Anti-tumor platinum (II) complex
A technology of complexes and coordination compounds, which can be used in antitumor drugs, active ingredients of heavy metal compounds, drug combinations, etc., can solve the problems of poor solubility of cisplatin, limited application, limited dose toxicity, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
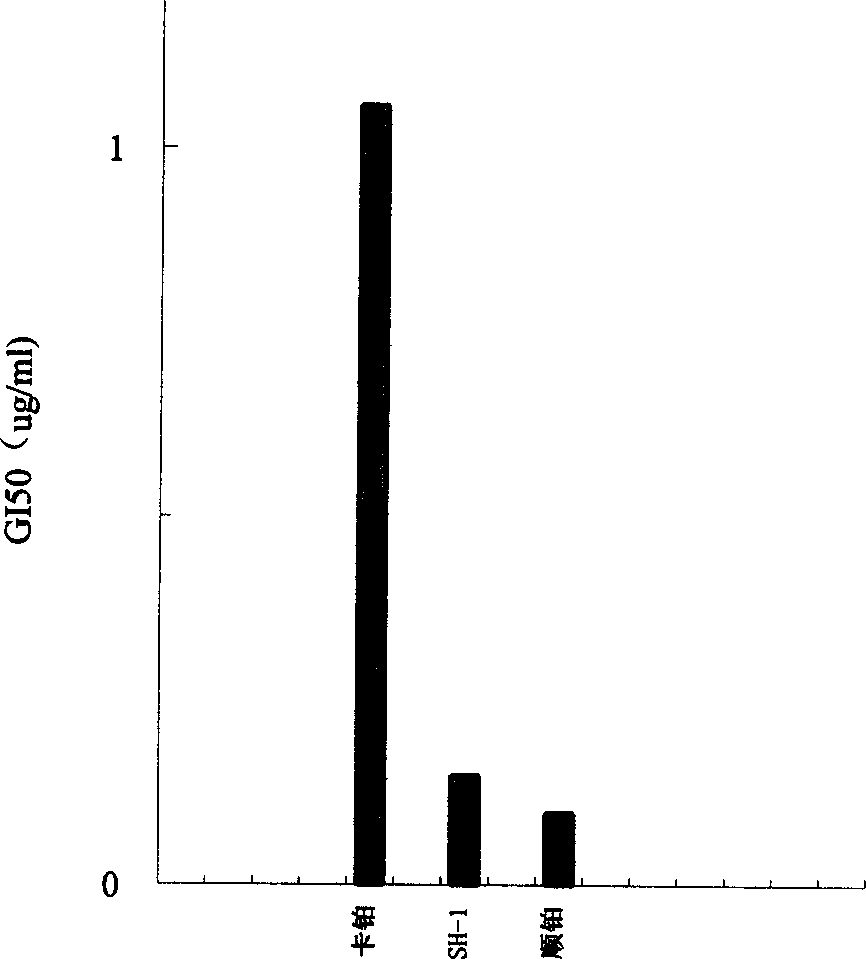
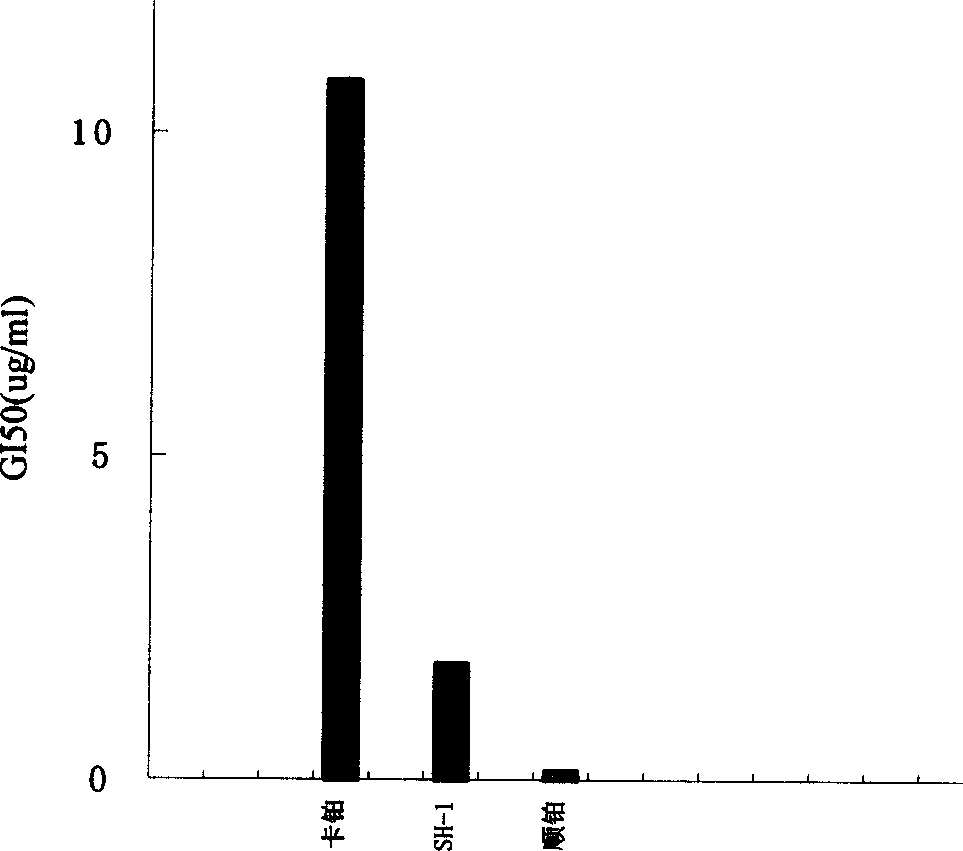
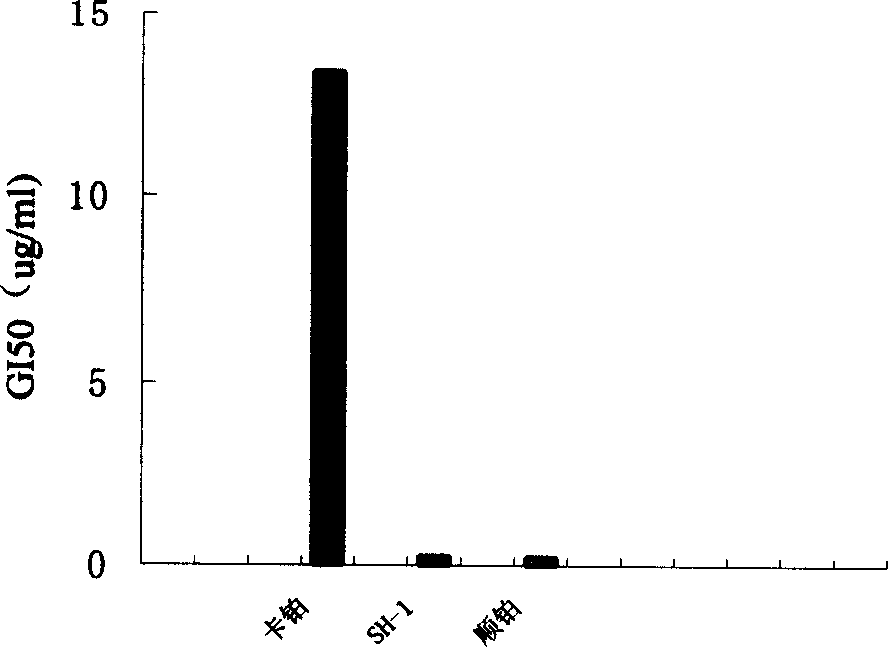
Image
Examples
Embodiment 1
[0017] Embodiment 1: Synthetic cis-bis(methoxy acetate) diammine platinum (II) with method B
[0018] Cis-diiododiammine platinum (II) (0.4 g, 0.84 mmol) was mixed with newly prepared silver methoxyacetate (0.33 g, 1.7 mmol), and water (40 ml) was added. Reacted for 24 hours, then assisted filtration with diatomaceous earth, concentrated the filtrate, precipitated an earthy white solid, filtered, and dried under vacuum at 60°C to obtain 0.12 g (35%) of the product.
[0019] IR(KBr): 3279s, 3222s, 3124s, 2989m, 2901m, 2827m, 1662vs, 1571vs, 1466m, 1423vs, 1393vs, 1335vs, 1284s, 1205s, 1117vs, 1015m, 989m
[0020] 1 H-NMR (D 2 O / TMS): δ3.34(m, 6H), 4.02(m, 4H)
[0021] ESI-MS: [M+Na] + =430(100%)
Embodiment 2
[0022] Embodiment 2: synthesizing cis-bis(methoxy acetate) diisopropylamine platinum (II) with method B
