Synthetic method of compound 2-cyclopropyl-1-(2-fluorophenyl)-2-carbonyl ethyl p-methylbenzensulfonate
A p-toluenesulfonate ester and synthetic method technology, applied in the preparation of sulfonate ester, organic chemistry, etc., can solve the problems of high toxicity, large environmental pollution, and low yield, and achieve short reaction cycle, environmental friendliness, and low toxicity Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
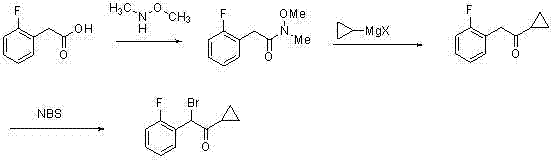
[0074] The method for synthesizing 2-cyclopropyl-1-(2-fluorophenyl)-2-carbonylethyl p-toluenesulfonate of the present invention will be described in detail below through specific experimental examples.
[0075] (1) Synthesis of o-fluoromandelic acid
[0076] Accurately weigh o-fluorobenzaldehyde (0.01mol, 1.24g), tetrabutylammonium bromide (0.0006mol, 0.19g) and put them into a three-necked flask equipped with 20mL of chloroform (both solvent and reactant) and a thermometer, Mix well and stir at 60°C for 15min. Then add 5.6g (mass concentration 40%) sodium hydroxide aqueous solution drop by drop from the dropping funnel to react, and keep stirring. After the addition, the temperature was maintained at 60°C to continue the reaction for 60 hours. After the reaction was completed, 100 mL of water was added to dilute and the reaction liquid was cooled to room temperature, and the lower layer of chloroform was separated. The aqueous phase was adjusted to pH 1 with 3mol / L hydroch...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com