 g of water was added dropwise for 1 hour. After static separation, the lower aqueous layer was separated, the upper organic layer was concentrated under reduced pressure, and 385 g of cyclohexane was collected. Add 220 g of ethanol, lower the temperature to 20° C., and stir for 1 hour. Filter with suction, wash with 30 g of ethanol, and dry the filter cake at 80° C. for 10 hours. 90.9 g of light yellow solid 2-methoxy-N-(2-nitro-5-phenylthio)phenylacetamide was obtained. Yield 93.8%. Melting point...
Embodiment 3
[0038] Add 350g toluene and 75.0g condensate (0.3mol), 32.43g (0.36mol) methoxyacetic acid and 14.24g (0.18mol) pyridine into a 1000ml four-neck flask, start stirring, raise the temperature to 75°C, and add 47.59g dropwise (0.4mol) thionyl chloride, and the addition was completed in about 4 hours. After the dropwise addition, the temperature was raised to reflux at 110° C., and the temperature was kept at reflux for 6.5 hours. The temperature was lowered to 80° C., and 90 g of water was added dropwise for 1 hour. After static separation, the lower aqueous layer was separated, the upper organic layer was concentrated under reduced pressure, and 315 g of toluene was collected. Add 320 g of methyl ethyl ketone, lower the temperature to 20° C., and stir for 1 hour. Filter with suction, wash with 30 g butanone, and dry the filter cake at 80° C. for 10 hours. 91.9 g of light yellow solid 2-methoxy-N-(2-nitro-5-phenylthio)phenylacetamide was obtained. Yield 94.8%. Melting point:...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com