[0023] Cis-diiododiisopropylamine platinum (II) (1.13 g, 2 mmol) was mixed with newly prepared silver methoxyacetate (0.79 g, 4 mmol), and water (100 ml) was added, and exposed to light at 40°C. Nitrogen was reacted for 18 hours, assisted by diatomaceous earth filtration, the filtrate was concentrated, a white solid was precipitated, filtered, and vacuum-dried at 60°C to obtain 0.35 g (36%) of the product.
[0024] IR(KBr): 3451m, 3207s, 3106m, 1630s, 1449w, 1384vs, 1333m, 1298m, 1173m, 1119s, 1065m, 1031m
[0025] 1 H-NMR (DMSO-d 6 / TMS): δ1.25(m, 12H), 3.22(m, 6H), 3.36-3.85(m, 6H)
[0026] ESI-MS: [M+Na] + =514(100%)
Embodiment 3
[0027] Example 3: Synthesis of cis-bis(methoxyacetate)[(1R,2R)-1,2-trans-cyclohexanediamine]platinum(II) by method A
[0028] Cis-dichloro[(1R, 2R)-1,2-trans-cyclohexanediamine] platinum (II) (1.10 g, 2.9 mmol), silver nitrate (0.99 g, 5.8 mmol) were mixed and added to water ( 50 milliliters), 55 DEG C, shielded from light, reacted with nitrogen for 36 h, assisted filtration with diatomaceous earth, added an aqueous solution of methoxyacetic acid (0.52 g, 5.8 mmol) and sodium hydroxide (0.23 g, 5.8 mmol) to the filtrate, React at 30°C for 8 hours in the dark under nitrogen, then concentrate the solution, and a large amount of light yellow solid is precipitated. It was filtered, washed repeatedly with water, ethanol and ether, and dried under vacuum at 60°C to obtain 0.89 g (63%) of the product.
[0029] IR(KBr): 3449br, 3218s, 3111m, 2937m, 2861w, 2821w, 2362w, 2342w, 1633vs, 1449w, 1385s, 1338m, 1305m, 1120s, 1064w, 1035w
[0030] 1 H-NMR (D 2 O / TMS): δ1.08(m, 2H), 1.08(m...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com